Abstract
Each pedal ganglion of the pteropod mollusc Clione limacina contains a cluster of serotonin-immunoreactive neurons that have been shown to modulate contractions of the slow-twitch musculature of the wing-like parapodia, and contribute to swim accelerations. Each cluster has a variable number of neurons, between 5 and 9, but there is no significant difference between right and left ganglia. In experiments with electrophysiological recordings followed by dye-injection (carboxyfluorescein), the clusters were found to contain two subsets of neurons. The majority innervate the ipsilateral wing via nerve n4. Two of the neurons in each cluster send processes out of the pedal ganglion in nerves n3 and n8. The processes in nerve n3 innervate the body wall of the neck region, while those in nerve n8 innervate the body wall of the tail. The baseline electrophysiological activity of the two subsets of neurons was different as “wing” neurons had constant barrages of small synaptic activity, while the “body wall” neurons had few synaptic inputs. The potential roles of the Pd-SW cluster in swim acceleration (wing neurons) and control of fluid pressure in the body and wing hemocoelic compartments (body wall neurons) are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Changes in locomotory speed typically involve increases in the frequency of movement of locomotory structures as well as increases in the force or amplitude of the movements. The neuronal basis of locomotory accelerations has been studied in detail in zebra fish, where interneurons are recruited into the central control circuitry in a predictable manner relative to locomotory speed (McLean et al. 2008; McLean and Fetcho 2009; Ausborn et al. 2012; Eklof-Ljunggren et al. 2012; Ampatzis et al. 2014; Bjornfors et al. 2019; Song et al. 2020). Similar results have been found in mice (Dougherty and Kiehn 2010; Zhong et al. 2010, 2011; Bjornfors et al. 2019). At the physiological level, motorneuron recruitment also follows a predictable order (Gabriel et al. 2011) that is matched with the recruitment of interneuron modules (Ausborn et al. 2012; Ampatzis et al. 2014; Bjornfors et al. 2019).
The pteropod mollusk, Clione limacina, shows two distinct swimming speeds, and the change from slow to fast swimming is equivalent to a gait change in vertebrates (Arshavsky et al. 1985a,b,c,d; Satterlie and Norekian 1997, 2001). The locomotory appendages of Clione consist of foot tissue that is laterally expanded into a pair of wing-like parapodia (called wings), which bend in symmetrical dorsal (upswing) and ventral (downswing) flapping movements (Arshavsky et al. 1985a; Satterlie and Spencer 1985; Szymik and Satterlie 2011). The body is normally oriented vertically so forward locomotion is in an upward direction. The animals are negatively buoyant, so they must swim to maintain their position in the water column.
During slow swimming, Clione typically maintain a steady vertical position, which is still considered forward locomotion due to their negative buoyancy. Mechanical stimulation of the tail results in a sudden acceleration from slow to fast swimming. This change includes an increase in wing-beat frequency as well as a significant increase in the force of wing movements (Arshavsky et al. 1985b,c; Satterlie, and Norekian 2001).
The roles of serotonergic neurons in the cerebral and pedal ganglia in triggering the change to fast swimming have been investigated electrophysiologically (Arshavsky et al. 1985a; Satterlie 1995; Satterlie and Norekian 1995; Satterlie et al. 1995, 2000). The “gear change” includes a re-wiring of the swim central pattern generator through changes in the cellular properties of component neurons and through recruitment of additional neurons into the circuit (Arshavsky et al. 1985d, 1989; Pirtle and Satterlie 2006). These changes result in the increase in pattern generator output to the wings, observed as an increase in wing-beat frequency (Arshavsky et al. 1985a,b,c,d; Satterlie and Norekian 1995, 2001). The associated increase in the strength of wing contractions is accomplished in two ways. First, additional motorneurons are recruited into activity (Satterlie 1993), and these neurons, in turn, recruit fast-twitch fatigable muscle cells in the wings to add to the force generated by the muscle cells active during slow swimming (slow-twitch fatigue-resistant fibers; Satterlie 1993). Second, bilaterally symmetrical clusters of serotonergic cells in the pedal ganglia are recruited and enhance contractility of the slow-twitch fibers through the modulatory action of serotonin (Satterlie 1995; Satterlie and Norekian 1995, 1997, 2001). These pedal cells (Pd-SW cells) are the subject of this investigation.
Mechanical stimulation of the body wall or a wing produces two distinct, and mutually exclusive, responses. If the stimulus is mild, both wings will continue to move within the slow swimming mode, but the wings show an increased force of contraction. If the stimulus is stronger, or repetitive, a wing-withdrawal reflex is triggered and swimming is inhibited (Huang and Satterlie 1990). The withdrawal can be partial or complete (pulling the wings totally into the body), and can be unilateral or bilateral.
The modest increase in forward movement following a mild stimulus occurs without a change in wing-beat frequency (Satterlie 1995), and therefore without a change to fast swimming. This change of swimming speed within the slow swimming mode is of interest since the accelerations exclusively involve an increase in the force of wing contractions without modification of the central pattern generator.
While we know a great deal about the change from slow to fast swimming, we know little about the neurophysiological basis of speed changes within the slow swimming “gear.” Since the Pe-SW neurons enhance contraction of the wing musculature without altering wing-beat frequency (Satterlie 1995), they are considered good candidates for producing changes in locomotory speed within the slow swimming mode. In our early electrophysiological work on these cells, we found that most, but not all of them, respond to wing stimulation below the threshold for wing withdrawal (Satterlie 1995). Within each cluster of Pd-SW neurons, most innervate the ipsilateral wing with a characteristic branching pattern. However, a subset of cells within the cluster send their axons out of the pedal ganglia in nerves other than the wing nerve. To better understand the organization of this neuronal group, we show that the overall number of cells in each cluster is variable, but two of the cells in each cluster innervate the body wall of the neck and tail. We also show the two subsets have slight differences in their baseline electrical activities such that we became successful in accurately identifying the morphological cell type from the resting electrophysiological activity alone, as verified with subsequent dye injections.
Materials and methods
Specimens of Clione limacina were collected from the breakwater of Friday Harbor Laboratories (University of Washington) and placed in 1 gallon jars of fresh seawater that were submerged in sea tables with flowing, natural seawater. Some animals were shipped to North Carolina and held in gallon jars of seawater in a refrigerator. Animals were anesthetized in a 2:1 mixture of seawater and isotonic MgCl2 and dissected in a petri dish coated with Sylgard (Dow Corning).In body-intact preparations, the body was opened dorsally and the digestive and reproductive organs were removed. The preparation was then pinned to minimize damage to the major nerves of the central ganglia while also immobilizing the pedal ganglia for electrophysiological recording. Reduced preparations consisted of the central ganglia (cerebral, pleural, and pedal pairs) and the attached wings. In all cases, tissue was pinned with cactus spines from the fruit of the prickly pear (Opuntia sp.)
For immunohistochemistry, body-intact and reduced preparations were fixed in 4% paraformaldehyde in phosphate-buffer saline (PBS) for 2–4 h followed by a 12-h wash period in PBS. Fixation occurred in the pinned configuration in the prep dishes. The tissue was then washed three times in PBS containing Tween 20. Following transfer of the tissue to small vials, the tissue was dehydrated in an ethanol series to 100% and then rehydrated by running backwards through the same series, to PBS/tween 20 (dehydration/rehydration). The tissue was then washed twice in PBS before a 24 h soak in 5% goat serum in PBS. This was followed by a 48-h incubation in primary antibody (serotonin; Chemicon, Inc.). After six washes in PBS (without tween 20), the tissues was incubated overnight in secondary antibody (goat anti-rabbit, conjugated to FITC or TRITC fluorescent labels; Sigma). After six washes in PBS, the tissue was cleared and mounted (whole mount) in a 9:1 mixture of glycerol and Tris buffer. Stained preparations were examined either by indirect fluorescence (Nikon Optiphot) or with a laser scanning confocal microscope (Sarastro 2000 argon laser confocal; Molecular Dynamics). The latter included computer-generated stacks of 3 μm optical sections. Cell counts of 5-HT immunoreactive cells and processes involved confocal examination of nine separate immunohistochemistry preparations, and included data from the 18 pedal ganglia (Table 1).
For electrophysiological recording and cell marking, recording microelectrodes were tip-filled with 5% carboxyfluorescein and further filled with 2 M potassium acetate (tip resistance between 15 and 30 MΩ). Once a stable penetration was achieved and electrical activity recorded (Axoscope software; Axon Instruments), dye was injected into the recorded neuron by passing negative pulses through the recording electrode (1nA, 0.5 s rectangular pulses at 1 Hz). The preparations were washed in anesthetizing solution (above) and mounted live on a microscope slide for examination with indirect fluorescence microscopy. Preparations were photographed with a digital camera (Spot, Diagnostic Instrument, Inc.).
Results
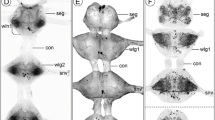
The central ring of ganglia includes pairs of cerebral, pleural, pedal, and intestinal ganglia. The pedal ganglia contain the central pattern generator circuitry for swimming movements, as well the motorneurons that activate the swim musculature of the wings. A schematic of the central ganglia with numbered nerves is shown in Fig. 1 (redrawn from Wagner (1885) and using his numbering system for nerves). Each pedal ganglion produces three major nerves of interest in this study. The largest is the wing nerve (nerve n4) which innervates the ipsilateral wing and contains all of the motor and modulatory neurons that innervate the wing musculature. Nerve n3 emerges laterally and innervates the neck and upper body wall. Nerve n8 runs posteriorly and innervates the body wall of the tail.
Schematic diagram of the central ganglia of Clione (redrawn from Wagner (1885), including his numbering of nerves). The buccal ganglia are not included. The nerves important for this study include n3, n4 (wing nerve), and n8. Ce, cerebral ganglion; Pl, pleural ganglion; Pe, pedal ganglion, I, intestinal ganglion
The pedal serotonin-immunoreactive neurons (Pd-SW neurons) form two tight clusters in the medial-anterior region of each pedal ganglion, near the emergence of the pedal commissure (Fig. 2). Cell bodies are between 20 and 80 μm in diameter, with most in the lower part of that range. Processes of the immunoreactive neurons either exited the pedal ganglion in the wing nerve (n4), in which case they branched throughout the entire wing, or exited from nerves n3 and n8. Nerve n3 split into two primary branches relatively close to its exit from the pedal ganglion (Fig. 1), while nerve n8 also split into two major branches that both ran posteriorly (not shown in Fig. 1 since the branching was detected in dye-injected neurons).
Confocal stacks of whole mount preparations using serotonin immunohistochemistry. The symmetrical clusters of pedal serotonergic neuron (Pd-SW neurons) are indicated by the arrows. a The pedal ganglion (Pe) on the right shows the three nerves containing processes of Pd-SW neurons, including nerves n3, N4, and n8. Ce, cerebral ganglion; Pl, pleural ganglion; I, Intestinal ganglion. b The heart excitor neuron (asterisk) is shown in addition to the Pd-SW clusters (arrows). The clusters are found near the emergence of the pedal commissure
The total number of neurons in each cluster was determined by examining confocal stacks of whole-mount preparations. Additional whole-mount preparations were examined with indirect immunofluorescence; however, accurate cell counts were difficult to obtain since the bright staining made it difficult to distinguish individual neuron somata. In the confocal-evaluated preparations, the number of immunoreactive neurons varied from preparation to preparation (Table 1; n = 18 pedal ganglia from 9 animals), and between right and left pedal ganglia in five of the nine preparations. The minimum and maximum numbers of neurons counted (per cluster) were 5 and 9 neurons, respectively, with an average of 6.67 neurons in the left pedal ganglia and 6.78 neurons in the right pedal ganglia. The difference between the two ganglia was not statistically significant (paired t test; p = 0.3405).
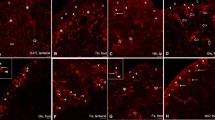
All but two of immunoreactive neurons innervated the ipsilateral wing via the wing nerve, verified by dye injections of individual and multiple neurons in single preparations (based on over 50 dye injections: Fig. 3). Once in the wing, the processes followed the characteristic branching pattern of the wing nerve such that branches of the cells extended throughout the entire wing (Fig. 3a, b). Individual neurons innervated either the dorsal or ventral musculature of the wing. This was best seen in carboxyfluorescein fills of cell pairs, where the processes of the two filled cells were either in exactly the same focal plane (both innervating the same side of the wing) or in distinctly different focal planes (innervating different sides of the wing; Fig. 3b, verified by changing the focal plane prior to photography). The main wing branches of the Pd-SW neurons ran together in the center of the wing hemocoel so it was not possible to distinguish the relative number of cells that innervated the dorsal and ventral musculature in either immunohistochemical or multiple-cell injection preparations. However, the density of terminals in all parts of the wing was similar suggesting the number of dorsal and ventral neurons was roughly equal.
Carboxyfluorescein fills of Pd-SW neurons. All filled neurons are “wing” neurons—they each innervate the ipsilateral wing, and show a similar branching pattern in the wings. Pl, pleural ganglion; Pe, pedal ganglion; S, statocyst; W, wing; n4, wing nerve. a The two neurons in the ganglion on the right are structurally similar. The processes in the wing of both cells are in the same focal plane. Also, the fine processes of both neurons (seen at higher magnification) were in the same focal plane as the dorsal swim musculature. b The wing processes of the two neurons were not in the same focal plane (verified at higher magnification). The cell indicated by the black arrows ran to the dorsal musculature while the cell indicated by the white arrows ran to the ventral musculature
In addition to the Pd-SW neurons that innervated the ipsilateral wing, a second morphological subtype was identified with carboxyfluorescein injections. These cells did not send processes into the wing nerve, but rather had a characteristic and consistent branching pattern. Processes of these cells exited the ganglion in ipsilateral nerves n3 and n8, including both major branches of each nerve (Fig. 4). Neurons from right and left pedal clusters exited the same ipsilateral nerves to produce a mirror-image branching pattern (compare Fig. 4a, b). The processes in n4 could be followed to the body wall of the neck and upper tail region, while those of n8 ran posteriorly into the tail. In preparations in which multiple neurons were filled with carboxyfluorescein no more than two neurons of this second subtype were found in any pedal ganglion (n = 23). In dual fills, in which both “body wall neurons” were filled, their primary processes followed the same major branching pattern and were in the same focal plane (Fig. 4b).
Double carboxyfluorescein fills of Pd-SW neurons. a Two neurons were injected; one “wing” neuron and one “body wall” neuron. The former sends a process to the wing in the wing nerve (n4; this process is out of focus). The “body wall” Pd-SW neuron shows the characteristic branching pattern, including processes in nerves n3 and n8. S, statocyst. b The two filled cells are both “body wall” Pd-SW neurons, and they both have the same, characteristic branching pattern. The branches of the two cells are slightly separated at the arrow, but otherwise run close together. The two panels together show that “body wall” Pd-SW neurons of the right and left ganglia have the same branching morphology
In the course of our electrophysiological investigations of the Pd-SW neurons (over 50 injected neurons), the spontaneous action potential activity, and the size and shape of the action potentials were similar in all recordings. The characteristics included resting potentials between −45 and −56 mV, large overshooting action potentials (up to 70 mV in amplitude; up to 10 ms in duration) with distinct after-hyperpolarizations, and irregular, low frequency action potential activity in unstimulated, slow swimming preparations (confirming Satterlie 1995). In addition, the lack of electrical coupling between Pd-SW neurons originally documented by Satterlie et al. (1985) was confirmed both within and between the two types of Pd-SW neurons. However, differences were noted in the background synaptic activity of the cells. Most cells had a continuous barrage of small synaptic potentials (2–5 mV: Fig. 5a). This background activity was absent in some of the cells (Fig. 5b). Both cell subtypes were verified as members of the Pd-SW cluster in double-label experiments (injection of carboxyfluorescein and serotonin immunohistochemical labeling: n = 12 from Satterlie et al. 1995). With subsequent dye injections of recorded neurons, those with the greater background activity were found to be “wing” neurons, and those with the lesser activity were found to be “body wall” neurons. We tested our predictive ability with the last 19 of these preparations by assigning the cell type based on background synaptic activity and prior to dye-injection and visualization of individual neurons. Our identification based on the electrophysiological activity was correct in 18 of the 19 preparations. The only error occurred in a preparation that was classified as a “body wall” neuron, but innervated the wing. On re-examination of the electrical record, the resting potential of the cell was −41 mV (outside of our previously acceptable range), and at higher gain, extremely small synaptic inputs (0.5–1 mV level) were noted.
Electrophysiological recording from two Pd-SW neurons. a All “body wall” Pd-SW neurons show very little background synaptic activity. b All “wing” Pd-SW neurons have constant barrages of small synaptic inputs. This difference allowed the accurate prediction of the type of Pd-SW neuron from the electrical recordings alone (always verified by dye injections)
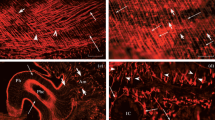
Serotonin immunolabeling of the body wall of the tail and neck revealed innervation of both longitudinal and circular body wall muscles (Fig. 6). In particular, longitudinal muscle bundles of the tail were innervated by multiple blebbed processes (Fig. 6a). Similarly, rich innervation was found in association with the circular muscles of the neck region (Fig. 6b). These processes could not be traced back to their ganglionic origins; however, the nerve n4 processes of the Pd-SW neurons appear to be the only source of peripheral nerves that run to the neck musculature, and their n8 processes represent two of three sources of serotonergic processes that extend into the tail. The third emerges from the intestinal ganglia in nerve n12.
Confocal stacks of serotonin immunohistochemical preparations of body wall musculature. a, b Longitudinal muscle bundles of the tail are innervated by blebbed processes largely running parallel to the muscle fibers. c The circular muscle of the neck region, which produces significant neck constriction during feeding behavior, is similarly innervated by a network of serotonin-immunoreactive processes, many of which run parallel to the muscle cells (arrows). Some of the nerve branches running to the tail are shown as brightly labeled processes
Discussion
The most noticeable change in muscular activity during locomotory acceleration is typically an increase in cycle frequency of locomotory appendages, this reflecting increases in the frequency of central pattern generator (CPG) output. In zebra fish, interneurons are divided into three distinct subcircuits that are recruited in an order that is related to the three muscle fiber types of the locomotory musculature (Ausborn et al. 2012; Ampatzis et al. 2014; Bjornfors et al. 2019). In contrast, the CPG of Clione operates as a two-phase acceleration system with a simple change from slow to fast swimming. This is accomplished through CPG reorganization through addition of interneurons that contribute to the shortening of the cycle period, an increase cycle frequency (Arshavsky et al. 1989; Pirtle and Satterlie 2006), and includes recruitment of large motorneurons which, in turn, activate fast twitch musculature (Arshavsky et al. 1985b,c; Satterlie 1993; Satterlie and Norekian 2001).
The Clione system includes an additional peripheral component to the acceleratory system—serotonergic modulation of the slow-twitch musculature produced by the clusters of pedal serotonergic neurons (PD-SW neurons) investigated here (Satterlie 1995; Satterlie et al. 1995). The Pd-SW neurons are recruited during the change from slow to fast swimming to contribute to the increase in muscle force during acceleration (Satterlie 1993). The Pd-SW neurons are similar in cell body position and innervation targets to the parapodial opener-phase neurons (POP neurons) of Aplysia brasiliana, which similarly swims with parapodial flapping movements (Parsons and Pinsker 1988, 1989; McPherson and Blankenship 1991, 1992). Like Pd-SW neurons, the POP neurons are serotonin-immunoreactive, and modulate contractile strength of parapodial muscle (McPherson and Blankenship 1992).
Peripheral modulation of skeletal muscle has been demonstrated in vertebrates, particularly through adrenergic input (Arreola et al. 1987; Emrick et al. 2010; Cairns and Borrani 2015). In addition, nitric oxide has been shown to modulate skeletal muscle activity (Kobzik et al. 1994; Dzoljic et al. 1997; Stamler and Meissner 2001). Most studies of skeletal muscle activity have focused on metabolic effects rather than on biomechanical activities, leaving the latter area less well understood.
The bilateral clusters of pedal serotonergic neurons (Pd-SW neurons) have been shown to enhance contractility of the swim musculature of Clione limacina without altering the frequency of swimming movements (Satterlie 1995). This purely peripheral modulation is hypothesized to be one method by which the animal could increase swim speed within the slow swimming mode (Satterlie and Plyler, in preparation). However, as noted here, not all of the cells in the clusters send processes to the wings via nerve n4 (wing nerve). Two of the cells in each cluster have processes that exit the pedal ganglion in nerves n3 and n8 which run to the body wall of the neck and tail, respectively, and the processes follow a consistent branching pattern in the body wall on the two sides of the animal. The target of these neurons, within the body wall, could not be determined with dye injections, but may be the musculature that helps control the fluid pressure in the hemocoelic compartments of Clione, which functions as a hydrostatic skeleton for the body and wings (Szymik and Satterlie 2017). Increases in fluid pressure in the body cavities can increase wing stiffness, which in turn, can alter the biomechanical activity of the wings, presumably contributing to a change in locomotory speed (Szymik and Satterlie 2011). Certainly, an increase in wing contractility brought on by an increase in activity of the serotonin neurons that innervate the wings could alter the force of wing contractions and thus increase locomotory speed.
The number of cells in the pedal clusters is variable from preparation to preparation and often between right and left ganglia. The average number is nearly seven neurons, with a maximum of two innervating the body wall, which suggests the majority of the neurons innervate the ipsilateral wing. The innervation pattern of the wing muscles is similar in all of the wing cells in that they extend throughout the entire wing, either on the dorsal or the ventral side. Similarly, the primary axonal branching pattern in the body wall neurons is identical for the two ipsilateral neurons and symmetrical on the two sides of the body. This raises questions about the redundancy of having multiple neurons of such similar morphology. At the level of the finest branches, the neurons may differ so that coverage of the innervation field is enhanced through innervation by multiple neurons. Also, the release of serotonin may be paracrine in nature so multiple neurons could increase the effectiveness of this modulation.
Satterlie et al. (1995) found that serotonergic terminals were exclusively associated with the slow-twitch musculature in the wings. These muscle fibers are active during slow swimming in the un-modulated state. The change to fast swimming involves recruitment of additional motorneurons and fast-twitch muscle fibers, enhanced activity in the slow-twitch muscle fibers, but also activation of the pedal serotonergic neurons (and thus up-modulation of the slow twitch fibers; Satterlie and Norekian 1995, 1997, 2001). The full significance of innervating only the slow-twitch muscle was initially unclear. However, if serotonin modulation is considered a possible mechanism for swim acceleration within the slow swimming speed, the specific targeting of slow-twitch musculature is necessary. This lends support to the suggestion that activation of the pedal serotonergic neurons represents one mechanism for such accelerations. Such an involvement is supported by the activation of a subset of pedal neurons by wing stimulation below the threshold for activation of the wing withdrawal reflex (Satterlie and Plyler, in preparation). This suggests that the pedal serotonergic cells have a dual role in swim acceleration; enhancement of contractility of the slow-twitch musculature to augment activation of the fast-twitch fibers during the change to fast swimming (Satterlie 1995), and activation by themselves, as a potential mechanism for swim acceleration within the slow swimming mode.
The full function of the “body wall” neurons will have to await identification of the target tissues and further physiological characterization. Their relative insensitivity to wing stimulation (Satterlie and Plyler, in preparation) suggests they may not produce changes in body wall activity during acceleration within the slow swimming gear. However, the similarity of firing activity in the two subsets of pedal serotonergic neurons following other forms of stimulation, including those that trigger fast swimming, suggest the body wall musculature may play a role in the change to fast swimming, likely in increasing hemocoelic pressure in the body and wings, as suggested by the increased wing stiffness during the change to fast swimming (Szymik and Satterlie 2011).
The organization of serotonergic systems in a number closely related opisthobranch molluscs appears to be highly conserved, even though the activity of the systems is variable and species-specific (Katz et al. 2001; Newcomb and Katz 2009). In particular, clusters of cerebral serotonergic cells share similar ganglionic positions in several species of swimming opisthobranchs, yet their influence is intrinsic to the swim CPG in Tritonia and extrinsic to the CPG in Melibe and Clione (Katz et al. 2001; Newcomb and Katz 2009). In Clione, these neurons can initiate swimming in non-swimming animals, but also trigger the change from slow to fast swimming, including recruitment of the Pd-SW neurons (Satterlie and Norekian 1995; Satterlie et al. 1995). Serotonin also modulates locomotion in zebra fish by increasing mid-cycle inhibition of the central pattern and decreasing the rising phase of excitation. The overall effect is to decrease burst frequency, an action opposite that in the molluscs (Gabriel et al. 2009).
References
Ampatzis K, Song J, Ausborn J, El Manira A (2014) Separate microcircuit modules of distinct V2a interneurons and motoneurons control the speed of locomotion. Neuron 83:934–943
Arreola J, Calvo J, Garcia MC, Sanchez JA (1987) Modulation of calcium channels of twitch skeletal muscle fibers of the frog by adrenaline and cyclic adenosine monophosphate. J Physiol 393:307–330
Arshavsky YI, Beloozerova IN, Orlovsky GN, Panchin YV, Pavlova GA (1985a) Control of locomotion in marine molluskClione limacina. I. Efferent activity during actual and fictitious swimming. Expl Brain Res 58:255–262
Arshavsky YI, Beloozerova IN, Orlovsky GN, Panchin YV, Pavlova GA (1985b) Control of locomotion in marine molluskClione limacina. II. Rhythmic neurons of pedal ganglia. Expl Brain Res 58:263–272
Arshavsky YI, Beloozerova IN, Orlovsky GN, Panchin YV, Pavlova GA (1985c) Control of locomotion in marine molluskClione limacina. III. On the origin of locomotory rhythm. Expl Brain Res 58:273–284
Arshavsky YI, Beloozerova IN, Orlovsky GN, Panchin YV, Pavlova GA (1985d) Control of locomotion in marine molluskClione limacina. IV. Role of type 12 interneurons. Expl Brain Res 58:285–293
Arshavsky YI, Orlovsky GN, Panchin YV, Pavlova GA (1989) Control of locomotion in marine molluskClione limacina. VII. Reexamination of type 12 interneurons. Expl Brain Res 78:398–406
Ausborn J, Mahmood R, El Manira A (2012) Decoding the rules of recruitment of excitatory interneurons in the adult zebrafish locomotor network. Proc Natl Acad Sci USA 109:E3631–E3639
Bjornfors ER, Picton LD, Song J, El Manira A (2019) Diversity of neurons and circuits controlling the speed of coordination of locomotion. Curr Opin Physiol 8:170–176
Cairns SP, Borrani F (2015) β-Adrenergic modulation of skeletal muscle contraction: key role of excitation-contraction coupling. J Physiol 593:4713–4727
Dougherty KJ, Kiehn O (2010) Firing and cellular properties of V2a interneurons in the rodent spinal cord. J Neurosci 30:24–37
Dzoljic E, DeVries R, Dzoljic MR (1997) New and potent inhibitors of nitric oxide synthase reduce motor activity in mice. Behav Brain Res 87:209–212
Eklof-Ljunggren E, Haupt S, Ausborn J, Dehnisch I, Uhlen P, Higashijima S, El Manira A (2012) Origin of excitation underlying locomotion in the spinal circuit of zebrafish. Proc Natl Acad Sci USA 109:5511–5516
Emrick MA, Sadilek M, Konoki K, Catterall WA (2010) Beta-adrenergic-regulated phosphorylation of skeletal muscle Ca(V)1.1 channel in the fight-or-flight response. Proc Natl Acad Sci USA 43:18712–18717
Gabriel JP, Mahmood R, Kyriakatos A, Soll I, Hauptmann G, Calabrese RL, El Manira A (2009) Serotonergic modulation of locomotion in zebrafish: endogenous release and synaptic mechanisms. J Neurosci 29:10387–10395
Gabriel JP, Ausborn J, Ampatzis K, Mahmood R, Eklof-Ljunggren E, El Manira A (2011) Principles governing recruitment of motoneurons during swimming in zebrafish. Nat Neurosci 14:93–99
Huang Z, Satterlie RA (1990) Neuronal mechanisms underlying behavioral switching in a pteropod mollusk. J Comput Physiol A 166:875–887
Katz PS, Fickbohm DJ, Lynn-Bullock CP (2001) Evidence that the central pattern generator for swimming in Tritonia arose from a non-rhythmic neuromodulatory arousal system: implications for the evolution of specialized behavior. Am Zool 41:962–975
Kobzik L, Reid MB, Bredt DS, Stamler JS (1994) Nitric oxide in skeletal muscle. Nature 372:546–548
McLean DL, Masino MA, Koh IY, Lindquist WB, Fetcho JR (2008) Continuous shifts in the active set of spinal interneurons during changes in locomotor speed. Nat Neurosci 11:1419–1429
McLean DL, Fetcho J (2009) Spinal interneurons differentiate sequentially from those driving the fastest swimming movements in larval zebrafish to those driving the slowest ones. J Neurosci 29:13566–13577
McPherson DR, Blankenship JE (1991) Neural control of swimming in Aplysia brasiliana. III. Serotonergic modulatory neurons. J Neurophysiol 66:1366–1379
McPherson DR, Blankenship JE (1992) Neuronal modulation of foot and body-wall contractions in Aplysia californica. J Neurophysiol 67:23–28
Newcomb JM, Katz PS (2009) Different functions for homologous serotonergic interneurons and serotonin in species-specific rhythmic behaviours. Proc R Soc London B 276:99–108
Parsons DW, Pinsker HM (1988) Swimming in Aplysia brasiliana: identification of parapodial opener-phase and closer-phase neurons. J Neurophysiol 59:717–739
Parsons DW, Pinsker HM (1989) Swimming in Aplysia brasiliana: behavioral and cellular effects of serotonin. J Neurophysiol 62:1163–1176
Pirtle TJ, Satterlie RA (2006) The contribution of the pleural type 12 interneuron to swim acceleration in Clione limacina. Invert Neurosci 6:161–168
Satterlie RA (1993) Neuromuscular organization in the swimming system of the pteropod mollusc Clione limacina. J Exp Biol 181:119–140
Satterlie RA (1995) Serotonergic modulation of swimming speed in the pteropod mollusc Clione limacina. II. Peripheral modulatory neurons. J Exp Biol 19:905–916
Satterlie RA, Norekian TP (1995) Serotonergic modulation of swimming speed in the pteropod mollusc Clione limacina. III. Cerebral neurons. J Exp Biol 198:917–930
Satterlie RA, Norekian TP (1997) Modulation of swimming speed in the pteropod mollusc, Clione limacina: role of a compartmental serotonergic system. Invert Neurosci 2:157–165
Satterlie RA, Norekian TP (2001) Mechanisms of locomotory speed change: the pteropod solution. Am Zool 41:1001–1008
Satterlie RA, Spencer AN (1985) Swimming in the pteropod mollusc, Clione limacina. II. Physiology. J Exp Biol 116:205–222
Satterlie RA, LaBarbera M, Spencer AN (1985) Swimming in the pteropod mollusc, Clione limacina. I. Behaviour and Morphology. J Exp Biol 116:189–204
Satterlie RA, Norekian TP, Jordan S, Kazilek CJ (1995) Serotonergic modulation of swimming speed in the pteropod mollusc Clione limacina. I. Serotonin immunoreactivity in the central nervous system and wings. J Exp Biol 198:895–904
Satterlie RA, Norekian TP, Pirtle TJ (2000) Serotonin-induced spike narrowing in a locomotor pattern generator permits increases in cycle frequency during accelerations. J Neurophysiol 83:2163–2170
Song J, Pallucchi I, Ausborn J, Ampatzis K, Bertuzzi M, Fontanel P, Picton LD, El Manira A (2020) Multiple rhythm-generating circuits act in tandem with pacemaker properties to control the start and speed of locomotion. Neuron 105:1048–1061
Stamler JS, Meissner G (2001) Physiology of nitric oxide in skeletal muscle. Physiol Rev 81:209–237
Szymik BG, Satterlie RA (2011) Changes in the wingstroke kinematics associated with a change in swimming in a pteropod mollusk, Clione limacina. J Exp Biol 214:3935–3947
Szymik BG, Satterlie RA (2017) Circulation of hemocoelic fluid during slow and fast swimming in the pteropod mollusc Clione limacina. Invert Biol 136:290–300
Wagner N (1885) Die Wirbellosen des Weissen Meeres: Zoologische Forschungen an der Kuste des Solowetzkischen Meerbusens in den Sommermonaten der Jahre 1877, 1878, 1879 und 1882. Verlag von Wilhelm Engelmann, Leipzig
Zhong G, Droho S, Crone SA, Dietz S, Kwan AC, Webb WW, Sharma K, Harris-Warrick RM (2010) Electrophysiological characterization of V2a interneurons and their locomotor-related activity in the neonatal mouse spinal cord. J Neurosci 30:170–182
Zhong G, Sharma K, Harris-Warwick RM (2011) Frequency-dependent recruitment of V2a interneurons during fictive locomotion in mouse spinal cord. Nat Commun 2:274
Acknowledgements
We thank the staff and Director of Friday Harbor Laboratories, University of Washington, for providing laboratory space and support for this research, and the Honors College of the University of North Carolina Wilmington for support for J.B.P. Primary support for this project came from NSF grant IBN-9904424.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Plyler, J.B., Satterlie, R.A. Pedal serotonergic neuron clusters of the pteropod mollusc, Clione limacina, contain two morphological subtypes with different innervation targets. Invert Neurosci 20, 21 (2020). https://doi.org/10.1007/s10158-020-00256-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10158-020-00256-0