Abstract
Some forms of tinnitus are believed to arise from abnormal central nervous system activity following a single or repeated noise exposure, for which there are no widely accepted pharmacological treatments. One central site that could be related to tinnitus awareness or modulation is the locus coeruleus, a brainstem structure associated with stress, arousal, and attention. In the present study, we evaluated the effects of cyclobenzaprine, a drug known to act on the rat locus coeruleus, on noise-induced tinnitus using Gap Prepulse Inhibition of the Acoustic Startle (GPIAS). In untreated rats, brief silent gaps presented prior to a 5–10-kHz bandpass startling stimulus produced robust GPIAS. Treatment with cyclobenzaprine alone had no effect on the ability of gaps to suppress the startle response. When animals were exposed to intense narrow-band (126 dB SPL, 16 kHz, 100 Hz BW) unilateral noise, GPIAS was significantly reduced, suggesting the presence of tinnitus. Following the noise exposure, a subset of rats that maintained a robust startle response continued to show GPIAS impairment at 6–20 kHz, 40 days post-noise, suggesting chronic tinnitus. When this subset of animals was treated with cyclobenzaprine, at a dose that had no significant effects on the startle response (0.5 mg/kg), GPIAS recovered partially or to near baseline levels at the affected frequencies. These results were consistent with the absence of tinnitus. By 48 h post-treatment, evidence of tinnitus re-emerged. Our results suggest that cyclobenzaprine was effective in transiently suppressing noise-induced tinnitus in rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Persistent tinnitus, a phantom auditory sensation often described as hissing, buzzing or ringing, is strongly correlated with noise-induced hearing loss. Approximately 50–85 % of individuals with tinnitus have some level of hearing loss (Kim et al. 2011). A recent report from the military further highlights the strong relationship between hearing loss and tinnitus, whereby 49 % of combat personnel exposed to blast noise experienced significant hearing loss and persistent tinnitus (McCombe et al. 2001; Cave et al. 2007; Shargorodsky et al. 2010). These alarming figures contribute to the enormous cost of tinnitus to the Veterans Administration (VA); tinnitus is its top service-connected disability (VA 2011). This cost is predicted by the American Tinnitus Association to exceed two billion dollars for the 2014 fiscal year alone.
In response to the need to find effective therapies for chronic tinnitus, several animal models were developed over the last 20 years and have been used to study drug- or noise-induced tinnitus (Jastreboff et al. 1988a; Bauer et al. 1999; Bauer and Brozoski 2001; Heffner and Harrington 2002; Guitton et al. 2003; Ruttiger et al. 2003; Lobarinas et al. 2004; Heffner and Koay 2005; Lobarinas et al. 2006; Turner et al. 2006; Turner and Parrish 2008; Heffner 2011; Yang et al. 2011). The animal models provide distinct advantages as (1) experiments with high-level noise exposures to induce tinnitus cannot be ethically carried out in humans, (2) tinnitus etiology is under the experimenter’s control, and (3) preclinical trials are necessary to establish both safety and efficacy of novel compounds.
Of these models, the Gap Prepulse Inhibition of the Acoustic Startle (GPIAS) has become widely used, as the model does not require overt training, food or water deprivation, or painful stimuli. Unfortunately, the loss of startle reactivity following noise exposure limits the GPIAS model (Lobarinas et al. 2013); i.e., many animals with unilateral hearing loss fail to maintain a robust acoustic startle response, potentially as a result of reduced loudness of the startling stimulus in frequency regions of hearing loss. However, in animals where startle reactivity is not significantly reduced after noise exposure and by using startle stimuli below the noise exposure frequency, GPIAS may be used to evaluate therapeutic interventions for tinnitus.
Previous clinical research efforts have evaluated a broad range of drugs for tinnitus treatment including, among others, benzodiazepines, anticonvulsants, GABA modulators, and NMDA receptor antagonists (Dobie 1999). To date, however, these studies have not identified pharmacological agents that can reliably suppress tinnitus. Moreover, attempts to replicate reports of potential tinnitus suppression often fail. In the animal literature, some drugs have been highly effective against salicylate-induced tinnitus (Puel 2007; Zheng et al. 2008; Lobarinas et al. 2011) and others have suppressed noise-induced tinnitus (Bauer and Brozoski 2001; Zheng et al. 2012). The extent to which these benefits overlap is largely unknown. Based on results from both animal and human studies, all treatments for either noise or salicylate-induced tinnitus have been only partially effective for long-term tinnitus reduction (Bauer and Brozoski 2006; Salvi et al. 2009).
Although no reliable drug treatment has emerged, clinical studies suggest that drugs that suppress neural activity may reduce the perception, loudness, or awareness of tinnitus (Brozoski et al. 2007a). Cyclobenzaprine, a tricyclic antidepressant analog (Lofland et al. 2001; Van Hoey 2005) and muscle relaxant (Basmajian 1978; Katz and Dube 1988) has been suggested as effective in reducing tinnitus in clinical reports (Coelho et al. 2012; Vanneste et al. 2012). Although widely used commercially as a muscle relaxant, cyclobenzaprine’s complex pharmacological actions on other functions are not well understood. In rats, reports indicate that cyclobenzaprine has effects on the locus coeruleus (Commissiong et al. 1981; Lang and Barnes 1983), a brainstem region associated with awareness, attention, arousal, wakefulness, vigilance, and memory function (Aston-Jones et al. 1991; Hansen and Manahan-Vaughan 2014). Based on its proposed effects on attention and partial success in early clinical trials, we hypothesized that cyclobenzaprine treatment would reduce evidence of noise-induced tinnitus assessed using GPIAS. In preliminary animal studies, we found that low to intermediate cyclobenzaprine doses (0.25–0.5 mg/kg) had no effect on distortion product otoacoustic emissions (DPOAE), startle amplitudes, GPIAS, or noise-burst pre-pulse inhibition (NBPIAS), suggesting that the drug itself has no negative effects on hearing or the startle response. Based on these initial findings, we assessed whether cyclobenzaprine could suppress noise-induced tinnitus. These data set the stage for subsequent experiments focused on potential mechanisms of cyclobenzaprine-induced tinnitus suppression.
Materials and Methods
Subjects
A total of 20 adult, male, albino Sprague Dawley SASCO rats (325–450 g) were used in this study. Rats were housed in Plexiglass cages, allowed free access to food and water, and were maintained on a normal 12-h light/dark cycle in a temperature-controlled room. All experimental procedures used in the present study were approved by the University at Buffalo-Institutional Animal Care and Use Committee (IACUC).
Test Apparatus
For startle reflex testing, a rat was placed in an acoustically transparent, wire-mesh (0.5 cm × 0.5 cm) cage (20 cm L, 7 cm W, 6 cm H) mounted on a Plexiglas base (20 cm × 10 cm) which rested on a pressure sensitive 35-mm piezoelectric transducer (MCM 28–745) that generated a voltage proportional to the magnitude of the startle response. The startle platform was placed in a custom-built, medium density fiber (MDF), sound-attenuating cubicle (57 cm L, 46 cm W, 46 cm H) that was lined with acoustic foam (noise floor <20 dB SPL at frequencies >4000 Hz). Sound stimuli were generated (TDT RX6, ~100-kHz sampling rate), amplified, and delivered via a free-field speaker (Fostex FT17H) placed above the startle platform (25 cm). Silent gaps in continuous sound were created using an RPVDS linear gate function to toggle the carrier sound “on” or “off” and the gap rise/fall times were 10-μs sound. Stimuli within the cubicle were calibrated using a Larson Davis sound level meter (SLM 824) and a one half- or one quarter-inch condenser microphone. The output of the startle platform was amplified (Behringer ADA8000), digitized, and low-pass filtered by an A/D converter (TDT RX8, ~6-kHz sampling rate), and stored on a computer for offline analysis.
Study Design
The overall aim of the study was to evaluate the efficacy of cyclobenzaprine in animals with behavioral evidence of noise-induced tinnitus. Consistent with our previous publications, a within-subjects design was implemented with a pre-noise drug control condition and exclusion criteria for animals with absent startle responses post-noise (responses not significantly different than noise floor) or animals with no behavioral evidence of tinnitus.
Acoustic Startle Reflex Input/Output
Noise exposure to just one ear can negatively impact startle reactivity to acoustic stimuli (Lobarinas et al. 2013). A reduced overall startle in both the gap and no gap conditions can confound GPIAS, as low overall startle (at or near the noise floor) can be incorrectly interpreted as a failure to inhibit. To overcome this limitation, we implemented two features in our protocol. First, the input–output function of the startle response to acoustic stimuli 70–115 dB SPL was evaluated before and after noise exposure. Animals that had statistically significant reductions in their startle response post-noise were removed from the study. A statistically significant reduction was identified as post-noise startle amplitudes that were outside the 95 % confidence interval associated with that animal’s pre-noise startle amplitude. These reduced post-noise startles are generally indistinguishable from the noise floor. Second, to optimize post-noise startle, we used an acoustic startling stimulus that was bandpass-filtered from 5–10 kHz, a range below the noise exposure stimulus frequency. The narrowband noise used in the exposure was centered at 16 kHz and was expected to produce hearing loss at and above 16 kHz (based on the half-octave shift identified by Davis et al. 1950 and data from Heffner et al. 2008). Thus, the acoustic startling stimulus would remain audible to both the unexposed and exposed ears given that the startle stimulus was below and outside the frequency range of the measured hearing loss.
The pre- and post-noise input–output schedule was composed of 100 trials with 10 randomized presentations at each startle intensity level. Startle stimulus levels were 70–115 dB SPL, in 5-dB increments. The inter-trial interval (ITI) was randomly varied from 5 to 15 s. Stimuli were generated using Tucker Davis Technologies (TDT) RPVDS and a Real-Time Processor (RP2.1). A continuous Gaussian noise function was digitized with a sampling rate of 100 kHz, bandpass-filtered to 5–10 kHz, converted to analog, and presented through a high-frequency speaker (Fostex FT17H horn tweeter) in a calibrated sound field. Baseline behavioral testing of the bandpass noise input–output function consisted of five sessions on non-consecutive days over a 2-week period. The startle input output function was determined pre-noise exposure (average of five baselines, saline, and cyclobenzaprine 0.5 mg/kg) and 48 h, 40 days, 42 days, and 45 days post-noise exposure as shown in Table 1.
Noise-burst Pre-pulse Inhibition of the Acoustic Startle (NBPIAS)
In addition to loss of startle, it is possible that unilateral noise exposure could affect audibility of both the acoustic startle stimulus and the pre-pulse carrier. To control for changes in audibility, animals were tested with NBPIAS. The NBPIAS has been previously described (Lobarinas et al. 2013). Briefly, an acoustic cue is presented before the onset of the startle stimulus. The acoustic cues were 60 dB SPL, 75-ms bandpass narrowband noises (NBN) centered at 6, 12, 16, 20, or 24 kHz, that were presented 100 ms prior to the onset of the startle stimulus (bandpass 5–10-kHz noise, 105 dB SPL). NBPIAS was assessed before and after the noise exposure in both the untreated and cyclobenzaprine treatment conditions.
GPIAS
The method for obtaining GPIAS in noise-exposed animals has been described in previous publications. The dependent measure in GPIAS is the amplitude change of the large motoric response to a loud (startling) stimulus. This startle response is suppressed when a silent gap is inserted in an otherwise continuous background sound prior to the presentation of the startle stimulus (Ison 1982; Ison et al. 1991). If the animal is experiencing tinnitus and the tinnitus is spectrally similar to or contains frequency elements of the continuous background noise, the ability to reliably detect the silent gap could be impaired. In contrast, when the continuous noise is spectrally dissimilar to the tinnitus, the silent gap may result in an audible change in spectra that could provide a cue of the imminent startle sound and thus reduce the startle response.
In the present study, GPIAS sessions were composed of 200 frequency and condition (gap versus no-gap) pseudo-randomized trials (20 trials gap and 20 no-gap trials at 5 frequencies) with a variable inter-trial intervals of 5–15 s and a total session time of 45–60 min. Each session trial started with a bandpass carrier noise and then a brief silent gap (75 ms) was inserted 100 ms prior to the delivery of the startle stimulus (5–10-kHz bandpass, 105 dB SPL) in half of the trials. The bandpass carrier sounds were narrowband noises (NBN) centered at 6, 12, 16, 20, or 24 kHz (bandwidth ranged from 100 to 5000 Hz to account for critical band differences). The presentation level of the carrier noise (6–24 kHz) was 60 dB SPL. This level was selected based on preliminary studies and is 30–35 dB above mean threshold performance for GPIAS.
GPIAS was evaluated pre-noise exposure (average of 5 baselines, saline), pre-noise exposure with 0.5 mg/kg cyclobenzaprine, 48 h post-noise exposure, 40 days post-noise exposure, 42 days post-noise exposure, 45 days post-noise exposure with 0.5 mg/kg cyclobenzaprine, and 47 days post-noise exposure as shown in Table 1.
Noise Exposure
The left ear of each rat (n = 20) was exposed to a 126 dB SPL narrowband noise centered at 16 kHz (BW = 100 Hz) for 1 h, a level shown to produce severe to profound unilateral hearing loss in rats at frequencies >16 kHz in previous noise-dose pilot studies and a level with a higher probability of producing behavior consistent with tinnitus. This noise exposure level was also selected because it maximizes unilateral high-frequency hearing loss without inducing changes in auditory brainstem response (ABR) thresholds, ABR input–output, or DPOAE in the non-exposed ear and because it does not affect NBPIAS.
In the present study, all subjects were anesthetized with isoflurane gas (5 % induction, 1–2 % maintenance), and placed on an automated heating pad within a calibrated sound field. Narrowband noise was created from a high- and low-pass filtered Gaussian noise (TDT RP2.1), amplified (Crown XLS-202), and presented via a free-field speaker (Fostex FT17H horn tweeter) positioned 2 cm from the entrance of the left ear canal at 270° azimuth. The right (contralateral) ear was protected with a pediatric ear probe filled with plumber tack, an approach that was shown in our previous study to prevent damage from the noise exposure (Kraus et al. 2010). This procedure was developed so that the acoustic stimuli during GPIAS remain audible in the non-exposed ear after unilateral noise exposure.
Distortion Product Otoacoustic Emissions
DPOAEs were screened in both ears using a commercial otoacoustic emission system (Intelligent Hearing System, Miami, FL, USA). Two primary tones, f1 and f2, were used with an f2/f1 ratio of 1.2 and DPOAE amplitude was measured at 2f1–f2. DPOAEs were obtained at f2 frequencies of 8, 12, 16, 20, 24, and 32 kHz (32 sweeps per frequency pair). The f1 intensity (L1) was presented at 70 dB SPL, a level 10 dB higher than the f2 intensity (L2). During DPOAE recording, animals were placed in a custom sound-attenuating chamber, on a heating pad, lightly anesthetized (5 % induction and 1 % maintenance with isoflurane gas) and the DPOAE probe assembly was placed in the animal’s external ear canal. DPOAE screenings were performed in each ear before and after unilateral noise exposure; DPOAE amplitudes that were 6 dB above the measured noise floor were defined as the pass criterion. The DPOAE were used to determine whether noise exposures resulted in significant cochlear damage based on levels previously shown to produce behavior consistent with tinnitus. In preliminary studies, the DPOAE screening was more sensitive than ABR threshold in identifying cochlear damage to the unexposed ear.
Cyclobenzaprine
Cyclobenzaprine hydrochloride (5-(3-dimethylaminopropylidene) dibenzo[a,e]cycloheptatriene hydrochloride, Sigma-Aldrich, St. Louis) was dissolved in a 0.9 % bacteriostatic saline to a 1 mg/ml concentration and injected intraperitoneally (i.p.) 1 h prior to behavioral testing. This time point was selected based on rat data from a previous comparative study of cyclobenzaprine absorption and excretion (Hucker et al. 1978). Doses were calculated as a function of body weight (mg/kg). In preliminary dose-response studies, we determined that the maximum dose of cyclobenzaprine that did not result in a significant reduction of the startle response was 0.5 mg/kg. Therefore, the current study evaluated the ability of the 0.5 mg/kg dose to attenuate behavioral evidence of noise-induced tinnitus.
Data Analysis
All subjects were evaluated for GPIAS, NBPIAS, and the startle amplitude input-output function prior to noise exposure. The startle amplitude input-output function obtained post-noise was analyzed relative to a 95 % confidence interval associated with the mean individual pre-noise baseline performance. Subjects that had a significant decrease in startle amplitude after noise exposure were excluded from the treatment portion of the study because loss of startle would confound interpretation and evaluation of GPIAS, NBPIAS, and treatment efficacy. Mean data for the subjects with no significant noise-induced decrease in startle were analyzed using a two-way repeated-measure analysis of variance (ANOVA) to ascertain the effect of treatment on startle reactivity. These subjects were then re-evaluated with GPIAS post-noise for evidence of tinnitus and NBPIAS as a control for the audibility of the bandpass carriers used in the GPIAS condition. Subjects with GPIAS data consistent with tinnitus were included in the study. Conversely, subjects with no significant decrease in GPIAS post-noise, suggesting that they did not develop noise-induced tinnitus, were not included in the treatment portion of the study. All included subjects were treated with cyclobenzaprine (0.5 mg/kg). GPIAS was then analyzed with a two-way repeated-measure ANOVA to determine the effects of treatment (baseline, post-noise, post-noise cyclobenzaprine), effects on frequency (6, 12, 16, 20, and 24 kHz), and interaction effects. All statistical comparisons used an alpha level of 0.05 and post hoc analysis was performed using Tukey tests to avoid type I errors associated with multiple comparisons. Sigma Stat 3.5 was used for all statistical analyses. All results are presented as mean ± standard deviation (SD).
Results
Pre-noise Startle Input-Output, NBPIAS, and GPIAS Measures
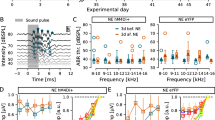
Prior to noise exposure, baseline startle input-output, NBPIAS, and GPIAS measures were established using 5-day averages, animals received a saline control injection, and 0.5 mg/kg pre-noise cyclobenzaprine treatment as shown in Table 1. Pre-noise saline and cyclobenzaprine had no significant effects on the startle input–output function, NBPIAS or GPIAS. The effects of pre-noise cyclobenzaprine on startle input/output and NBPIAS are shown in Figures 1 and 2 respectively.
Effect of cyclobenzaprine and noise on startle input–output function. Mean startle amplitude (±SD) as a function of startle stimulus intensity showed no significant change following pre-noise cyclobenzaprine treatment (p > 0.05 n.s.). In contrast, startle amplitudes 40 days post unilateral noise exposure showed significant decreases in a subset of animals (p < 0.05). These animals were rejected from the study (open triangles) as the startle responses were indistinguishable from the noise floor. The remaining animals showed no significant decreases 40 days post noise (p = 0.101 n.s.) or 45 days post-noise cyclobenzaprine treatment.
Effect of noise and cyclobenzaprine on NBPIAS. Treatment with cyclobenzaprine (0.5 mg/kg) had no effect on pre-noise NBPIAS in the subgroup of animals with no significant reduction in startle amplitude post-noise (p > 0.05 n.s.). There were no significant effects on NBPIAS following unilateral noise exposure or post-noise cyclobenzaprine treatment (p = 0.518 n.s).
Post-noise DPOAE
All animals that entered the study (n = 20) were exposed unilaterally to 16 kHz, 126 dB SPL narrow band noise (BW = 100 Hz) for 1 h, a level previously shown to produce data consistent with tinnitus in a subset of animals. As expected, following noise trauma, DPOAEs were absent in the exposed (left) ears at frequencies 16 kHz and higher across all study subjects (DPOAES were in the noise floor). In contrast, DPOAEs in the right ear remained present and relatively unchanged from baseline; i.e. all subjects passed right ear screening with DPOAE >6 dB above noise floor. The DPOAE results were consistent with significant hearing loss >40 dB in the exposed ear and no significant change in the protected ear. Because our outcome measures were the presence or absence of tinnitus with the preservation of NBPIAS and hearing in the non-exposed ear, we did not specifically quantify the degree of hearing loss in the exposed ear.
Post-noise Startle Input-Output Function
In all noise-exposed animals (n = 20), sound overstimulation was presented unilaterally to ensure that the rats would retain hearing in one ear so that all startle and GPIAS stimuli would remain audible. When the amplitude of the startle response was compared between the baseline and post-noise conditions, unilateral noise exposure resulted in a significant reduction in the startle response (outside 95 % baseline confidence interval) at 85–115 dB SPL in more than half of animals (n = 13). These animals were removed from the study based on post-noise startle amplitudes outside the 95 % confidence interval for their own baseline data. In the remaining animals (n = 7), noise exposure did not significantly reduce the measured startle response amplitude at the 105 dB SPL presentation level used for the GPIAS condition. The mean pre and post-nose startle amplitude input–output response functions (±SD) are shown in Figure 1. A two-way repeated-measure ANOVA showed no statistically significant overall difference among baseline, pre-noise cyclobenzaprine, post-noise, and post-noise cyclobenzaprine startle amplitudes elicited by our bandpass startle stimulus for the experimental group [F(3,54) = 3.759, p = 0.101]. In contrast, the mean startle amplitude for the excluded animals (n = 13) was indistinguishable from the noise floor.
Post-noise NBPIAS
NPIAS was evaluated in the seven subjects that did not have a significant reduction in startle response amplitudes post-noise exposure. NBPIAS as a function of treatment and frequency is shown in Figure 2. A two-way repeated measures ANOVA showed no significant reduction in NBPIAS as a function of pre-noise cyclobenzaprine, noise, or post-noise cyclobenzaprine [F(3,60) = 0.791, p = 0.518 n.s.]
Post-noise GPIAS
GPIAS was evaluated in the seven subjects that did not have a significant reduction in startle response amplitudes or a significant change in NBPIAS following noise exposure. Whereas six of the subjects showed a reduction in GPIAS at one or more tested frequencies (outside 95 % confidence interval), one subject failed to show any difference in GPIAS post-noise at all test frequencies. This subject was excluded from further analysis, although we note the potential for tinnitus at other untested frequencies. The mean baseline, pre-noise cyclobenzaprine and post-noise GPIAS for the remaining six animals are shown in Figure 3 (±SD). A two-way repeated-measure ANOVA showed a statistically significant difference in GPIAS as a function of treatment [F(4,40) = 12.454, p < 0.001]. A post hoc analysis using Tukey showed a statistically significant overall treatment difference between baseline and post-noise GPIAS (p < 0.001), a statistically significant effect of noise on frequencies 6–20 kHz (p < 0.05), and a statistically significant difference between the post-noise (tinnitus) and pre-noise cyclobenzaprine conditions (p < 0.001). Pairwise comparisons for baseline, pre-noise cyclobenzaprine, and post-noise (tinnitus) are shown in Table 2.
Noise-induced reduction in GPIAS. Unilateral noise exposure resulted in a reduction of GPIAS 6–20 kHz, 40 days post-exposure in the subset of animals with no significant changes in post-noise startle input–output or NBPIAS (p < 0.001). These results were consistent with the presence of tinnitus interfering with GPIAS at multiple frequencies. Treatment with cyclobenzaprine, 45 days post-exposure, increased GPIAS to baseline levels (p = 0.116). However, a 48-h post-treatment assessment showed a reemergence of behaviors consistent with tinnitus suggesting only transient drug-induced suppression (p < 0.05). Statistically significant overall treatment effects are shown by brackets and asterisks. Mean GPIAS is shown ± SD.
Post-noise Effect of Cyclobenzaprine
The six subjects that showed reduced GPIAS in at least one frequency, behavior that was consistent with our operational definition of tinnitus, were treated with a 1-ml saline injection. Treatment with saline had no effect on post-noise GPIAS (not shown). In contrast, when subjects were treated with a single dose of 0.5 mg/kg cyclobenzaprine 45 days post-noise, GPIAS increased to near baseline levels. As shown in Figure 3, treatment with cyclobenzaprine significantly increased GPIAS at frequencies 6–20 kHz relative to post-noise (tinnitus). A two-way repeated-measure ANOVA [F(4,40) = 12.454, p < 0.001] showed a statistically significant effect of treatment. A post hoc analysis using Tukey showed a statistically significant difference between post-noise tinnitus and post-noise tinnitus + cyclobenzaprine (p = 0.048), but no overall difference between post-noise + cyclobenzaprine and baseline (p = 0.116) or pre-noise cyclobenzaprine (p = 0.109). These effects suggest that treatment with cyclobenzaprine reduced noise-induced tinnitus.
To evaluate whether cyclobenzaprine-induced suppression of tinnitus was transient or long term, subjects were reassessed for the presence of tinnitus 48 h post-treatment. As shown in Figure 3, there was no significant difference between the post-noise values and the 48-h post-cyclobenzaprine values. These post-cyclobenzaprine results suggest that the chronic tinnitus was only transiently suppressed. The 48-h post-treatment measure was based on the effective half-life for cyclobenzaprine (18 h with a rapid elimination half-life of 3.1 h and average terminal elimination half-life of 31.9 h). However, it is possible that tinnitus suppression may be shorter than 48 h. Additional experiments will explore the effects on tinnitus at shorter time points and whether repeated treatments can extend suppression.
Discussion
Whereas a number of drugs have been proposed as effective treatments for tinnitus, few have shown consistent and long-term results across a broad spectrum of tinnitus patients. The purpose of this study was to evaluate the efficacy of cyclobenzaprine on unilateral noise-induced tinnitus in an animal model. There is a growing consensus that tinnitus may be the result of neural hyperactivity (Norena 2011; Middleton and Tzounopoulos 2012). Consequently drugs that reduce central activity may reduce some forms of tinnitus (Brozoski et al. 2007b, a; Zheng et al. 2012). In the present experiment, we evaluated the effect of cyclobenzaprine on the subset of noise-exposed animals that had behavioral evidence of tinnitus. The presence of tinnitus was assessed at 48 h and at 40 days post-exposure. Based on our GPIAS design, we had expected that evidence of tinnitus would emerge at the exposure frequency or within one octave above the exposure (narrowband noise centered at 16 kHz). In contrast, our results indicated significant post-noise GPIAS performance deficits at 6–20 kHz but not at 24 kHz. Although we were surprised by these findings, evidence of tinnitus at frequencies lower than the noise exposure have been reported in rats under similar GPIAS conditions and in chinchillas using an operant conditioning tinnitus paradigm (Brozoski et al. 2002; Bauer et al. 2008; Engineer et al. 2011). The agreement between two different models and two different species suggests that the noise-induced tinnitus may significantly interfere with detection of silent intervals at multiple frequencies.
In the present study, for the subset of animals with GPIAS deficits consistent with the presence of tinnitus, treatment with cyclobenzaprine significantly reduced evidence of tinnitus (i.e., GPIAS returned toward baseline levels). The hypothesis that cyclobenzaprine would reduce noise-induced tinnitus was based on evidence from early clinical reports regarding the potential efficacy of this compound on persistent tinnitus. In two recent open-label studies, cyclobenzaprine significantly reduced tinnitus distress and tinnitus handicap (Coelho et al. 2012; Vanneste et al. 2012). More importantly, however, is that in one of the studies, 24 % of cyclobenzaprine treated participants, who responded to treatment, showed a 53 % reduction in tinnitus loudness (Vanneste et al. 2012). This secondary finding would be consistent with a reduction of the tinnitus signal itself or a reduction in its perception as opposed to a reduction in the response to tinnitus. The data presented here appear to support a reduction in the tinnitus perception or loudness as evidenced by a significant improvement in GPIAS during treatment. Our data suggest that the improvement in GPIAS is a result of a reduction in the tinnitus percept and not a generalized improvement in GPIAS as pre-noise cyclobenzaprine treatment failed to enhance GPIAS over baseline levels and cyclobenzaprine treatment did not enhance NBPIAS. The effect on GPIAS appears to be the direct result of cyclobenzaprine’s action since cessation of treatment was followed by evidence of reemerging tinnitus as shown in Figure 3.
The positive effects of cyclobenzaprine on tinnitus open a host of additional questions as the pharmacological actions of cyclobenzaprine are complex. Whereas it is chemically similar to tricyclic antidepressants, it is also widely used as a muscle relaxant with some use as an attenuator of fibromyalgic pain. In this regard, cyclobenzaprine may reduce tinnitus induced by hearing loss via mechanisms akin to those of drugs that reduce phantom pain, a deafferentation model proposed for tinnitus (Jastreboff et al. 1988b; Muhlnickel et al. 1998; Lockwood et al. 1999; Sahley et al. 2013).
Conversely, the drug’s known effects on the locus coeruleus in rats may instead elucidate a potential new site related to tinnitus as a form of abnormal sound perception. Stimulation to the locus coeruleus has been shown to alter firing patterns of neurons in the auditory cortex (Edeline et al. 2011) and has been suggested to limit unnecessary cerebral activity associated with sound stimulation (Justice et al. 1989). In the epilepsy literature, vagal nerve stimulation (VNS) has been used to reduce aberrant activity (Connor et al. 2012), potentially via modulation of activity of the locus coeruleus (Maurin et al. 1986; Giorgi et al. 2006). More recently VNS has been shown to reduce behaviors consistent with the presence of tinnitus in animals (Engineer et al. 2013) and is being evaluated in clinical trials as a tinnitus treatment (NCT01962558 2014). Collectively, these findings suggest that the locus coeruleus may play a role in some forms of tinnitus.
An alternative explanation for our results could stem from cyclobenzaprine’s muscle relaxant effects. However, our results showed no significant effects on the startle motor output or GPIAS prior to the noise exposure. In contrast, cyclobenzaprine treatment showed significant improvement in GPIAS relative to the post-noise condition. Because GPIAS returned to baseline conditions post-treatment, the results suggest the temporary drug-induced suppression was the result of cyclobenzaprine treatment. These data would be consistent with tinnitus models that propose the potential contribution of non-auditory pathways as essential in recruiting attention to aberrant neural activity (Hazell and Jastreboff 1990; Jastreboff 1990; Moller et al. 1992; Roberts et al. 2013).
The effects of cyclobenzaprine observed here are relevant to current basic assumptions regarding attention to the phantom percept of tinnitus as a hallmark of the clinical condition. If current data are indicative of an attentional mechanism, then these results could provide a partial explanation as to why some animals experience tinnitus after the same noise-induced hearing loss whereas others do not. That the perception and sensation of tinnitus varies across individuals exposed to identical noise conditions is clear (Le Prell et al. 2012; Spankovich et al. 2013). Perhaps, it is the abnormal gating, in addition to the degree of cochlear damage that results in the tinnitus percept (Zhang 2013). These possibilities may partially explain the efficacy of cyclobenzaprine on some patients whose tinnitus could be dominated by abnormal gating. The contribution of the locus coeruleus, changes in activity in tinnitus-positive animals, and the role of cyclobenzaprine will need to be explored within the same animals in more detail in subsequent experiments to further investigate this possible pathway.
References
Aston-Jones G, Chiang C, Alexinsky T (1991) Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Prog Brain Res 88:501–520
Basmajian JV (1978) Cyclobenzaprine hydrochloride effect on skeletal muscle spasm in the lumbar region and neck: two double-blind controlled clinical and laboratory studies. Arch Phys Med Rehabil 59:58–63
Bauer CA, Brozoski TJ (2001) Assessing tinnitus and prospective tinnitus therapeutics using a psychophysical animal model. J Assoc Res Otolaryngol 2:54–64
Bauer CA, Brozoski TJ (2006) Effect of gabapentin on the sensation and impact of tinnitus. Laryngoscope 116:675–681
Bauer CA, Brozoski TJ, Rojas R, Boley J, Wyder M (1999) Behavioral model of chronic tinnitus in rats. Otolaryngol Head Neck Surg 121:457–462
Bauer CA, Turner JG, Caspary DM, Myers KS, Brozoski TJ (2008) Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. J Neurosci Res 86:2564–2578
Brozoski TJ, Bauer CA, Caspary DM (2002) Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J Neurosci 22:2383–2390
Brozoski TJ, Spires TJ, Bauer CA (2007a) Vigabatrin, a GABA transaminase inhibitor, reversibly eliminates tinnitus in an animal model. J Assoc Res Otolaryngol 8:105–118
Brozoski TJ, Ciobanu L, Bauer CA (2007b) Central neural activity in rats with tinnitus evaluated with manganese-enhanced magnetic resonance imaging (MEMRI). Hear Res 228:168–179
Cave KM, Cornish EM, Chandler DW (2007) Blast injury of the ear: clinical update from the global war on terror. Mil Med 172:726–730
Coelho C, Figueiredo R, Frank E, Burger J, Schecklmann M, Landgrebe M, Langguth B, Elgoyhen AB (2012) Reduction of tinnitus severity by the centrally acting muscle relaxant cyclobenzaprine: an open-label pilot study. Audiol Neuro-otol 17:179–188
Commissiong JW, Karoum F, Reiffenstein RJ, Neff NH (1981) Cyclobenzaprine: a possible mechanism of action for its muscle relaxant effect. Can J Physiol Pharmacol 59:37–44
Connor DE Jr, Nixon M, Nanda A, Guthikonda B (2012) Vagal nerve stimulation for the treatment of medically refractory epilepsy: a review of the current literature. Neurosurg Focus 32:E12
Davis H, Morgan CT, Hawkins JE Jr, Galambos R, Smith FW (1950) Temporary deafness following exposure to loud tones and noise. Acta Otolaryngol Suppl 88:1–56
Dobie RA (1999) A review of randomized clinical trials in tinnitus. Laryngoscope 109:1202–1211
VA DoVA (2011) Annual Benefits Report. In: http://www.vba.va.gov/reports/abr/2011_abr.pdf
Edeline JM, Manunta Y, Hennevin E (2011) Induction of selective plasticity in the frequency tuning of auditory cortex and auditory thalamus neurons by locus coeruleus stimulation. Hear Res 274:75–84
Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, Borland MS, Kilgard MP (2011) Reversing pathological neural activity using targeted plasticity. Nature 470:101–104
Engineer ND, Moller AR, Kilgard MP (2013) Directing neural plasticity to understand and treat tinnitus. Hear Res 295:58–66
Giorgi FS, Mauceli G, Blandini F, Ruggieri S, Paparelli A, Murri L, Fornai F (2006) Locus coeruleus and neuronal plasticity in a model of focal limbic epilepsy. Epilepsia 47(Suppl 5):21–25
Guitton MJ, Caston J, Ruel J, Johnson RM, Pujol R, Puel JL (2003) Salicylate induces tinnitus through activation of cochlear NMDA receptors. J Neurosci 23:3944–3952
Hansen N, Manahan-Vaughan D (2014) Locus coeruleus stimulation facilitates long-term depression in the dentate gyrus that requires activation of beta-adrenergic receptors. Cereb Cortex. doi:10.1093/cercor/bht429
Hazell JW, Jastreboff PJ (1990) Tinnitus. I: auditory mechanisms: a model for tinnitus and hearing impairment. J Otolaryngol 19:1–5
Heffner HE (2011) A two-choice sound localization procedure for detecting lateralized tinnitus in animals. Behav Res Methods 43:577–589
Heffner HE, Harrington IA (2002) Tinnitus in hamsters following exposure to intense sound. Hear Res 170:83–95
Heffner HE, Koay G (2005) Tinnitus and hearing loss in hamsters (Mesocricetus auratus) exposed to loud sound. Behav Neurosci 119:734–742
Heffner HE, Koay G, Heffner RS (2008) Comparison of behavioral and auditory brainstem response measures of threshold shift in rats exposed to loud sound. J Acoust Soc Am 124:1093–1104
Hucker HB, Stauffer SC, Balletto AJ, White SD, Zacchei AG, Arison BH (1978) Physiological disposition and metabolism of cyclobenzaprine in the rat, dog, rhesus monkey, and man. Drug Metab Dispos: Biol Fate Chem 6:659–672
Ison JR (1982) Temporal acuity in auditory function in the rat: reflex inhibition by brief gaps in noise. J Comp Physiol Psychol 96:945–954
Ison JR, O’Connor K, Bowen GP, Bocirnea A (1991) Temporal resolution of gaps in noise by the rat is lost with functional decortication. Behav Neurosci 105:33–40
Jastreboff PJ (1990) Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci Res 8:221–254
Jastreboff PJ, Brennan JF, Sasaki CT (1988a) An animal model for tinnitus. Laryngoscope 98:280–286
Jastreboff PJ, Brennan JF, Coleman JK, Sasaki CT (1988b) Phantom auditory sensation in rats: an animal model for tinnitus. Behav Neurosci 102:811–822
Justice A, Feldman SM, Brown LL (1989) The nucleus locus coeruleus modulates local cerebral glucose utilization during noise stress in rats. Brain Res 490:73–84
Katz WA, Dube J (1988) Cyclobenzaprine in the treatment of acute muscle spasm: review of a decade of clinical experience. Clin Ther 10:216–228
Kim DK, Park SN, Kim HM, Son HR, Kim NG, Park KH, Yeo SW (2011) Prevalence and significance of high-frequency hearing loss in subjectively normal-hearing patients with tinnitus. Ann Otol Rhinol Laryngol 120:523–528
Kraus KS, Mitra S, Jimenez Z, Hinduja S, Ding D, Jiang H, Gray L, Lobarinas E, Sun W, Salvi RJ (2010) Noise trauma impairs neurogenesis in the rat hippocampus. Neuroscience 167:1216–1226
Lang IM, Barnes CD (1983) Cyclobenzaprine effects on locus coeruleus cells in tissue slice. Neuropharmacology 22:249–252
Le Prell CG, Dell S, Hensley B, Hall JW 3rd, Campbell KC, Antonelli PJ, Green GE, Miller JM, Guire K (2012) Digital music exposure reliably induces temporary threshold shift in normal-hearing human subjects. Ear Hear 33:e44–e58
Lobarinas E, Sun W, Cushing R, Salvi R (2004) A novel behavioral paradigm for assessing tinnitus using schedule-induced polydipsia avoidance conditioning (SIP-AC). Hear Res 190:109–114
Lobarinas E, Yang G, Sun W, Ding D, Mirza N, Dalby-Brown W, Hilczmayer E, Fitzgerald S, Zhang L, Salvi R (2006) Salicylate- and quinine-induced tinnitus and effects of memantine. Acta Otolaryngol Suppl 13–19
Lobarinas E, Dalby-Brown W, Stolzberg D, Mirza NR, Allman BL, Salvi R (2011) Effects of the potassium ion channel modulators BMS-204352 Maxipost and its R-enantiomer on salicylate-induced tinnitus in rats. Physiol Behav 104:873–879
Lobarinas E, Hayes SH, Allman BL (2013) The gap-startle paradigm for tinnitus screening in animal models: limitations and optimization. Hear Res 295:150–160
Lockwood AH, Salvi RJ, Burkard RF, Galantowicz PJ, Coad ML, Wack DS (1999) Neuroanatomy of tinnitus. Scand Audiol Suppl 51:47–52
Lofland JH, Szarlej D, Buttaro T, Shermock S, Jalali S (2001) Cyclobenzaprine hydrochloride is a commonly prescribed centrally acting muscle relaxant, which is structurally similar to tricyclic antidepressants (TCAs) and differs from amitriptyline by only one double bond. Clin J Pain 17:103–104
Maurin Y, Enz A, Le Saux F, Besson MJ (1986) Supernumerary locus coeruleus neurons as a determinant of inherited epilepsy in the convulsive mutant mouse quaking. Brain Res 366:379–384
McCombe A, Baguley D, Coles R, McKenna L, McKinney C, Windle-Taylor P (2001) Guidelines for the grading of tinnitus severity: the results of a working group commissioned by the British Association of Otolaryngologists, Head and Neck Surgeons, 1999. Clin Otolaryngol Allied Sci 26:388–393
Middleton JW, Tzounopoulos T (2012) Imaging the neural correlates of tinnitus: a comparison between animal models and human studies. Front Syst Neurosci 6:35
Moller AR, Moller MB, Yokota M (1992) Some forms of tinnitus may involve the extralemniscal auditory pathway. Laryngoscope 102:1165–1171
Muhlnickel W, Elbert T, Taub E, Flor H (1998) Reorganization of auditory cortex in tinnitus. Proc Natl Acad Sci U S A 95:10340–10343
NCT01962558 (2014) A blinded randomized pilot study assessing vagus nerve stimulation (VNS) paired with tones for tinnitus vs. VNS with unpaired tones. http://clinicaltrials.gov/show/NCT01962558
Norena AJ (2011) An integrative model of tinnitus based on a central gain controlling neural sensitivity. Neurosci Biobehav Rev 35:1089–1109
Puel JL (2007) Cochlear NMDA receptor blockade prevents salicylate-induced tinnitus. B-Ent 3(Suppl 7):19–22
Roberts LE, Husain FT, Eggermont JJ (2013) Role of attention in the generation and modulation of tinnitus. Neurosci Biobehav Rev 37:1754–1773
Ruttiger L, Ciuffani J, Zenner HP, Knipper M (2003) A behavioral paradigm to judge acute sodium salicylate-induced sound experience in rats: a new approach for an animal model on tinnitus. Hear Res 180:39–50
Sahley TL, Hammonds MD, Musiek FE (2013) Endogenous dynorphins, glutamate and N-methyl-d-aspartate (NMDA) receptors may participate in a stress-mediated Type-I auditory neural exacerbation of tinnitus. Brain Res 1499:80–108
Salvi R, Lobarinas E, Sun W (2009) Pharmacological treatments for tinnitus: new and old. Drugs Future 34:381–400
Shargorodsky J, Curhan GC, Farwell WR (2010) Prevalence and characteristics of tinnitus among US adults. Am J Med 123:711–718
Spankovich C, Griffiths SK, Lobarinas E, Morgenstein KE, De la Calle S, Ledon V, Guersio D, Le Prell CG (2013) Temporary threshold shift after impulse-noise during video game play: laboratory data. International J Audiol Accepted
Turner JG, Parrish J (2008) Gap detection methods for assessing salicylate-induced tinnitus and hyperacusis in rats. Am J Audiol 17:S185–S192
Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K, Hughes LF, Caspary DM (2006) Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav Neurosci 120:188–195
Van Hoey NM (2005) Effect of cyclobenzaprine on tricyclic antidepressant assays. Ann Pharmacother 39:1314–1317
Vanneste S, Figueiredo R, De Ridder D (2012) Treatment of tinnitus with cyclobenzaprine: an open-label study. Int J Clin Pharmacol Ther 50:338–344
Yang S, Weiner BD, Zhang LS, Cho SJ, Bao S (2011) Homeostatic plasticity drives tinnitus perception in an animal model. Proc Natl Acad Sci U S A 108:14974–14979
Zhang J (2013) Auditory cortex stimulation to suppress tinnitus: mechanisms and strategies. Hear Res 295:38–57
Zheng Y, Hooton K, Smith PF, Darlington CL (2008) Carbamazepine reduces the behavioural manifestations of tinnitus following salicylate treatment in rats. Acta Otolaryngol 128:48–52
Zheng Y, McNamara E, Stiles L, Darlington CL, Smith PF (2012) Evidence that memantine reduces chronic tinnitus caused by acoustic trauma in rats. Front Neurol 3:127
Conflict of Interest
The authors report no conflict of interest with the research presented in the manuscript.
All work was supported by the Tinnitus Research Initiative (TRI).
Author information
Authors and Affiliations
Corresponding author
Additional information
EL designed the experiments, EL and CB performed data collection and analysis, and all authors contributed to writing the manuscript.
Rights and permissions
About this article
Cite this article
Lobarinas, E., Blair, C., Spankovich, C. et al. Partial to Complete Suppression of Unilateral Noise-Induced Tinnitus in Rats after Cyclobenzaprine Treatment. JARO 16, 263–272 (2015). https://doi.org/10.1007/s10162-014-0500-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-014-0500-x