Abstract
Descending neural pathways in the mammalian auditory system are known to modulate the function of the peripheral auditory system. These pathways include the medial olivocochlear (MOC) efferent innervation to outer hair cells (OHCs) and the acoustic reflex pathways mediating middle ear muscle (MEM) contractions. Based on measurements in humans (Marks and Siegel, companion paper), we applied a sensitive method to attempt to differentiate MEM and MOC reflexes using contralateral acoustic stimulation in mice under different levels of anesthesia. Separation of these effects is based on the knowledge that OHC-generated transient evoked otoacoustic emissions (TEOAE) are delayed relative to the stimulus, and that the MOC reflex affects the emission through its innervation of OHC. In contrast, the MEM-mediated changes in middle ear reflectance alter both the stimulus (with a short delay) and the emission. Using this approach, time averages to transient stimuli were evaluated to determine if thresholds for a contralateral effect on the delayed emission, indicating potential MOC activation, could be observed in the absence of a change in the stimulus pressure. This outcome was not observed in the majority of cases. There were also no statistically significant differences between MEM and putative MOC thresholds, and variability was high for both thresholds regardless of anesthesia level. Since the two reflex pathways could not be differentiated on the basis of activation thresholds, it was concluded that the MEM reflex dominates changes in TEOAEs induced by contralateral noise. This result complicates the identification of purely MOC-induced changes on OAEs in mice unless the MEM reflex is inactivated surgically or pharmacologically.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
For many years, researchers have studied the two major descending neural pathways to the auditory periphery: the olivocochlear (OC) efferent reflex and the middle ear muscle (MEM) reflex. The medial olivocochlear (MOC) efferents originate from the superior olivary complex and innervate outer hair cells (OHCs) in the cochlea (Guinan 2006). In addition, the MEM reflex is associated with two muscles: the stapedius innervated by the facial nerve (seventh cranial) and the tensor tympani innervated by the trigeminal (fifth cranial) nerve (Borg 1973). Although both reflexes can be activated by acoustic stimulation, there is evidence that a more intense stimulation is required to trigger the MEM reflex in humans, which is generally believed to be mediated by the stapedius muscle (Moller 1984; Collet et al. 1990). Whether this threshold difference holds in mice is currently unknown. Mice are widely used to study cochlear function through genetic manipulations, making it important to learn the relative thresholds of these two descending pathways in wild-type mice as a prelude to further experimental investigations. Our study, therefore, attempted to evaluate the relative thresholds of both MEM and MOC reflexes in unanesthetized mice. We also evaluated the degree to which the effects of contralateral noise are influenced by anesthesia, which has been reported to attenuate the strength of the MOC efferent reflex in guinea pigs and mice (Boyev et al. 2002; Chambers et al. 2012).
It is sometimes difficult to distinguish MOC- and MEM-mediated effects since they can be co-activated by the same acoustic stimuli. Although the MOC efferent pathway is known to influence cochlear function and reduce cochlear responses, the suppressive effects of the MOC reflex on otoacoustic emissions (OAEs) are difficult to distinguish from the effects of MEM contractions, which alter the reflectance of the middle ear. Muscle contractions can change the energy absorbed by the cochlea, as well as the reverse transmission of OAE signals through the middle ear (Mukerji et al. 2010).
In this study, we performed a time-domain measurement to detect MEM/MOC thresholds in mice. The reflexes were elicited by contralaterally presented broadband noise. Separation of MEM from MOC effects is based on the knowledge that changes in OAEs are delayed, reflecting the round-trip time needed for the traveling wave to propagate to the cochlear region where the OAEs are generated and then for the emission to be coupled back out to the ear canal. In contrast, MEM contractions affect eardrum impedance and the associated pressure almost instantaneously (Borg 1973). Thus, MEM activation can be identified by its early effect on the ear canal pressure within the time interval of the transient stimulus (Marks and Siegel, companion paper). This activation of the MEM reflex can also induce subsequent changes in the delayed OAEs.
Even though conventional clinical tests of MEM contraction thresholds in humans are not sensitive enough to detect small changes in eardrum impedance, the MEM reflex can still be sufficient to suppress OAEs, thereby confounding the measurement of MOC-mediated suppression of OAEs (Neumann et al. 1996). A sensitive method for detecting MEM contractions could provide a more rigorous way to monitor MEM contraction. For instance, MEM-mediated suppression of OAEs evoked with a pure tone has been identified via group delay measurements of the vector change induced by contralateral acoustic stimuli (CAS) in a frequency-domain protocol (Guinan et al. 2003). In the present study, we applied a sensitive alternative approach that detects MEM activation that may contaminate MOC efferent effects on transient evoked OAEs (TEOAEs) (Goodman et al. 2013; Boothalingam and Purcell 2015; Mertes and Goodman 2016) (Marks and Siegel, companion paper). Considering that anesthesia attenuates the strength of both MOC and MEM reflexes (Borg and Moller 1975; Chambers et al. 2012), we developed a protocol to perform the measurement in unanesthetized mice.
METHODS
Animal Preparation
We employed CBA/J and 129/C57BL/6J mice (both sexes; average age 51.4 ± 17.1 days) since these genetic backgrounds are widely used in auditory research. To record from unanesthetized mice, it is necessary to prevent head movements. A titanium head plate was, therefore, affixed to the skull of each mouse under surgical anesthesia, so that we could immobilize the head during emission measurements (Fig. 1). Data collection began at least 3 days after these minor survival surgeries, guaranteeing the animal’s successful recovery. During measurements, mice were initially anesthetized to place the emission probe and the contralateral sound system, but were tested under reduced or no anesthesia. There were four experimental groups. Mice in group A (n = 8) were injected with ketamine (100 mg/kg ip) and xylazine (10 mg/kg ip), and measurements were taken both under anesthesia (typically 15 min post-induction) and during recovery from anesthesia (typically 40–60 min post-induction). Groups B (n = 18), C (n = 24), and D (n = 6) were given isoflurane (2–3 %) during experimental setup, but then received different percentages of isoflurane during recording. For example, in groups B (0.5 %) and D (1.0 %), isoflurane was provided to minimize movement artifacts and vocalizations in order to facilitate the recordings. Mice in group B showed toe pinch reflexes, whisker, and uncoordinated limb movements during the recording, while animals in group D were areflexive. Animals in group C (0 %) were fully conscious during recordings. Although they received no isoflurane, the same tubing with room air flowing was presented for group C animals to ensure a similar level of background noise. The number of CBA/J mice was 4, 8, 7, and 3 in groups A, B, C, and D, respectively. The number of 129/C57BL/6J mice was 4, 10, 17, and 3 in groups A, B, C, and D, respectively. All procedures were approved by the Northwestern University’s Institutional Review Board and by the National Institutes of Health.
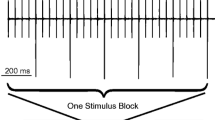
A cartoon of the animal with head plate along with the time-domain measurement procedures. Titanium head plates were affixed to the mouse skull to prevent head movements during recordings. The time-domain measurement contains two parts: (1) TEOAE screening and (2) CAS recording. During the CAS measurements, the 1429 ms broadband noise was presented to the contralateral ear as a sequence interleaved with silent periods of equal length. CAS levels ranged from 55 to 110 dB SPL in 5 or 10 dB steps. Tone pips were presented to the ipsilateral ear 593 ms after the onset and offset of the CAS. Blocks of stimulus presentations were repeated to reach the final number of presentations in the time average used to compute ΔP stim or ΔP TEOAE.
Placement of the Acoustic Systems
During the initial anesthesia, the acoustic systems were coupled to the animal’s ears. For the ipsilateral ear, a custom probe equipped with a sensitive microphone (Knowles Electronics, FG-3652-CX) was placed close to the eardrum. A sound calibration was then performed using a chirp generated in SYSRES (Neely and Stevenson 2002). For the contralateral ear, a separate speaker was coupled to the pinna using a soft tube. After placing the tip of the soft tube close to the eardrum, the tube was glued to the ear canal to maintain its position during the measurements. The sound sources were modified Radio Shack, Realistic #40-1310B Super Tweeters.
Recording Procedures
Animals in all four groups received a stimulus frequency OAE (SFOAE) screening and an initial TEOAE measurement while under anesthesia. For example, mice in group A received the injectable anesthesia, while mice in groups B, C, and D were given 1.5 % isoflurane, a standard maintenance dose. Discrete tones ranging from 4 to 38 kHz in 1.2-kHz steps were used to elicit SFOAEs using the suppression method (Shera and Guinan 1999) where the suppressor tone was 23 Hz below the probe. Probe and suppressor tones were presented at 30 and 60 dB sound pressure level (SPL), respectively. The stimulus producing the largest SFOAE was selected as the probe frequency for the TEOAE recordings since it appears that these two OAEs originate from the same phenomenon (Siegel et al. 2011). This choice tended to assure that the TEOAEs were relatively large, allowing any CAS effects to be observed. The average probe frequency was 20.8 ± 3.1 kHz and corresponds to the cochlear region where mice generate strong OAEs and where the MOC efferent innervation density peaks in mice (Maison et al. 2003, 2007).
These time-domain measurements contained two steps (Fig. 1). The first was a TEOAE measurement using the compression method (Kemp and Chum 1980). The emission evoked by a moderate-level tone pip (the “probe”) was extracted from the stimulus by predicting the stimulus pressure from the response to a higher-level tone pip (the “reference”). In this approach, the TEOAE is presumed to be saturated at the higher level, thereby contributing insignificantly to the total pressure. Probe stimuli were 0.25-ms tone pips gated by cosine ramps with 0.125-ms rise/fall times. The probe was initially presented at 50 dB peak equivalent SPL (peSPL) to evoke a TEOAE. If the response was more than 6 dB above the noise floor (NF), then this probe level was used for the subsequent recordings. However, if the TEOAE level was less than 6 dB above NF floor, a 60-dB peSPL probe was used to elicit TEOAEs. In all cases, the evoked TEOAEs were at least 6 dB above the NF. Reference stimuli were 20 dB above the level of the probe.
The second step involved measurements of ear canal pressure changes during the presentation of different levels of CAS, which consisted of a broadband noise (4 to 40 kHz) with a duration of 1429 ms. This CAS was interleaved with silent periods of equal length (as shown in Fig. 1), such that tone pips were presented to the ipsilateral ear with and without the CAS. In order to allow MOC and/or MEM effects to be activated, the CAS was turned on 593 ms prior to the presentation of a train of probe stimuli to the ipsilateral ear, which appears to be sufficient for the effects to reach steady state (Valero et al. 2016). Similarly, control responses were measured 593 ms after the beginning of the silent period following the CAS (see Fig. 1). Because these measurements were repeated 128 times for each with and without CAS, it took ∼6 min to collect the data for a single level of CAS. In other words, two intervals of 1.429 s = 2.858 s times 128 samples = 356.8 s. Software developed using Visual Basic 6.0 also incorporated a trial rejection feature that allowed noisy samples to be eliminated. Since the degree of limb movement or vocalization ranged widely, the additional time added to the data collection varied but was usually less than 30 s. Customized analysis programs also allowed us to subtract ear canal pressures recorded when the CAS was on versus off, thereby extracting the changes of ear canal pressure induced by CAS. For each measurement, the averaged responses were divided into two sub-averages from which separate “signal” and “noise” buffers were computed from the average and difference between the two buffers (Marks and Siegel, companion paper). The signal buffer was used to identify the pressure changes due to central reflex activation, and the noise buffer was used to estimate the NF. Digital band-pass filters centered at the probe frequency were applied to the recorded TEOAEs and ear canal pressure changes induced by CAS to optimize the signal-to-noise ratio prior to further analysis. The bandwidth was selected based on the spectrum of the probe stimulus for each individual recording. The bandwidth was approximately 10-kHz wide and centered at the probe frequency so that the stimulus magnitudes at the corner frequencies were ∼10 dB lower than the stimulus magnitude at the center frequency. The signal and noise buffers were processed identically. Additional details are provided in the companion paper by Marks and Siegel.
Analysis
Pressure changes in the ipsilateral ear canal with various levels of CAS were examined in two intervals: (1) pressure changes during the time interval of the probe stimulus, referred to as ΔP stim and (2) pressure changes during the time interval of the delayed TEOAE, referred to as ΔP TEOAE. In order to separate these two pressure changes in time, two analysis windows were selected. The first window (0.5 ms in length) was centered on 0 ms to include ΔP stim (red-shaded region from −0.25 to 0.25 ms in Figures 2, 3, 4, 5, and 6). The second window was centered approximately on the midpoint of the TEOAE envelope (purple shaded region in Figs. 2, 3 and 6). Although the center of the latter window varied slightly depending on probe frequency, the window length was 0.5 ms and centered at a time that avoided overlap between the analysis windows to provide a clear separation between ΔP stim and ΔP TEOAE in time. Sometimes ΔP TEOAE in the earliest portion of the TEOAE waveform could not be included in the analysis. This compromise was required, given that the delay between the stimulus and the TEOAE in mice is much shorter than for humans (Marks and Siegel, companion paper).
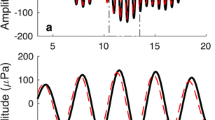
An example of CAS-induced ΔP stim and/or ΔP TEOAE in an animal (43 days of age) from group C. The probe and reference were 60 and 80 dB peSPL tone pips at 21.57 kHz. a Time waveforms of probe, TEOAE, and changes in the ear canal pressure induced by different levels of CAS. Shaded regions designate time intervals for the stimulus (red) and the response/TEOAE (Franklin et al. 2007). a On the right side of the ordinate, we also indicate the change in pressure as ΔP in micropascal. b RMS values of ΔP stim (red), ΔP TEOAE (purple) and the average (±1 standard deviation) rms value of the NF (gray) for each tested CAS level. NF is the average noise floor in 50 non-overlapping windows (0.5 ms in duration) randomly selected in the non-stimulus portion of each recording. The gray shaded area represents rms values below the average NF. Notice that ΔP stim at CAS levels of 60, 65, and 70 dB SPL is within this region. c CC, the Pearson product-moment correlation coefficient, of the probe and ΔP stim during CAS at 75 dB SPL (red), as well as the TEOAE and ΔP TEOAE for CAS at 60, 65, 70, and 75 dB SPL (purple). CCs below 0.3 were marked as no correlation. When rms values are below the NF, the corresponding CC is not plotted. In other words, no CC is plotted for ΔP stim when the CAS was presented at 60, 65, and 70 dB SPL.
An example of CAS-induced ΔP stim and/or ΔP TEOAE in an animal (43 days of age) from group C. The figure format is identical to that of Fig. 2. The probe and reference were 60 and 80 dB peSPL, respectively, and the stimulus frequency was 16.88 kHz. a Time waveforms of probe, TEOAE, and CAS-induced changes in ear canal pressure. b RMS values of ΔP stim (red), ΔP TEOAE (purple) and the average (±1 standard deviation) rms NF (gray) for each tested CAS level. When the CAS was 50 dB SPL, both ΔP stim and ΔP TEOAE were in this range. c CC of the probe and the ΔP stim (red), as well as the TEOAE and the ΔP TEOAE (purple) during CAS at 60, 70, and 80 dB SPL. Because rms values for both ΔP stim and ΔP TEOAE were below the NF when the CAS was presented at 50 dB SPL, a CC was not plotted.
a The average ΔP stim (red, n = 50) and ΔP TEOAE (purple, n = 15) thresholds were 94.4 ± 13.5 and 90.0 ± 7.1 dB SPL in group A, 78.8 ± 13.1 and 70.6 ± 13.1 dB SPL in group B, and 75.3 ± 14.7 and 69.4 ± 14.0 dB SPL in group C. Error bars represent standard deviations. b The proportion of animals showing ΔP TEOAE effects without ΔP stim in groups A (25 %), B (27.8 %), and C (33.3 %). c The average differences of ΔP stim (red) and ΔP TEOAE thresholds in animals who showed exclusive ΔP TEOAE were 7.5 ± 3.5 (n = 2), 9.2 ± 4.7 (n = 5), and 9.8 ± 5.7 (n = 8) dB in groups A, B, and C respectively.
TEOAEs recorded from group A when the animal was anesthetized and during recovery. The probe and reference levels were 50 and 70 dB peSPL tone pips at 23 kHz. a Probe time waveform. bTEOAE time waveform. c, e Changes induced by CAS at 110 dB SPL. d, f Changes induced by CAS at 100 dB SPL. There was no acoustic cross talk at either 100 or 110 dB SPL.
To assess whether a pressure change was CAS related, two additional analyses were conducted based on the magnitude and morphology of the ΔP stim and ΔP TEOAE, since the spectral analysis of these pressure changes cannot clearly quantify whether they were CAS induced as shown in Marks and Siegel (companion paper). This sequential analysis was required because mouse data are more prone to movement artifact than human data. If mouse movements do occur, they can cause amplitude and/or time differences between the sub-averages used to calculate signal and noise buffers, with the result that signal-like responses appear in the noise buffer even though trial rejection minimizes this complication. Using this approach, we first examined whether a ΔP stim or ΔP TEOAE was above the noise. In order to estimate the NF, 50 non-overlapping windows (0.5 ms in length) were randomly selected in the non-stimulus portion, i.e., the interstimulus interval, of the time averages of the noise buffer. For each recording, the rms value of the noise within these windows was calculated and averaged to serve as the NF level. A pressure change was determined to be CAS related only when the rms value of ΔP stim or ΔP TEOAE was larger than the corresponding NF level by any amount. Comparisons were also made between the rms value and the average NF plus one standard deviation. Threshold determinations were not different when calculated using these two methods.
For ΔP stim and/or ΔP TEOAE that passed the rms criterion, the morphology of its time waveform was evaluated in the second phase of the analysis. The Pearson product-moment correlation coefficient (CC) of the waveforms of ΔP stim and probe stimulus, as well as of the ΔP TEOAE and TEOAE were calculated. The ΔP stim delay was calculated as the time difference between the center of the ΔP stim envelope and the center of the stimulus (0 ms). The slight delay in ΔP stim relative to the probe stimulus pressure, presumed to be due to the conduction time of the reflected stimulus pressure in the ear canal, was corrected when calculating the CC. For example, if the ΔP stim delay relative to the probe stimulus were 0.01 ms, then the probe stimulus pressure from −0.25 to 0.24 ms and the ΔP stim from −0.24 to 0.25 ms would be used to calculate the CC. The CC shows the degree to which the pressure change, either ΔP stim or ΔP TEOAE, represents the morphology of the original waveform, i.e., the probe or the TEOAE. This waveform analysis served as a second criterion, the CC criterion, to assess whether a pressure change is due to CAS: CC > 0.8 indicates a strong correlation; 0.3 < CC < 0.8 indicates a moderate correlation; CC < 0.3 indicates no correlation. Only a moderate or strong correlation would indicate that a pressure change is due to CAS.
Using the rms and CC criteria, it was possible to assess whether a ΔP stim or ΔP TEOAE was induced by CAS and to determine a threshold for this effect. The rms of the magnitude of ΔP stim or ΔP TEOAE was initially compared with its corresponding NF measure. If either ΔP stim or ΔP TEOAE passed the magnitude criterion, then its morphology was examined through CC calculations. If not, the pressure change was considered negligible and CC was not calculated. Only when the CC showed a moderate to strong correlation was a pressure change determined to be CAS related. The lowest tested CAS levels required to activate a ΔP stim or ΔP TEOAE were defined as the ΔP stim or ΔP TEOAE threshold for each animal. It was reasoned that the presence of a CAS evoked ΔP stim (usually followed by a CAS evoked ΔP TEOAE) indicated a MEM-mediated eardrum impedance change at this particular CAS level. Alternatively, it was reasoned that the presence of a CAS that evoked ΔP TEOAE without a corresponding ΔP stim for a particular CAS level would indicate that the TEOAE was affected without MEM activation, as would be expected if the change resulted from MOC activation.
RESULTS
Temporal Separation of CAS Effects on Ear Canal Pressure Measurements
In some animals, both CAS-induced ΔP stim and ΔP TEOAE were observed, as shown in Figure 2 where a complete set of data from one animal in group C is used to illustrate the analysis. The TEOAE (Fig. 2a) evoked in this animal had an envelope with a midpoint of approximately 0.7 ms. Thus, a time window centered at 0.7 ms was selected (purple shaded region in Figure 2) to assess ΔP TEOAE, while ΔP stim waveforms were centered at 0 ms (red shaded region in Fig. 2). Because the onset of the TEOAE occurred at ∼0.2 ms, the first part of the TEOAE and ΔP TEOAE were not included in the analysis.
CAS-induced ΔP stim and ΔP TEOAE were seen when the CAS was 75 dB SPL based on rms and CC criteria: the rms values of ΔP stim and ΔP TEOAE were larger than the average NF level (Fig. 2b), and the CC values confirmed a strong correlation between the waveforms of ΔP stim and the probe, as well as a moderate correlation between the waveforms of ΔP TEOAE and the TEOAE (Fig. 2c). The existence of ΔP stim implies that the MEM reflex was likely responsible for the subsequent ΔP TEOAE. Thus, the MEM reflex was activated by a 75 dB SPL CAS, producing the detected ΔP stim, and contributing at least partially to the following ΔP TEOAE presumably through bidirectional changes in middle ear transmission. When the CAS level was 60, 65, or 70 dB SPL, it induced a ΔP TEOAE without a detectable ΔP stim: the rms values of the ΔP stim were smaller than their corresponding NF levels, whereas the ΔP TEOAE had rms values that exceeded the NF (Fig. 2b). When CAS level was 65 and 70 dB SPL, ΔP TEOAE demonstrated moderate correlations with TEOAEs, but no correlation was seen when CAS was 60 dB SPL (Fig. 2c). In this animal, the ΔP stim threshold was designated as 75 dB SPL, the ΔP TEOAE threshold as 65 dB SPL. Although similar results arose in groups A, B, and C, pressure changes in the delayed TEOAE window independent of those in the early stimulus time window were not common.
In other animals, no CAS-induced ΔP TEOAE was seen without CAS-induced ΔP stim as shown by a representative example from group C in Figure 3. In this animal, ΔP stim waveforms were centered at 0 ms and ΔP TEOAE waveforms at 0.7 ms. Thresholds for both time intervals were 60 dB SPL and similar behavior was observed in groups A, B, and C. Although the results in Figures 3b, c indicate that the growth in rms values and in the CCs was different for ΔP stim and ΔP TEOAE, the functional significance of these differences or whether they could be probed to disentangle the contribution of MEM versus MOC reflexes is currently unknown.
ΔP stim and ΔP TEOAE Threshold Comparisons
The lowest CAS level required to generate ΔP stim or ΔP TEOAE was defined as the ΔP stim or ΔP TEOAE threshold. As shown in Figure 4b, ΔP TEOAE thresholds could be lower than ΔP stim thresholds, but only in 25 % (group A, n = 2) of the mice recovering from ketamine/xylazine, 27.8 % (group B, n = 5) of mice maintained on 0.5 % isoflurane and 33.3 % (group C, n = 8) of the animals receiving 0 % isoflurane. For groups A, B, and C, only 15 of 50 mice (30 %) showed a lower estimated ΔP TEOAE threshold compared to ΔP stim. For this subset of animals (n = 15), the average threshold difference between the two pressure changes (Fig. 4c) was 7.5 ± 3.5, 9.2 ± 4.7, 9.8 ± 5.7 dB in groups A, B, and C, respectively. A Student’s t test showed no significant difference between the ΔP stim and ΔP TEOAE thresholds in groups A (p = 0.49), B (p = 0.23), and C (p = 0.48), respectively. In Figure 4a, average ΔP stim thresholds (red) are presented for all mice along with the average ΔP TEOAE thresholds (purple) for mice where ΔP TEOAE occurred at lower CAS levels than for the ΔP stim. In group A, ΔP stim thresholds varied from 65 to 110 dB SPL (n = 8), while the ΔP TEOAE thresholds ranged from 85 to 95 dB SPL (n = 2). In group B, ΔP stim thresholds ranged from 60 to 100 dB SPL; the ΔP TEOAE thresholds from 55 to 85 dB SPL. In group C, ΔP stim thresholds varied from 55 to 110 dB SPL, and the ΔP TEOAE thresholds from 55 to 100 dB SPL. Comparisons between groups suggest that ΔP stim thresholds were significantly higher in group A compared with groups B (p = 0.0072) and C (p = 0.0034), indicating that the injectable anesthesia had a lingering suppressive effect on the strength of ΔP stim thresholds. A similar comparison was not made for ΔP TEOAE thresholds because there were only two mice in group A with a ΔP TEOAE threshold lower than that for ΔP stim. We should point out, however, that there was no significant difference between the ΔP stim and ΔP TEOAE thresholds in groups B and C. This observation suggests that 0.5 % isoflurane had no obvious influence on ΔP stim and ΔP TEOAE thresholds.
Anesthesia Diminishes Brainstem Acoustic Reflexes
Our results showed that 1 % isoflurane reduces TEOAEs in mice (Fig. 5). Animals had reduced TEOAEs (Fig. 5b, c) after 60 min under 1 % isoflurane, which is below the 1.5 % maintenance level commonly used for OAE recordings in mice. The average TEOAE reduction for mice in group D was 9.0 ± 7.4 dB. Larger TEOAE level decreases were observed with higher dosages or longer exposures to isoflurane.
Anesthesia Suppresses CAS Effects
Our results confirm that anesthesia subdues CAS effects on TEOAEs. For example, changes induced by CAS were measured during ketamine/xylazine anesthesia and during recovery in group A, as shown in Figure 6. Probe stimulus and TEOAE response waveforms are shown in Figure 6a, b, respectively. Pressure changes were induced by CAS levels at 110 and 100 dB SPL when the animal was under anesthesia (Fig. 6c, d) (15 min after initial injection of ketamine/xylazine) and recovering from anesthesia (Fig. 6e, f) (45 min after initial injection). ΔP TEOAE and ΔP stim were not robust during anesthesia when the CAS was at 110 dB SPL, but emerged 30 min later as the animal recovered from anesthesia (Fig. 6c, e). ΔP stim for CAS at 100 dB SPL also occurred during recovery (Fig. 6d, f). Similar effects have been observed in all mice from group A. The magnitude of ΔP (including both ΔP stim and ΔP TEOAE) induced by the highest tested CAS increased about 8.0 ± 7.6 dB after animals recovered from anesthesia. These results confirm that anesthesia suppresses CAS effects in mice (Chambers et al. 2012).
DISCUSSION
This is the first study to attempt to evaluate both MOC and MEM reflex thresholds in awake mice using a sensitive method to identify contralaterally induced MEM contractions that may contaminate MOC efferent effects on TEOAEs. CAS-induced ΔP stim indicated changes in stimulus pressure, which likely relate to activation of the MEM reflex. Thus, ΔP stim thresholds are taken to represent MEM thresholds. Although the existence of ΔP stim implies that the MEM reflex was likely responsible for the subsequent ΔP TEOAE, it is unclear whether there is an additional mechanism involved in the ΔP TEOAE. For example, a small proportion of animals showed CAS-induced ΔP TEOAE in the absence of obvious ΔP stim. Although uncommon, this observation implies that there may be an additional central mechanism triggered by CAS and ultimately influencing TEOAEs. Previous investigations observed CAS effects on distortion product otoacoustic emissions (DPOAEs) in unanesthetized mice and claimed that these effects were mediated by the MOC reflex (Chambers et al. 2012). Based on these results, one possible explanation for the exclusive ΔP TEOAE measured here is that it is mediated by the MOC efferent reflex. Another possible contributor to ΔP TEOAE is the “unknown pathway” known to suppress DPOAEs measured with CAS (Maison et al. 2012). The relative contributions from the contralateral MOC reflex and/or the unknown pathway are difficult to quantify. It is, therefore, unclear whether purely MOC effects were recorded even though ΔP TEOAE thresholds somewhat below the ΔP stim thresholds were seen in ∼30 % of the animals. In most of the animals, the difference between the two thresholds, if any, did not exceed the 5–10 dB CAS step size used in the experiments. It is also possible that small MEM contractions did not produce a ΔP stim that met our detection criteria, but that the effect on the emission was detected because the MEM contraction affects both inward propagation of the stimulus and outward propagation of the emission. In spite of these caveats, however, it is at least feasible that a mixed effect occurred in a few cases, similar to a previous report on stimulus frequency OAEs in humans (Guinan et al. 2003).
Our data also show that lower ΔP TEOAE thresholds were seen more frequently with decreasing anesthesia level: including groups A and B in which animals were partially anesthetized, as well as group C in which mice were unanesthetized. However, even the frequency of observing this for group C is still too low to conclude that purely MOC effects can be recorded in unanesthetized mice without disabling the MEM effects. In fact, there were no statistically significant differences between ΔP stim and ΔP TEOAE thresholds across groups A, B, and C. Assuming the exclusive ΔP TEOAE measured was mediated by MOC reflex, then one possible explanation is that MEM and MOC thresholds may be activated by similar levels of contralateral broadband noise in mice, as reported previously in rats (Relkin et al. 2005; Mukerji et al. 2010). Independent of whether or not the MOC reflex was involved in the exclusive ΔP TEOAE seen on rare occasions, we cannot reject the hypothesis that the MEM reflex dominates CAS effects on TEOAEs in partially and fully unanesthetized mice. Similar MEM-dominated CAS effects on DPOAEs have been reported in anesthetized mice (Valero et al. 2016). Taken together, these results complicate the use of CAS to study the MOC efferent pathway in mice unless the MEM reflex is inactivated surgically or pharmacologically.
Based on the time-domain measurements reported here, the MEM (ΔP stim) thresholds varied across animals in groups A, B, and C. Although MEM reflex thresholds can be affected by different levels of anesthesia (Borg and Moller 1975), this variation in MEM thresholds was observed independent of the type and the degree of anesthesia in our experiments. The MOC thresholds, assuming that the ΔP TEOAE was in fact MOC-mediated, showed large threshold variations as well. In contrast to humans, a particular CAS level cannot be used a priori to evoke MOC but not MEM reflexes due to the wide range of thresholds, and the lack of clear separation between the putative MEM and MOC effects. This variability further complicates MOC studies in mice.
The time-domain approach revealed MEM thresholds in mice as low as 55 dB SPL, consistent with previous studies where MEM contractions were monitored using the cochlear microphonic (CM) and electromyography (EMG) (van den Berge et al. 1990). SFOAE-based techniques also indicate that MEM thresholds in humans are between 55 and 60 dB SPL (Guinan et al. 2003; Goodman and Keefe 2006; Zhao and Dhar 2010). This SFOAE-based noninvasive method showed unequivocally better sensitivity than the middle ear analyzers used clinically. Unfortunately, SFOAE-based techniques rely on time-consuming offline analysis of vector changes in the ear canal pressure induced by CAS to assess whether changes are MEM associated (Sun 2008). In contrast, the time-domain measurement adopted here provides a real-time method to assess MEM thresholds with the sensitivity of OAE recordings and could be adapted for both clinical and research purposes. In fact, monitoring the MEM reflex may be valuable in the early detection of cochlear neuropathy (Valero et al. 2016).
Results from this study also indicate that MEM effects can be observed at high frequencies in mice. In our experiments, the tone pips used to generate TEOAEs ranged from 12 to 28 kHz, and the effects of MEM contractions were evident at these frequencies. Hence, these observations confirm the results of Valero and colleagues, where MEM-mediated changes were seen at frequencies up to 32 kHz (Valero et al. 2016). Effects of middle ear muscle contractions can, therefore, be readily measured in the 30 kHz range, even though the maximal middle ear attenuation was reported to occur at ∼1 kHz in mice (van den Berge et al. 1990).
Our results also confirm that anesthesia reduces the effects of contralateral noise on TEOAEs that appear to be dominated by the MEM reflex but with possible contributions from the MOC reflex that are difficult to separate/distinguish (Borg and Moller 1975; Chambers et al. 2012). The injectable anesthesia used in our study, ketamine and xylazine, increased average reflex thresholds by 15–20 dB. This anesthesia-induced attenuation prevents a thorough evaluation of the mechanisms and functions of these two descending pathways in anesthetized mice. Anesthesia effects have also been documented in the auditory afferent pathways. Neurons in the inferior colliculus in awake rabbits showed higher spontaneous activity and greater sustained responses, both excitatory and inhibitory, than in anesthetized animals (Chung et al. 2014). Although auditory neural circuits have been extensively studied in anesthetized animals, anesthesia serves as a confounding factor for investigating neural responses. Thus, there is a need to develop methods to study the auditory neural pathways in awake animals.
Our results also showed that isoflurane (1 % and above) has a suppressive effect on TEOAEs in mice that usually engages after ∼15 min and ultimately minimizes TEOAEs after ∼1 h. Similar effects have been observed in DPOAEs in mice (Cederholm et al. 2012) with a similar time course (Charaziak and Siegel, private communication). Similar effects have also been observed on other types of OAEs. In contrast, low-level isoflurane (0.5 %) and the injectable anesthesia, ketamine, and xylazine, have little effect on DPOAEs (Kim et al. 2012). A thorough investigation regarding the suppressive effect of different levels of isoflurane on OAEs, as well as the underlying mechanism, is needed.
In conclusion, the time-domain approach provides a sensitive method to identify central reflex effects on TEOAEs induced by CAS. Although this method can theoretically be used to assay MOC effects on OAEs, it was not possible in this study to repeatedly demonstrate an exclusive MOC activation independent of MEM contamination even in unanesthetized animals. In the absence of clear MOC effects on TEOAEs, the CAS appears to co-activate both pathways with the MEM reflex dominating the effects in unanesthetized mice. Future studies on mice should, therefore, employ procedures to directly inactivate the MEMs in order to assay the influence of the MOC reflex on cochlear function.
References
Boothalingam S, Purcell DW (2015) Influence of the stimulus presentation rate on medial olivocochlear system assays. JAcoust Soc Am 137:724–732
Borg E (1973) On the neuronal organization of the acoustic middle ear reflex. A physiological and anatomical study. Brain Res 49:101–123
Borg E, Moller AR (1975) Effect of central depressants on the acoustic middle ear reflex in rabbit. A method for quantitative measurements of drug effect on the CNS. Acta Physiol Scand 94:327–338
Boyev KP, Liberman MC, Brown MC (2002) Effects of anesthesia on efferent-mediated adaptation of the DPOAE. J Assoc Res Otolaryngol: JARO 3:362–373
Cederholm JM, Froud KE, Wong AC, Ko M, Ryan AF, Housley GD (2012) Differential actions of isoflurane and ketamine-based anaesthetics on cochlear function in the mouse. Hear Res 292:71–79
Chambers AR, Hancock KE, Maison SF, Liberman MC, Polley DB (2012) Sound-evoked olivocochlear activation in unanesthetized mice. J Assoc Res Otolaryngol: JARO 13:209–217
Chung Y, Hancock KE, Nam SI, Delgutte B (2014) Coding of electric pulse trains presented through cochlear implants in the auditory midbrain of awake rabbit: comparison with anesthetized preparations. J Neurosci. Off J Soc Neurosci 34:218–231
Collet L, Kemp DT, Veuillet E, Duclaux R, Moulin A, Morgon A (1990) Effect of contralateral auditory stimuli on active cochlear micro-mechanical properties in human subjects. Hear Res 43:251–261
Franklin JC, Moretti NA, Blumenthal TD (2007) Impact of stimulus signal-to-noise ratio on prepulse inhibition of acoustic startle. Psychophysiology 44:339–342
Goodman SS, Keefe DH (2006) Simultaneous measurement of noise-activated middle-ear muscle reflex and stimulus frequency otoacoustic emissions. J Assoc Res Otolaryngol: JARO 7:125–139
Goodman SS, Mertes IB, Lewis JD, Weissbeck DK (2013) Medial olivocochlear-induced transient-evoked otoacoustic emission amplitude shifts in individual subjects. Journal of the Association for Research in Otolaryngology : JARO
Guinan JJ Jr (2006) Olivocochlear efferents: anatomy, physiology, function, and the measurement of efferent effects in humans. Ear Hear 27:589–607
Guinan JJ Jr, Backus BC, Lilaonitkul W, Aharonson V (2003) Medial olivocochlear efferent reflex in humans: otoacoustic emission (OAE) measurement issues and the advantages of stimulus frequency OAEs. J Assoc Res Otolaryngol: JARO 4:521–540
Kemp DT, Chum R (1980) Properties of the generator of stimulated acoustic emissions. Hear Res 2:213–232
Kim JU, Ahn YS, Suh JK, Chung JW (2012) Effect of isoflurane on the hearing in mice. Korean Journal of Audiology 16:14–17
Maison SF, Adams JC, Liberman MC (2003) Olivocochlear innervation in the mouse: immunocytochemical maps, crossed versus uncrossed contributions, and transmitter colocalization. J Comp Neurol 455:406–416
Maison SF, Vetter DE, Liberman MC (2007) A novel effect of cochlear efferents: in vivo response enhancement does not require alpha9 cholinergic receptors. J Neurophysiol 97:3269–3278
Maison SF, Usubuchi H, Vetter DE, Elgoyhen AB, Thomas SA, Liberman MC (2012) Contralateral-noise effects on cochlear responses in anesthetized mice are dominated by feedback from an unknown pathway. J Neurophysiol 108:491–500
Mertes IB, Goodman SS (2016) Within- and across-subject variability of repeated measurements of medial olivocochlear-induced changes in transient-evoked otoacoustic emissions. Ear Hear 37:e72–e84
Moller AR (1984) Neurophysiological basis of the acoustic middle-ear reflex. Orlando, Academic (1984): 1–35. In: the acoustic reflex: basic principles and clinical applications (Silman S, ed). Orlando: Academic Press
Mukerji S, Windsor AM, Lee DJ (2010) Auditory brainstem circuits that mediate the middle ear muscle reflex. Trends Amplif 14:170–191
Neely ST, Stevenson R (2002) SysRes. Technical memo No. 19, boys town national research hospital. Omaha, Nebraska
Neumann J, Uppenkamp S, Kollmeier B (1996) Detection of the acoustic reflex below 80 dB HL. Audiol Neuro-Otol 1:359–369
Relkin EM, Sterns A, Azeredo W, Prieve BA, Woods CI (2005) Physiological mechanisms of onset adaptation and contralateral suppression of DPOAEs in the rat. J Assoc Res Otolaryngol: JARO 6:119–135
Shera CA, Guinan JJ Jr (1999) Evoked otoacoustic emissions arise by two fundamentally different mechanisms: a taxonomy for mammalian OAEs. J Acoust Soc Am 105:782–798
Siegel JH, Charaziak K, Cheatham MA (2011) Transient- and tone-evoked otoacoustic emissions in three species. In: Shera CA, Olson ES (eds) What fire is in mine ears: progress in auditory biomechanics, XIth international mechanics of hearing workshop. American Institute of Phhysics, Melville, pp 307–314
Sun XM (2008) Contralateral suppression of distortion product otoacoustic emissions and the middle-ear muscle reflex in human ears. Hear Res 237:66–75
Valero MD, Hancock KE, Liberman MC (2016) The middle ear muscle reflex in the diagnosis of cochlear neuropathy. Hear Res 332:29–38
van den Berge H, Kingma H, Kluge C, Marres EH (1990) Electrophysiological aspects of the middle ear muscle reflex in the rat: latency, rise time and effect on sound transmission. Hear Res 48:209–219
Zhao W, Dhar S (2010) The effect of contralateral acoustic stimulation on spontaneous otoacoustic emissions. J Assoc Res Otolaryngol: JARO 11:53–67
Acknowledgments
Work supported by NIDCD Grant #DC000089 to M.A. Cheatham. The authors acknowledge Dr. Dhar and Dr. Charaziak for suggesting some of the analysis methods, and Dr. Zhou for collecting some of the data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, Y., Cheatham, M.A. & Siegel, J.H. Identifying the Origin of Effects of Contralateral Noise on Transient Evoked Otoacoustic Emissions in Unanesthetized Mice. JARO 18, 543–553 (2017). https://doi.org/10.1007/s10162-017-0616-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-017-0616-x