Abstract
Stimulus frequency otoacoustic emissions (SFOAEs) are produced by cochlear irregularities reflecting energy from the peak region of the traveling wave (TW). Activation of medial olivocochlear (MOC) efferents reduces cochlear amplification and otoacoustic emissions (OAEs). In other OAEs, MOC activation can produce enhancements. The extent of MOC enhancements of SFOAEs has not been previously studied. In anesthetized guinea pigs, we electrically stimulated MOC fibers and recorded their effects on SFOAEs. MOC stimulation mostly inhibited SFOAEs but sometimes enhanced them. Some enhancements were not near response dips and therefore cannot be explained by a reduction of wavelet cancelations. MOC stimulation always inhibited auditory-nerve compound action potentials showing that cochlear-amplifier gain was not increased. We propose that some SFOAE enhancements arise because shocks excite only a small number of MOC fibers that inhibit a few scattered outer hair cells thereby changing (perhaps increasing) cochlear irregularities and SFOAE amplitudes. Contralateral sound activation is expected to excite approximately one third of MOC efferents and may also change cochlear irregularities. Some papers suggest that large SFOAE components originate far basal of the TW peak, basal of the region that receives cochlear amplification. Using a time-frequency analysis, we separated SFOAEs into components with different latencies. At all SFOAE latencies, most SFOAE components were inhibited by MOC stimulation, but some were enhanced. The MOC inhibition of short-latency SFOAE components is consistent with these components being produced in the cochlear-amplified region near the TW peak.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Activation of medial olivocochlear (MOC) efferents, through synapses on outer hair cells (OHCs), reduces cochlear amplification. This is expected to reduce stimulus frequency otoacoustic emissions (SFOAEs) and other otoacoustic emissions (OAEs). However, in other OAEs, MOC-induced increases have been reported many times since the initial report of increases by Siegel and Kim (1982). For SFOAEs, most previous studies of MOC effects measured the change in the SFOAE from the baseline sound pressure (ΔSFOAE), which must always be positive and does not reveal if the SFOAE may have increased in amplitude. Zhao et al. (2015) measured actual SFOAEs during MOC stimulation (SFOAEmoc’s) and showed MOC enhancements only near SFOAE dips, but few relevant data were presented. MOC-induced enhancements of SFOAEs are expected near SFOAE response dips that come from the cancelation of out-of-phase SFOAE wavelets, or cancelation of out-of-phase reflection and distortion components (at sound levels high enough to produce SFOAE distortion) because MOC activity produces phase shifts and inhibits some SFOAE sources more than others thereby reducing the cancelations (Goodman et al. 2003). There has, however, been no study that systematically measured MOC effects on SFOAEs across frequency to determine the prevalence of enhancements and whether the reduced-cancelation explanation accounts for all MOC-induced enhancements of SFOAEs.
Although it is generally accepted that stimulus frequency otoacoustic emissions (SFOAEs) come from cochlear irregularities that coherently reflect energy in the peak region of the traveling wave (Zweig and Shera 1995), arguments have been made that some SFOAE delay components arise from the basal part of the traveling wave (Siegel et al. 2005; Choi et al. 2008; Charaziak and Siegel 2014, 2015). SFOAE delay components that arise basal of the cochlear region where tones are amplified are termed “far-basal SFOAEs” (Berezina-Greene and Guinan 2015). In our recent analysis of SFOAEs from guinea pigs, chinchillas and models we argued that (1) SFOAEs collected with a suppressor tone near the probe tone (termed “near-suppressor SFOAEs” (nsSFOAEs)) predominantly arise from the peak region of the traveling wave and, (2) if nsSFOAEs have far-basal delay components, then these unamplified components would be very small. In addition, since far-basal components do not receive cochlear amplification, they should not be affected by activation of MOC efferents that reduces cochlear amplification.
Since SFOAEs can be measured noninvasively in humans, understanding MOC effects on SFOAEs may be clinically useful. MOC efferents may help to protect hearing from damage due to traumatic sounds and to reduce the effects of auditory aging (Kujawa and Liberman 2009; Liberman et al. 2014). Since this protection depends on the strength of the MOC reflex, there is interest in techniques for evaluating the strength of MOC efferents. Ideally, such tests would detect individuals with weak MOC reflexes who are particularly susceptible to damage due to loud sounds so that they can be counseled to avoid noisy conditions or to employ hearing protection. MOC activity may also help in hearing signals in noisy conditions, and variation in this ability may be partly due to variations in MOC strength. Evaluation of MOC effects on SFOAEs provides a way of assessing MOC strength (Backus and Guinan 2007). However, before we can determine how well MOC effects on SFOAEs assess MOC strength and predict MOC protection from acoustic trauma, there is a need to understand the ways in which MOC efferents affect SFOAEs in the first place.
Here, we present data on MOC effects on SFOAEs measured across a wide range of frequencies in guinea pigs with the MOC fibers activated by brainstem shocks. We analyzed MOC effects on different SFOAE delay components using a time-frequency analysis. Although shocks excite MOC fibers in synchronous volleys but sound excites MOC fibers separately and without synchronization, it has generally been thought that sound and shock activation produces similar MOC effects. As will be seen, our finding of SFOAE enhancements in areas of broad peaks points to a difference due to the number of MOC fibers excited.
Methods
Most of our methods have been described previously (Stankovic and Guinan 1999; Lin and Guinan 2000; Berezina-Greene and Guinan 2015). Briefly, albino guinea pigs were anesthetized with Nembutal, followed by fentanyl and haloperidol (initial doses 25, 0.2, and 10 mg/kg, respectively) with additional doses given as necessary. Animals were tracheotomized and mechanically ventilated. The heart rate, expired CO2, body temperature, and electroencephalogram were continuously monitored. The rectal temperature was maintained at ~37 to 38 °C. The bulla was opened and a silver electrode was placed near the round window to monitor auditory-nerve (AN) compound action potentials (CAPs) in response to tone pips (5-ms duration, 0.5-ms rise/fall times, repeated every 100 ms). An automated up/down procedure determined CAP threshold as the sound level needed to produce 10-μV peak-to-peak (PP) CAPs. After removing the overlying bone, the cerebellum was aspirated over the floor of the fourth ventricle and a custom-made, three-prong platinum MOC stimulating electrode was placed at the midline near the crossing olivocochlear bundle. MOC fibers were stimulated by 0.3-ms voltage pulses at 200/s delivered across two electrode prongs via a transformer with a series resistor (usually 2 kΩ). The shock voltage was adjusted to be just below the level that caused muscle movement (except that very weak whisker twitches and eye movements were allowed). During data collection, animals were paralyzed using gallamine to eliminate spontaneous or evoked middle ear muscle (MEM) or other muscle contractions. Experimental protocols were approved by the Massachusetts Eye and Ear Infirmary Animal Care Committee.
SFOAE frequency sweeps with and without MOC stimulation were collected for 40-dB SPL probe tones at frequencies from 0.5 to 9 kHz in 83-Hz steps. To minimize the effects of response drift, the frequency range was divided into blocks, each containing 16 probe frequencies, e.g., the first block had 16 frequencies from 0.5 to 1.745 kHz and the seventh block had 16 frequencies from 8.468 to 9.713 kHz. A single block included three trials of the 16 frequencies presented in different randomized orders, with the data at each frequency averaged across the trials. For each block, the two sets of measurements required to measure SFOAE changes from MOC stimulation (see below) were done separately but close in time. To control for drift, MOC effects on click level functions were measured before and after every block. We only used data from animals and frequency regions that (1) had CAP thresholds ≤40 dB SPL before and after the data were collected, (2) had stable MOC effects (i.e., MOC effects were present and changed ≤1 dB during a block), and (3) showed no spontaneous or shock-induced MEM contractions (animals were paralyzed using gallamine to eliminate MEM contractions).
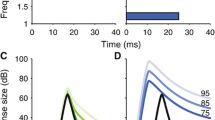
The MOC-induced changes in SFOAEs were collected in two steps. First, the baseline SFOAE (i.e., the SFOAE without MOC stimulation) was measured at every probe frequency using a suppressor paradigm. In Berezina-Greene and Guinan (2015), SFOAEs measured in this way were called nsSFOAEs. For each probe frequency, a suppressor tone (50 ms every 100 ms) was presented at 50 Hz and 20 dB above the level and frequency of a continuous probe tone as in Fig. 1c. Three data sets, each with 20 responses, were obtained and averaged. From each average, the baseline total pressure and the probe source pressure at the probe frequency were extracted by fast Fourier transforms (FFTs). The vector difference between these yielded the baseline SFOAE without MOC stimulation (Fig. 1b). In the second step, the MOC-induced change in the SFOAE was measured by presenting a continuous probe tone (no suppressor present) along with 500-ms bursts of MOC shocks every 1500 ms (Fig. 1a). Four to 8 responses were averaged. The baseline total Pressure and the MOC-induced total pressure at the probe frequency were extracted by FFTs from responses in a 100-ms window before MOC stimulation and a 300-ms window that ended at the end of the MOC stimulation (Fig. 1a). The vector difference between these two measures yielded the change in SFOAE (ΔSFOAE) due to MOC stimulation (Fig. 1b). The two measurement steps were put together by the vector addition of the ΔSFOAE and the baseline SFOAE, which yields the SFOAE with MOC stimulation (SFOAEmoc) (Fig. 1b). The MOC effect on SFOAEs was measured as the difference in SFOAE levels, i.e., SFOAEmoc magnitude minus the baseline SFOAE magnitude (both in dB), and the phase angle change from the baseline SFOAE to SFOAEmoc in Fig. 1b (positive is a phase advance from SFOAE to SFOAEmoc).
The measurement of SFOAEmoc (the stimulus frequency otoacoustic emission (SFOAE) during stimulation of medial olivocochlear (MOC) efferents). a Relative to the baseline SFOAE, the MOC-induced change in the SFOAE (termed ΔSFOAE) was measured by presenting a continuous probe tone along with 500-ms duration bursts of MOC shocks every 1500 ms (shading = the shock period). The baseline ear-canal sound pressure was averaged over a 100-ms window at the beginning of the trace and vectorially subtracted from the measured pressure to yield the ΔSFOAE, as shown in b. c With no MOC stimulation, a continuous probe tone and a 50-ms suppressor tone were presented every 100 ms. The vector difference between the ear canal pressure at the probe frequency in the first and last 50 ms (called “Pbaseline” and “Probe Source Pressure”, respectively) is the baseline SFOAE without MOC stimulation. b A vector diagram showing the relationships of the various measured quantities. SFOAEmoc was calculated from the addition of the baseline SFOAE and ΔSFOAE. Vector quantities in a and c were calculated by fast Fourier transforms applied to appropriate windows of the averaged ear-canal sound pressure waveforms.
To determine MOC effects on cochlear amplification for comparison with those on SFOAEs, we measured MOC-induced changes in tone-evoked CAPs at a few frequencies. CAPs were evoked by 5-ms tone pips (0.5-ms rise/fall times), repeated every 100 ms throughout a 1500-ms repetition period with the MOC activity evoked by the same 500-ms burst of MOC shocks used for measuring MOC effects on SFOAEs. Four to 8 sets of CAP responses were averaged. Tone-pip-evoked CAP amplitudes were measured at several sound levels near threshold as the N1-P1 PP amplitudes in time windows before and during the MOC shocks. The MOC effect was quantified as the sound level shift, i.e., the change in the sound level (in dB) with MOC stimulation needed to achieve the same CAP amplitude as was produced by tone pips without MOC stimulation. The test was done at the same sound level used for determining the MOC effect on SFOAEs (a sound level 10–15 dB above CAP threshold, usually 30 or 35 dB SPL).
As a control for variations in MOC effect across time, a measurement of the MOC-induced level shift was done using the CAPs from clicks at several sound levels near threshold. This click-level shift was used to quantify the overall strength of the shock-induced MOC effect and was done frequently throughout the experiment.
To separate SFOAE components with different delays, we did the time-frequency analysis described in Berezina-Greene and Guinan (2015). We used a 0.5-kHz window duration, in 83-Hz steps, done separately for the measurements with and without MOC stimulation. The main component was defined as the component whose latency agreed with the SFOAE latency calculated from the two-line fit to the single-fiber delays from guinea pig AN responses to clicks (Versnel et al. 1990). The AN single-fiber delays minus neural conduction times were assumed to equal the forward traveling-wave delay and were doubled to account for the forward and backward delays of SFOAEs (described in detail in Berezina-Greene and Guinan 2015). Delay components were classified as either a main component, or as a component with a longer or a shorter delay than the main component, with the dividing line ±0.6 ms from the main component as in Berezina-Greene and Guinan (2015).
Results
Data that satisfied all of the control criteria were obtained from 12 ears of 10 animals, totaling 37 blocks each with 16 probe frequencies. In many animals, the threshold at mid-frequencies was over 40 dB SPL which prevented us from obtaining a continuous series of good data from the 0.5–10 kHz region of interest. For the five ears with good low-frequency (<3 kHz) data, the MOC strength (the MOC-induced level shift in click-evoked CAPs) averaged 3.85 ± 1.1 dB (range 2.15 to 5 dB). For the nine ears with good high frequency data, the MOC strength averaged 3.4 ± 0.94 dB (range 1.97 to 4.49 dB). The drift of the MOC strength was <0.5 dB on all, but four frequency blocks and in these, it was <1 dB.
SFOAE amplitudes and phases with and without MOC stimulation from a representative ear are shown in Fig. 2. The data from this ear show both MOC inhibition and MOC enhancement. Furthermore, the enhancements are not restricted to frequencies close to dips (e.g., enhancements are seen throughout the region from 3 to 4 kHz in Fig. 2). In this ear, the MOC effect on SFOAE phase was generally small (Fig. 2b).
MOC effects on SFOAE magnitudes and phases in a representative ear. a SFOAE magnitudes without (blue line) and with (red line) MOC stimulation (i.e., baseline SFOAE and SFOAEmoc in Fig. 1 b); black dots = noise floor. b SFOAE phase without (blue line) and with (red line) MOC stimulation. c Purple circles = MOC-induced changes in SFOAE amplitudes (with-MOC minus without-MOC SFOAE magnitudes from the top panel). Asterisks (*) indicate MOC-induced enhancements not in dip regions. Data from GP104L.
All of the good SFOAE amplitude data from all other ears, with and without MOC stimulation, are shown in Fig. 3. The corresponding amplitude and phase changes induced by MOC stimulation are shown in Fig. 4. In every ear, MOC stimulation produced both SFOAE inhibitions and SFOAE enhancements (Figs. 2–4). In a few ears, MOC stimulation produced enhancements that were in broad SFOAE peaks (asterisks in Figs. 2–4). MOC stimulation also produced both advances and delays in SFOAE phase (Fig. 4). A few MOC-induced phase changes were >90°, but most were <45° (Fig. 4). There was only a weak correlation between the MOC-induced changes in SFOAE phase and magnitude (correlation coefficient of −0.145, p = 0.001) (Fig. 5).
SFOAE amplitudes with (red) and without (blue) MOC stimulation across ears and frequencies. Data from 9 ears in which there were 12 good frequency blocks over which SFOAEs were collected with and without MOC stimulation (the data in Fig. 2 are not included). Note that the scales vary across the panels. Asterisks (*) indicate MOC-induced enhancement not in dip regions. In each panel, the guinea pig number is at the lower right.
Changes in SFOAE amplitudes (purple) and phase (green) due to MOC stimulation. Phase is expressed in periods (P) times 10, i.e., 1 = 36°. Same data source as Fig. 3, in same order. Note that the scales vary across the panels. Asterisks (*) indicate MOC-induced enhancement not in dip regions. In each panel, the guinea pig number is at the lower right.
Since we found MOC-induced SFOAE enhancements that were not near dips, we questioned whether these enhancements might be due to MOC-induced increases in cochlear-amplifier gain. Changes in cochlear amplification can be measured by the MOC-induced change in the sound level needed to produce a fixed CAP amplitude from near-threshold tone pips (i.e., the MOC-induced sound level shift). Obtaining such data was not a goal of this project, but we did measure MOC effects on CAPs at 6–11 kHz in some animals. These MOC-induced CAP level shifts are shown in Fig. 6b, and the MOC-induced changes in SFOAE magnitudes for 10 ears are shown in Fig. 6a. The MOC effects on the tone-pip CAPs were always inhibitory (Fig. 6b). In one ear, we measured MOC effects on tone-pip CAPs at two frequencies where SFOAEs were enhanced (filled red circles in Fig. 6). At these two frequencies and at the same sound levels, the MOC effects on CAPs were inhibitory even though the effects on SFOAEs were enhancing. This shows that the MOC enhancement was not present at the level of cochlear amplification.
MOC-induced level shifts in SFOAE magnitudes (a) and tone-pip CAP amplitudes (b) vs. frequency from 10 ears. Blue x’s = data from all ears except 120 L. Red circles = data from ear 120 L. Filled red circles = frequencies at which MOC effects on SFOAEs and CAPs were both evaluated. The inhibitory CAP level shifts show that the MOC enhancements were not due to MOC-induced increases in cochlear amplification.
MOC Effects on SFOAE Delay Components
In Berezina-Greene and Guinan (2015), we found that every guinea pig ear has frequency regions over which a time-frequency analysis shows two, or sometimes three, SFOAE wavelet components that arrive in the ear canal with different delays. For the ear that was shown in Fig. 1, the SFOAE delay components, with and without MOC stimulation, are shown in Fig. 7. In these data, and the data from the other guinea pigs, the MOC effect on the main delay component (Fig. 7b) was usually similar to the MOC effect on the whole SFOAE (Fig. 7a). That is, the main component was inhibited over regions where the total SFOAE was inhibited and was enhanced in regions where the total SFOAE was enhanced. This was true in general (Fig. 8c). Delay components other than the main component were also affected by MOC stimulation (Fig. 7c). There was, however, no consistent pattern as to whether a nonmain component was enhanced or inhibited in regions where the overall SFOAE was enhanced or inhibited (Fig. 8a, b). The short-delay component was significantly correlated with the main component, but the correlation was low (R = 0.24, p = 0.04). The long-delay component was almost randomly distributed relative to the main component (R = −0.0034, p = 0.95). In contrast, the overall SFOAE amplitude was highly correlated with the main component (R = 0.7, p < 0.001) (Fig. 8c).
MOC-induced changes in SFOAE delay components. a Total-SFOAE magnitudes without MOC stimulation (blue line) and with MOC stimulation (red line) (same data as Fig. 2a). b Magnitudes of the main SFOAE delay components without MOC stimulation (blue line) and with MOC stimulation (red line). c Magnitudes of SFOAE delay components longer than the main component without MOC stimulation (dark green line) and with MOC stimulation (light green line). Magnitude of SFOAE delay component shorter than main component without MOC stimulation (dark purple line) and with MOC stimulation (light purple line). All panels: black dots = noise floor. Data from GP104L.
Discussion
Our data clearly show that MOC effects on SFOAEs can be both inhibitory and enhancing. When an enhancement is near a SFOAE dip, the SFOAE enhancement can be due to a reduction in the cancelation of the out-phase wavelets or the out-phase distortion and reflection components that produced the dip (Goodman et al. 2003). However, this reduction-of-cancelation mechanism does not explain the MOC-induced increases we found in broad peaks of SFOAE amplitude. Furthermore, we never saw a MOC-induced increase in AN CAP responses to low-level tone bursts, which indicates that SFOAE enhancement is not a result of an increase of cochlear amplification. The CAP data we present here are relatively few, but many other publications have reported MOC effects on AN CAP and single-fiber responses as well as on basilar membrane (BM) responses, and all of these found purely inhibitory MOC effects at low sound levels (e.g., Gifford and Guinan 1987, Guinan and Gifford 1988a, 1988b; Murugasu and Russell 1996; Stankovic and Guinan 1999; Lin and Guinan 2000; Cooper and Guinan 2006; Guinan 2006). MOC-induced increases in both AN and BM responses have been found near dips at moderate to high sound levels (Gifford and Guinan 1983; Dolan et al. 1997; Guinan and Cooper 2003). These increases appear to be due to reductions of cancelations between two out-of-phase components, but the components involved in these cancelations are quite different from those that produce the cancelations and dips in SFOAEs for probe levels of 40 dB SPL and below. For probe tones around CF, cancelations and MOC-induced response increases in BM motion or AN responses have not been found near threshold (the region where cochlear amplification is most apparent). We know of no evidence that MOC stimulation ever increases cochlear amplification. Although the best evidence would be many paired measurements showing SFOAE enhancements and CAP inhibition in the same preparation and at the same frequency and level, the many other separate measurements showing that at low-levels, MOC stimulation only reduces cochlear amplification make a strong case that the SFOAE enhancements are not due to MOC-induced increases in cochlear amplifiction. Thus, neither a reduction of cancelations nor an increase in cochlear amplification provide good explanations for MOC-induced SFOAE enhancements in broad frequency regions where there are no dips. The MOC inhibitory effects, on the other hand, are consistent with a wide range of evidence that shows that activation of MOC efferents reduces cochlear amplification.
Our working hypothesis is that MOC stimulation can increase SFOAEs by changing (possibly increasing) cochlear irregularities. Several lines of evidence are important for this hypothesis: First, in unpublished work, McCue and Guinan stimulated MOC fibers with shocks at the midline of the fourth ventricle and recorded responses of individual MOC fibers in the bundle of Oort. They found that shocks excited only a small fraction of MOC fibers, even when the efferent inhibition was over 10 dB. McCue and Guinan also found that MOC-fiber action potentials followed each shock at rates up to 200/s (but not at 400/s). Second, in our experiments, we stimulated MOC fibers at high shock rates (200/s), which is a much higher rate than MOC fibers are expected to fire in response to sound stimulation at nontraumatic sound levels. We often used low-level shocks that elicited inhibitions <5 dB because we wanted measurements with MOC attenuations similar to those found with sound stimulation in humans (i.e., attenuations of a few dB). Thus, for most of our data, it seems likely that our shocks excited only a small number of MOC fibers at high rates. A final relevant observation is that individual MOC fibers have scattered irregular innervation patterns along the length of the cochlea (Brown 2014). Electrical stimulation of a few MOC fibers at high rates, each with an irregular innervation pattern, will strongly inhibit scattered individual OHCs while leaving the other OHCs unchanged. In localized cochlear regions, this may significantly change the pattern of irregularities along the cochlea (as well as decreasing cochlear amplification). In some cochlear regions, the pattern of irregularities may be changed toward a “rougher” pattern and result in a local increase in cochlear reflectance which in turn would result in an increased SFOAE amplitude. In summary, our working hypothesis is that in some cases, shocks excite only a small number of MOC fibers that affect a few scattered OHCs thereby changing the pattern of cochlear irregularities along the cochlea, and this change in the pattern of irregularities translates into localized decreases or enhancements of SFOAE amplitudes.
Are there SFOAE enhancements in humans? In most of our previous work measuring sound-evoked MOC effects on SFOAEs in humans (e.g. Backus and Guinan 2007; Lilaonitkul and Guinan 2009), we measured ΔSFOAE (see Fig. 1) which does not show whether the SFOAE increased or decreased. In Lilaonitkul and Guinan (2012), we measured both the baseline SFOAE and ΔSFOAE, and from these calculated SFOAEmoc as was done here. Lilaonitkul and Guinan (2012) did not report seeing a statistically significant SFOAE increase (i.e., |SFOAEmoc| > |SFOAE|), but there were conditions in which at several adjacent frequency points, the SFOAE averaged across subjects showed a small (<1 dB) increase. This small average increase is enough to suggest that at least some of these increases are more than just noise, which implies that MOC-induced SFOAE increases are present in humans. SFOAEmoc measurements in humans were reported by Zhao et al. (2015). They showed enhanced SFOAEmoc responses in frequency regions near dips but not at broad maxima; however, they only showed data from one subject at two sound levels over a 1-kHz frequency range. Other relevant data might be obtained from studies of MOC effects on transient-evoked otoacoustic emissions (TEOAEs). However, in most published measurements showing an increase in TEOAE amplitude with MOC stimulation compared to without MOC stimulation, the SNR was not adequate to determine whether the increase in TEOAE amplitude was due to a MOC effect or was a variation due to noise. Changes in TEOAEs are also difficult to interpret to discern an increase in the emission at a particular frequency because the responses from different frequencies overlap in time and changes in their phase relationships can also change TEOAE amplitudes, particularly at their peak amplitudes. More work is needed to determine to what extent sound-evoked MOC activity enhances SFOAEs in frequency regions without response dips in humans.
Electrical vs. Sound Stimulation of MOC Efferents
The increase-in-cochlear-irregularities explanation for the MOC-induced enhancement of SFOAEs suggests that the enhancement may be prominent in shock-evoked MOC effects but less prominent in sound-evoked MOC effects. Animal data indicate that binaural sound stimulation may excite all, or nearly all, MOC fibers and therefore not increase cochlear irregularities by much (Liberman and Brown 1986; Liberman 1988; Brown 1989; Brown et al. 1998). Because of this more uniform MOC stimulation, MOC activity evoked by binaural sound should produce smaller changes in cochlear irregularities than electrically evoked MOC activity. However, the sound stimulus used in most studies is contralateral sound which only excites about one third of MOC fibers in animals (Liberman and Brown 1986; Liberman 1988; Brown 1989). The fraction of MOC fibers excited by contralateral sound in humans is unknown and may be slightly higher, but it is not likely to be more than one half (reviewed by Guinan 2012). In human studies using contralateral sound to evoke changes in SFOAEs, Backus and Guinan (2007) found inhibition that changed greatly within a narrow frequency range and they suggested that this pattern was due to the MOC activity changing the pattern of cochlear irregularities. Thus, changes in cochlear irregularities may also be a factor in human measurements with sound-evoked MOC activity.
When we started this study, we expected that our shock-evoked MOC activity would produce SFOAE changes that would be equivalent to those from sound-evoked MOC stimulation when both produced similar reductions in cochlear amplification. It now seems likely that shocks produce more changes in cochlear irregularities than sound because they excite fewer MOC fibers and excite them at high rates. MOC-induced changes in cochlear irregularities, whether produced by contralateral sound or by shocks, lessen the correlation between the observed MOC effect on SFOAEs and the actual MOC effect in reducing cochlear amplification. This phenomenon will affect both SFOAEs and transient-evoked otoacoustic emissions (TEOAEs) and to a lesser extent distortion-product OAEs (DPOAEs) through their reflection component. How important this phenomenon is for measuring MOC strength in a clinical setting is unclear. Experiments in humans are needed to determine how well sound-evoked changes in SFOAEs, TEOAEs, or DPOAEs provide useful measures of MOC strength in individuals that can predict the degree of MOC protection from acoustic trauma or hearing aging and/or predict other MOC effects such as enhancement of the ability to hear complex signals in noise. Despite this newly revealed imprecision in OAE measures of MOC effects, OAEs still appear to be the preferred way to measured efferent effects in humans because measuring MOC effects with CAPs or by psychophysical methods takes much longer (Lichtenhan et al. 2016; Aguilar et al. 2015).
MOC Effects on SFOAE Delay Components
We examined the MOC effects on the SFOAE delay components discerned by our time-frequency analysis. In general, the main SFOAE delay component showed the same direction of MOC effect as the overall SFOAE at the same frequency, either increases or decreases. This is because the main component usually dominates the others in producing the overall SFOAE. We saw no strong relationship between the direction (enhancement or inhibition) of MOC effects on the main delay component and the direction of MOC effects on additional delay components (Fig. 8). The fact that MOC stimulation inhibited the additional SFOAE delay components revealed by time-frequency analysis suggests that these components also received cochlear amplification, i.e., they were not generated in the basal end of the traveling wave, the region termed “far-basal” by Berezina-Green and Guinan (2015). However, our data do not prove this. A MOC-generated change in irregularities in the basal part of the cochlea might increase local cochlear reflectance which would increase SFOAEs with short delays. This effect would be small because the BM traveling wave is very small far basal of CF. Furthermore, the cochlear irregularities most likely generated by MOC activity are changes in OHC electrical-to-mechanical output. Such differences in OHC motilities can greatly affect the gain and reflectance in the traveling-wave peak region but would have little effect on SFOAEs in the region far-basal of CF where the cochlear-partition impedance is dominated by BM stiffness. A recent analysis of human SFOAEs also concluded that short-latency SFOAE components originate from within the peak region of the traveling wave (Moletti and Sisto 2016).
An alternate interpretation for the origin of short-delay, far-basal SFOAE delay components is that cochlear amplifier gain extends far more basal than current papers suggest. This is the explanation we proposed when we first reported far-basal SFOAE residuals from applying a far-basal second tone (Guinan 1990). However, many lines of evidence indicate that the region of cochlear amplification does not extend many octaves basal to CF, and there are more likely explanations for the origin of far-basal SFOAE residuals (for more detail, see Berezina-Greene and Guinan 2015).
Conclusions
Shock excitation of MOC fibers most often decreases SFOAEs, but it sometimes enhances SFOAEs and enhancements can be in frequency regions that are not near response dips. Measurements of MOC effects on low-level AN CAPs always showed inhibition, so the SFOAE enhancements are not due to enhanced cochlear-amplifier gain. We hypothesize that SFOAE increases that are not near response dips are due to MOC-induced changes in the local pattern of cochlear irregularities. We expect that sound-evoked MOC activity will produce changes in cochlear irregularities but that these will be smaller than for shock-induced MOC activity. More work is needed to fully understand the extent to which changes in cochlear irregularities affect MOC-induced changes in OAEs.
Shock excitation of MOC fibers predominantly decreases SFOAEs in all of the SFOAE delay components shown by our time-frequency analysis. The predominance of inhibition in the short delay SFOAE components argues for these components having received cochlear amplification and having originated within the cochlear-amplified region of the traveling wave.
References
Aguilar E, Johannesen PT, Lopez-Poveda EA (2015) Contralateral efferent suppression of human hearing sensitivity. Front Syst Neurosci 8:251
Backus BC, Guinan JJ (2007) Measurement of the distribution of medial olivocochlear acoustic reflex strengths across normal-hearing individuals via otoacoustic emissions. J Assoc Res Otolaryngol 8:484–496
Berezina-Greene MA, Guinan JJ Jr (2015) Stimulus frequency otoacoustic emission delays and generating mechanisms in Guinea pigs, chinchillas, and simulations. J Assoc Res Otolaryngol 16:679–694
Brown MC (1989) Morphology and response properties of single olivocochlear fibers in the Guinea pig. Hearing Res. 40:93–110
Brown MC (2014) Single-unit labeling of medial olivocochlear neurons: the cochlear frequency map for efferent axons. J Neurophysiol 111:2177–2186
Brown MC, Kujawa SG, Duca ML (1998) Single olivocochlear neurons in the Guinea pig. . I. Binaural facilitation of responses to high-level noise. J Neurophysiol 79:3077–3087
Charaziak KK, Siegel JH (2014) Estimating Cochlear frequency selectivity with stimulus-frequency otoacoustic emissions in chinchillas. J Assoc Res Otolaryngol 15:883–896
Charaziak KK, Siegel JH (2015) Tuning of SFOAEs evoked by low-frequency tones is not compatible with localized emission generation. J Assoc Res Otolaryngol 16:317–329
Choi YS, Lee SY, Parham K, Neely ST, Kim DO (2008) Stimulus-frequency otoacoustic emission: measurements in humans and simulations with an active cochlear model. J Acoust Soc Am 123:2651–2669
Cooper NP, Guinan JJ Jr (2006) Efferent-mediated control of basilar membrane motion. J Physiol 576:49–54
Dolan DF, Guo MH, Nuttall AL (1997) Frequency-dependent enhancement of basilar membrane velocity during olivocochlear bundle stimulation. J Acoust Soc Am 102:3587–3596
Gifford ML, Guinan JJ Jr (1983) Effects of crossed-olivocochlear-bundle stimulation on cat auditory nerve fiber responses to tones. J Acoust Soc Am 74:115–123
Gifford ML, Guinan JJ Jr (1987) Effects of electrical stimulation of medial olivocochlear neurons on ipsilateral and contralateral cochlear responses. Hear Res 29:179–194
Goodman SS, Withnell RH, Shera CA (2003) The origin of SFOAE microstructure in the Guinea pig. Hear Res 183:7–17
Guinan JJ Jr (1990) Changes in stimulus frequency otoacoustic emissions produced by two-tone suppression and efferent stimulation in cats. In: Dallos P, Geisler CD, Matthews JW, Steele CR (eds) Mechanics and biophysics of hearing. Springer Verlag, Madison, Wisconsin, pp. 170–177
Guinan JJ Jr (2006) Olivocochlear efferents: anatomy, physiology, function, and the measurement of efferent effects in humans. Ear Hear 27:589–607
Guinan JJ Jr (2012) Efferent System. In: Tremblay KL, Burkard R (eds) Translational perspectives in hearing science. Plural Pub. Inc., San Diego, pp. 283–323
Guinan JJ Jr, Cooper NP (2003) Fast effects of efferent stimulation on basilar membrane motion. In: Gummer AW, Dalhoff E, Nowotny M, Scherer MP (eds) The biophysics of the cochlea: molecules to models. World Scientific, Singapore, pp. 245–251
Guinan JJ Jr, Gifford ML (1988a) Effects of electrical stimulation of efferent olivocochlear neurons on cat auditory-nerve fibers. I. Rate-level functions. Hearing Res 33:97–114
Guinan JJ Jr, Gifford ML (1988b) Effects of electrical stimulation of efferent olivocochlear neurons on cat auditory-nerve fibers. III. Tuning curves and thresholds at CF. Hearing Res 37:29–46
Kujawa SG, Liberman MC (2009) Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci 29(45):14077–14085
Liberman MC (1988) Response properties of cochlear efferent neurons: monaural vs. binaural stimulation and the effects of noise. J Neurophysiol 60:1779–1798
Liberman MC, Brown MC (1986) Physiology and anatomy of single olivocochlear neurons in the cat. Hearing Res 24:17–36
Liberman MC, Liberman LD, Maison SF (2014) Efferent feedback slows Cochlear aging. J Neurosci 34(14):4599–4607
Lichtenhan JT, Wilson US, Hancock KE, Guinan JJ Jr (2016) Medial olivocochlear efferent reflex inhibition of human cochlear nerve responses. Hear Res 333:216–224
Lilaonitkul W, Guinan JJ Jr (2009) Reflex control of the human inner ear: a half-octave offset in medial efferent feedback that is consistent with an efferent role in the control of masking. J Neurophysiol 101:1394–1406
Lilaonitkul W, Guinan JJ Jr (2012) Frequency tuning of medial-olivocochlear-efferent acoustic reflexes in humans as functions of probe frequency. J Neurophysiol 107:1598–1611
Lin T, Guinan JJ Jr (2000) Auditory-nerve-fiber responses to high-level clicks: interference patterns indicate that excitation is due to the combination of multiple drives. J Acoust Soc Am 107:2615–2630
Moleti A, Sisto R (2016) Localization of the reflection sources of stimulus-frequency otoacoustic emissions. J Assoc Res Otolaryngol. (in press)
Murugasu E, Russell IJ (1996) The effect of efferent stimulation on basilar membrane displacement in the basal turn of the Guinea pig cochlea. J Neurosci 16:325–332
Siegel JH, Kim DO (1982) Efferent neural control of cochlear mechanics? Olivocochlear bundle stimulation affects cochlear biomechanical nonlinearity. Hearing Res 6:171–182
Siegel JH, Cerka AJ, Recio-Spinoso A, Temchin AN, van Dijk P, Ruggero M (2005) Delays of stimulus-frequency otoacoustic emissions and cochlear vibrations contradict the theory of coherent reflection filtering. J Acoust Soc Am 118:2434–2443
Stankovic KM, Guinan JJ Jr (1999) Medial efferent effects on auditory-nerve responses to tail-frequency tones I: rate reduction. J Acoust Soc Am 106:857–869
Versnel H, Prijs VF, Schoonhoven R (1990) Single-fibre responses to clicks in relationship to the compound action potential in the Guinea pig. Hear Res 46:147–160
Zhao W, Dewey JB, Boothalingam S, Dhar S (2015) Efferent modulation of stimulus frequency otoacoustic emission fine structure. Front Syst Neurosci 9:168
Zweig G, Shera CA (1995) The origin of periodicity in the spectrum of evoked otoacoustic emissions. J Acoust Soc Am 98:2018–2047
Acknowledgments
We thank Drs. C.A. Shera, M.C. Brown, and D.C. Mountain for comments on earlier versions of the manuscript. This work was supported by RO1 DC000235, R01 DC003687, T32 DC00038, P30 DC005209, and a NSF GRFP, and was part of M. B-G’s PhD thesis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Berezina-Greene, M.A., Guinan, J.J. Electrically Evoked Medial Olivocochlear Efferent Effects on Stimulus Frequency Otoacoustic Emissions in Guinea Pigs. JARO 18, 153–163 (2017). https://doi.org/10.1007/s10162-016-0593-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-016-0593-5