Abstract
The crayfish stretch receptor consisting of the single mechanoreceptor neurons enveloped by satellite glial cells is the simplest functioning neuroglial preparation. However, during isolation, its axons are usually transected that eliminates afferent regulation and induces complex axotomy-related signaling responses in neurons and satellite glia. We developed new microsurgical method of crayfish stretch receptor isolation, which preserves connections of sensory neurons to the ventral nerve cord ganglion. The stretch receptor may either remain on the abdominal carapace, or be completely isolated. In both cases, it may be either intact, or axotomized. The integrity of axons was confirmed by firing recording from proximal and distal axon points. Normal, necrotic and apoptotic cells were visualized using double fluorochroming with Hoechst 33342 and propidium iodide. The isolated mechanoreceptor neurons maintain regular firing during 8–10 or more hours. Glial cells surrounding non-axotomized neurons demonstrate lower necrosis and apoptosis levels than the axotomized ones. Unlike the existing method, in which the sensory neurons were axotomized, the present method preserves links between the sensory neurons and the ganglion and makes possible to avoid consequences of axotomy in neurons and satellite glia. The present neuroglial preparation may be used as a simple but informative model object in studies of axotomy-induced degeneration and survival of peripheral neurons, the role of glia in neuron injury, the signaling mechanisms of neuroglial interactions, and the effects of diverse physical and chemical factors on neuronal and glial cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic brain injury is among main factors of young and middle-age human mortality (Pearson et al. 2012; McIntyre et al. 2013). Every neurotrauma is accompanied with axonal transection, axotomy that leads either to neuron death, or to regeneration and restoration of nerve connections. Therefore, a comprehensive mechanistic study of neurodegeneration and neuroprotection after neurotrauma is necessary (Richardson et al. 2009; Rodríguez-Muela and Boya 2012; Conforti et al. 2014; Rishal and Fainzilber 2014). Such study is better to carry out on simple invertebrate model systems, where neurons are easily identified and their functional activity is controlled.
The isolated crayfish stretch receptor (CSR) discovered by Alexandrowicz (1951) is very suitable for such study. It is the classical neurophysiological preparation consisting of a single mechanoreceptor neuron enveloped by satellite glial cells. CSR consists of two mechanoreceptor neurons: slowly and rapidly adapting ones (SN and RN, respectively). Their dendrites penetrate into the correspondent receptor muscles (SM and RM, respectively) attached to the chitin carapace between neighboring abdominal segments and are in contact with muscle fibers (Tao-Cheng et al. 1981). The stretching of SM and RM elicits transient impulse responses of SN and RN that are transmitted to the abdominal ganglion. The ganglion neurons generate commands for the abdominal muscles that control movements of the crayfish tail. SN informs the ganglion neurons on the relative position of abdominal segments. Its firing frequency is proportional to elongation of SM. RN informs the ganglion neurons on the rate of abdomen movement. In response to segment displacement, it generates a transient firing burst, in which the frequency and number of spikes correlate with the rate of RM extension (Eyzaguirre and Kuffler 1955; Kuffler and Eyzaguirre 1955; Purali 2005; Rydqvist et al. 2007).
The studies of neuronal activity and its inhibition (Eyzaguirre and Kuffler 1955; Kuffler and Eyzaguirre 1955; Purali 2005), metabolism (Giacobini 1969), mechanosensory transduction (Rydqvist et al. 2007), neuroglial interactions (Fedorenko and Uzdensky 2009), responses of neurons and satellite glial cells to different physical and chemical factors (Giacobini 1969; Uzdensky and Kutko 1997; Lin and Rydqvist 1999; Uzdensky 1993, 2011), and signal transduction processes in neurons and glia (Lobanov and Uzdensky 2009; Komandirov et al. 2011; Uzdensky et al. 2007; Uzdensky 2011) were performed on the CSRs isolated according to the technique suggested by Florey and Florey (1955). In this method, axons of mechanoreceptor neurons are transected before CSR isolation. The axotomized neurons survive and maintain regular activity during 6–10 h after isolation. However, the isolation of CSR leads to delayed death, necrosis or apoptosis, of sensory neurons and satellite glial cells. These processes are mediated by complex signaling processes. It would be reasonable to study neuronal functions in the undamaged neuroglial preparation. Furthermore, the mechanoreceptor is innervated by neurons from the same ventral nerve cord (VNC) ganglion (G). Axotomy eliminates this regulation. Therefore, these experiments lack the sufficient control samples, in which the stretch receptor neurons preserve contacts with the ganglion neurons.
In the present work, we developed a novel technique of CSR isolation, in which the sensory neurons are isolated together with the appropriate ventral cord ganglion (SN-G complex). The neurons in this preparation maintain regular firing and demonstrate higher resistance to necrosis and apoptosis after isolation as compared with axotomized neurons. This neuroglial preparation may be used as a simple but informative model object in studies of axotomy-induced neuronal degeneration and neuroprotection, the role of glia in neuron injury, the signaling mechanisms of neuroglial interactions that control each other’s survival, and effects of diverse physical and chemical factors on neuronal and glial cells.
Materials and methods
The abdominal crayfish stretch receptor
The crayfishes Astacus leptodactylus from the Don River and its affluents were obtained from the local market. In each abdominal segment, the stretch receptor neurons send axons to the appropriate VNC ganglion as a part of the second (in the caudal direction) n.dorsalis (Fig. 1). The latter also comprises afferent axons that innervate these neurons and abdominal muscles (Florey and Florey 1955; Ilyinsky 1975). The detailed description of the crayfish nerve system anatomy is given by Fomichev (1986).
The crayfish abdominal stretch receptors. Each abdominal segment contains a pair of symmetric stretch receptors consisting of two sensory neurons mounted on the receptor muscles. Their axons are sent to the appropriate ganglia of the ventral nerve cord. Left micrograph of slowly adapting neuron; its dendrites ramify between the fibers of the receptor muscle. Right the ventral nerve cord ganglion (A1). It contains a pair of n.dorsalis (arrowheads). A2 stretch receptors, A3 the sites of attachment of receptor muscle to the carapace. B1 slowly adapting receptor muscle, B2 slowly adapting neuron, B3 axon, B4 satellite glial cells
Isolation of the SN-G complex
Removal of the abdominal deep muscles is carried out in the plexiglass chamber (3 cm × 3 cm × 5 cm) filled with a 1:1 paraffin/wax mixture (P/W) so that its bottom has a 45° slope (chamber 1). Further isolation of the SN-G complex is performed in the Petri dish filled with 7–10 mm P/W layer (chamber 2). The dissection is performed under the stereomicroscope control (16–20× magnification) using glass needles, pointed ophthalmic scissors, and forceps. All manipulations are performed in van Harreveld’s saline (mM: NaCl—205; KCl—5.4; NaHCO3—0.2; CaCl2—13.5; MgCl2—5.4; pH 7.2–7.4).
The crayfish is killed by transection of the anterior thorax behind eyes at the level of the subesophageal ganglion. The abdomen is transected through the line a–a, and the telson, through the line b–b (Fig. 2a). The lateral protrusions of tergites and pleopods are cut off. In order to gain access to the VNC, the ventral carapace is cut along the line (d–d) located 2–3 mm laterally from the abdominal midline c–c, under which VNC is situated (Fig. 2b). The dorsal carapace is cut along the midline e–e (Fig. 2c), and deep abdominal muscles are cut along the plane d–e–e–d (Fig. 2d). One of two abdominal halves (left on Fig. 2d) contains 5 stretch receptors connected to the corresponded VNC ganglia. It is then fixed by pins to the inclined bottom of the chamber 1 filled with van Harreveld’s saline (Fig. 3a).
Isolation of the crayfish abdomen. a Transection of the abdomen along the line a–a, and telson, along b–b. b The ventral carapace is transected along the line d–d located at 2–3 mm from the midline c–c. c Transection of the dorsal carapace along the midline e–e. d Transection of the abdominal deep muscles through the plane d–e–e–d. The resulting abdominal halves are unequal. One of them (left) contains the undamaged stretch receptor neurons linked to the VNC ganglia
Removal of deep muscles in the chamber 1 with an inclined bottom. Right the scheme of the preparation. a Fixation of the abdomen half. b Amputation of o.l.abd. and p.m.o. (m1 and m2, respectively) at the ventral carapace side along the dashed line. c Amputation of tr.abd (m3) along the dashed line at the ventral side of the carapace. c VNC connective; circles VNC ganglia. 1 deep muscles, 2 carapace; 3 d.o.abd. and d.r.abd
Using ophthalmic scissors, we first transect the deep abdominal muscles: m. obliques lateralis abdominis (o.l.abd), m. posterior medialis obliquus (p.m.o.), and m. transverses abdominis (tr.abd.) from the ventral side of the carapace (Fig. 3b, c: m1, m2, and m3, respectively). Then, tr.abd., o.l.abd. m. obliquus mediolateralis abdominis (o.ml.abd.), m. anterior bliquus medialis, (a.o.m) and p.m.o. (Fig. 4: m4, m5, m6, and m7, respectively) are cut off from the dorsal side of the carapace. This provides the access to n.dorsalis (Fig. 4b, arrows).
Further removal of deep muscles. a Transection of tr.abd., o.l.abd. o.m.abd, a.o.m., and p.m.o. (denoted as m3, m4, m5, m6, and m7, respectively). These are not distinguished in the preparation and transected altogether in the places of their attachment to the ventral carapace. White dashed lines show the transection path on the visible side, black dashed lines the transection path hidden by deep muscles. b The scheme of transection of deep muscles tr.abd., o.l.abd. o.m.abd, a.o.m., and p.m.o. Arrows n. dorsalis. Muscles d.o.abd. и d.r.abd are denoted as m8 and m9, respectively. st1 the stump remaining after transection of o.l.abd., p.m.o., and tr. abd. c The preparation in the chamber 1 after removal of deep muscles. The VNC becomes visible (dashed oval), circles ganglia
After that, the preparation is transferred into the Petri dish (chamber 2) and fixed with pins to the P/W bottom. The sternites are transected (Fig. 5a), and fragments of the ventral carapace are unbent and fixed by additional pins (Fig. 5b, c). The following procedures are made under the stereomicroscope control. At the medial preparation, side m. dorsales obliquus abdominis (d.o.abd.) and m. dorsales rectus abdominis (d.r.abd.) are cut out (Fig. 5c, d: m8 and m9, respectively).
The unbending of carapace and removal of remaining deep muscles. a The bases of sternites are transected along the dashed lines. b The scheme of carapace unbending. c The straightened carapace is fixed by pins. Dashed oval shows VNC. Dashed lines indicate the transection of d.o.abd. and d.r.abd (m8 and m9, respectively). d The scheme of d.o.abd. and d.r.abd transection. Circles ganglia
After removal of thick deep muscles, the thin dorsal muscles (m. dorsales superficialis abdominis, m.d.s.abd., or m10 at Fig. 6a, b), which are expanded between two neighboring carapace segments, are exposed (Fig. 6a). This muscle layer consists of two parts: lower and upper ones (Fig. 6b). The upper layer is shorter, because it starts from the stump of deep abdominal muscles that were cut off beforehand from the carapace (St2). The stretch receptor muscles and sensory neurons lie just under m.d.s.abd (Fig. 6a, b).
The final steps of SN-G isolation: the liberation of n.dorsalis and extracellular recording of neuronal activity. a The micrograph of the thin dorsal muscles (m. dorsales superficialis abdominis, m.d.s.abd., or m10). b The scheme of m.d.s.abd. amputation and liberation of n.dorsalis. The connective tissue ligaments between the mechanoreceptor muscles and m.d.s.abd. are broken by glass needles (B1). The lower layer of m.d.s.abd is transected along the dashed line and removed. Then, the connective tissue ligaments between the mechanoreceptor axon and the upper layer of m.d.s.abd are broken by glass needles and this m.d.s.abd layer is transected along the dashed line and removed (B2). The stump st1 is carefully broken by glass needles, and n.dorsalis is released (B3). c, d The scheme and micrograph showing the extracellular recording of neuronal activity with glass pipette electrodes (E) that suck to n.dorsalis near the ganglion SN axon and/or near the neuron soma (S). Dashed lines on a, b show the transection of m.d.s.abd. Dot-dashed line on a indicates the position of the stretch receptor under m.d.s.abd. Arrows on a, d indicate n.dorsalis
In order to liberate the receptor muscles SM and RM, which lie together under the lower layer of m.d.s.abd., one should insert two glass needles between the medial edge of m.d.s.abd. and receptor muscles and carefully break thin connective tissue bundles that link the receptor neuron and dorsal muscles. After liberation, the thin receptor muscles and the connective tissue triangle, which contains the bodies and proximal axon parts of SN and RN (Fig. 6b), become visible. The lower layer of m.d.s.abd. is carefully transected up to the st2 (on the left at the Fig. 6a, b) so as not to damage the underlying receptor muscles. This muscle layer is bent by a forceps to the opposite direction (to the right at the Fig. 6a, b) in order to expose the bound axon bundle. The connective tissue bundles that link the axons and m.d.s.abd. are carefully broken by glass needles, and this muscle bundle is cut off. The upper layer of m.d.s.abd. is removed similarly, but the muscle layer is bent to the opposite direction. Afterward, the stump st1 is carefully broken by two glass needles so as not to damage n.dorsalis and release it completely (Fig. 6b). This provides recording of bioelectric activity of SN and RN at any point of n.dorsalis (Fig. 6c, d).
The following preparation variants (Fig. 7) may be used in further experiments:
The preparation variants: I Partial liberation of n.dorsalis without amputation of dorsal muscles; neuronal activity may be recorded near the ganglion (point 1) and between muscle bundles (point 2). II After amputation of dorsal muscles and liberation of n.dorsalis, neuronal activity may be recorded throughout the axons including the proximal point 3. III After liberation and transection of n.dosalis (axotomy), the neuronal activity may be recorded at the truncated axon. In variants, I–III CSR remains on the carapace. IV Full isolation of the stretch receptor from the carapace; only small carapace pieces, to which the SM and RM are attached, remain. The neurons are not axotomized. V Complete isolation of the stretch receptor from the carapace; only small carapace pieces, to which the SM and RM are attached, remain. The neurons are axotomized
-
I—Partial liberation of n.dorsalis without amputation of dorsal muscles; the neuronal activity may be recorded near the ganglion (point 1) and between the muscle bundles (point 2).
-
II—Removal of dorsal muscles and liberation of n.dosalis; the neuronal activity may be recorded throughout the axons including the proximal point 3.
-
III—Liberation and transection of n.dosalis (axotomy). The neuronal activity may be recorded at the truncated axon.
-
In variants I–III CSR remains on the carapace.
-
IV—Complete isolation of the stretch receptor from the carapace; only small carapace pieces, to which the SM and RM are attached, remain. The neurons are not axotomized.
-
V—Complete isolation of the stretch receptor from the carapace; only small carapace pieces, to which the SM and RM are attached, remain. The neurons are axotomized.
Recording of neuronal activity
Neuronal activity was recorded extracellularly from axons by glass suction micropipettes filled with van Harreveld’s saline. Spikes were amplified, digitized by the analog-to-digital converter L-761 (L-Card, Moscow, Russia), and processed by a personal computer using the homemade software that provided continuous monitoring of firing. The experiments were carried out at 22 ± 4 °C.
Cell death assay
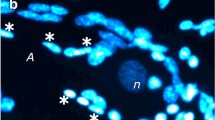
In order to visualize dead neurons and glial cells, 20 µM propidium iodide and 10–20 µM Hoechst 33342 are added into the experimental chamber (Uzdensky 2011). Then, preparations are washed with van Harreveld’s saline, fixed with 0.2 % glutaraldehyde, repeatedly washed and mounted in glycerol. Fluorescent images are studied using the fluorescence microscope Axiolab (K. Zeiss, FRG) equipped with a digital photocamera. Propidium iodide, a membrane impermeable fluorochrome, imparts red fluorescence to the nuclei of necrotic cells with the compromised plasma membrane. Hoechst 33342 imparts blue fluorescence to the nuclear chromatin. It visualizes intact nuclei of living cells and fragmented nuclei of apoptotic cells (Fig. 1). Nucleus fragmentation is the final stage of apoptosis when the no-return point has passed. The fraction of red nuclei of necrotic glial cells stained by propidium iodide is counted in the predetermined standard field (the proximal 2 mm axon region). The number of fragmented nuclei of glial cells per 2 mm of proximal axon region indicates the level of apoptosis. The standard statistical evaluation based on the Student’s t test was performed. The number of experiments on the cell death evaluation for different time intervals was 5–8. Data are presented as mean ± SD.
Results
Neuronal activity
Recording of SN activity near the VNC ganglion at point 1 (experimental variants I, II, and IV) confirms the axon integrity and conductivity along its lengths. The axon integrity is confirmed also by synchronous SN firing activation in response to a brief stimulation (extension) of SM. Recorded with two electrodes at points 1 and 3 (Fig. 8).
Morphology and cell death in the SN-G complex
The panoramic fluorescent image of the isolated SN-G complex composed from 17 fields of view (Fig. 9) demonstrates the preparation integrity from the neuron body (on the left) to the VNC ganglion (on the right). In this image, the nuclei of neuronal, glial, muscle, and connective tissue cells fluorochromed with Hoechst-33342 are blue. A few necrotic nuclei fluoresce in red. Just after isolation, fragmented nuclei of apoptotic cells (Fig. 9b) are rare. The most of cells in this preparation are alive and functioning.
It was of interest to compare the time course of cell death in axotomized (variant III on Fig. 7) and intact CSR preparations (variant II) incubated in 4 ml of van Harreveld’s saline during different time intervals after isolation. Since the number of glial cells in this preparation is much higher than the number of neurons, we compared death of glial cells surrounding axons. In both experimental variants, necrosis of glial cells occurred at 15 h after isolation (Fig. 10a). In the axotomized preparation, it was significantly higher than in the intact one. Apoptosis of glial cells that was estimated as a relative number of fragmented nuclei (Fig. 9b) appeared earlier, 4–8 h after isolation (Fig. 10b). Interesting, in the intact, non-axotomized preparation, the level of apoptosis did not change during following 8-h incubation. In contrast, the level of glial apoptosis in the axotomized preparation gradually increased from 4 to 15 h after isolation (Fig. 10b). It became significantly higher than in control samples at 12–15 h (Fig. 10b). Therefore, axotomy induced both necrosis and apoptosis of glial cells, which envelop axons of the CSR neurons. Apoptosis and necrosis of satellite glial cells in axotomized CSR neurons develop later and differently than in intact preparations.
The development of necrosis (a) and apoptosis (b) of glial cells surrounding the 2 mm proximal region of the stretch receptor axon. Intact non-axotomized neurons (variant II), AT axotomized neurons (variant III on Fig. 7). Significant difference between axotomized and non-axotomized neurons: *p < 0.05; **p < 0.01; ***p < 0.001 (Student’s t test; n = 5–8 for different time intervals)
Discussion
Traditional technique of isolation of the CSR requires axon transection of the sensory neurons (Florey and Florey 1955; Kuffler and Eyzaguirre 1955; Leksrisawat et al. 2010). Nevertheless, this neurotrauma is healed, and the isolated SN can regularly fire during following 6–10 h with almost stable frequency. This provides sufficient time to study its neuronal activity (Eyzaguirre and Kuffler 1955; Kuffler and Eyzaguirre 1955; Purali 2005; Rydqvist et al. 2007) and effects of various physical and chemical factors (Giacobini 1969; Uzdensky and Kutko 1997; Lin and Rydqvist 1999; Uzdensky 1993, 2011). However, axotomy by itself induces complex neuronal and glial responses mediated by numerous intracellular signaling cascades (Richardson et al. 2009; Rodríguez-Muela and Boya 2012; Conforti et al. 2014; Rishal and Fainzilber 2014). In order to avoid additional axotomy-induced effects, the axon transection should be excluded, and the experiments be performed on intact, non-axotomized preparations. On the other hand, the isolated CSR provides the opportunity to study molecular mechanisms of axotomy-induced processes in neurons and surrounding glial cells, and related neuroglial interactions. However, the data obtained on the axotomized preparations should be compared with the data obtained on the non-axotomized control preparations.
The present technique of isolation of the CSR, which preserves connections of the sensory neurons with the VNC ganglia, provides such control. In this preparation, one can register neuronal activity at any points of the axon. Simultaneous recording of SN firing at the proximal and distal parts of its axon has shown that the SN axon does not lose its integrity and firing capability. Application of inhibitors or activators of various signaling proteins and immuno-fluorescent visualization of various signaling proteins provide information on molecular mechanisms of the axotomy-induced responses of neurons and glial cells. Double fluorochroming with Hoechst 33342 and propidium iodide reveals nuclei of all alive and dead cells in this preparation. This technique clearly demonstrates the glial envelope around the axons and its changes under diverse impacts.
As an example, we considered the dynamics of axotomy-induced apoptosis or necrosis of glial cells after isolation of CSR. In the non-axotomized (intact) preparation, apoptosis of few glial cells occured at 4–8 h after isolation and remained further at the low and constant level (Fig. 10). It was possibly a result of occasional damage to some glial cells during isolation. In the axotomized CSR, glial apoptosis was higher and permanently increased after isolation. It was possibly the direct result of AT-induced injury of some glial cells and/or indirect result of the neuron damage. One can suggest that in the first case, the damage of some glial cells, which envelop the cut axon end, led to massive penetration of Ca2+, which can induce both necrosis and apoptosis (Trump and Berezesky 1996; Uzdensky et al. 2007). The indirect way may be related to Ca2+ influx through the transected axon end (LoPachin and Lehning 1997), complex biochemical response of the damaged neuron (Rishal and Fainzilber 2014), and neuron-to-glia signaling (Goldberg and Barres 2000). These processes were directed to axon repair and regeneration, but in the isolated CSR preparation, the recovery was not reached and cells died.
Necrosis of glial cells in both preparations occurred after 15-h incubation, later than apoptosis. Apoptosis is known to require the energy supply. When the ATP level is low, cells die from necrosis (Nicotera et al. 1998). One can suggest that prolonged incubation of the isolated stretch receptor in the limited saline volume (4 ml) exhausted energetic substrates in glial cells and depleted ATP that led to necrosis. This process occurred faster in glial cells surrounding the axotomized neurons, which consumed a lot of ATP for sealing of the transected axon end and maintaining the axon integrity, than in glial cells around the non-axotomized neurons. In contrast, apoptosis of some cells occurred earlier, when the ATP level was still sufficient.
Only few works in the literature were devoted to characterization of the time course of the death of satellite cells after peripheral axotomy. In adult rat, this process took weeks and months (McKay Hart et al. 2002). The nature of neuroglial and interglial signals involved in neuron degeneration and death of glial cells is to be cleared.
The present technique of CSR isolation that preserves the link between axons of the sensory neurons and the VNC ganglion provides a simple but informative neuroglial preparation that may be used as a model object in studies of axotomy-induced degeneration and protection of peripheral neurons, the role of glia in the neuron damage, the mechanism of neuroglial interactions, the occurring signal transduction processes, and the effects of diverse physical and chemical factors on neuronal and glial cells.
References
Alexandrowicz JS (1951) Muscle receptor organs in the abdomen of Homarus vulgaris and Palinurus vulgaris. Quart J Microsc Sci 92:163–199
Conforti L, Gilley J, Coleman MP (2014) Wallerian degeneration: an emerging axon death pathway linking injury and disease. Nat Rev Neurosci 20:394–409. doi:10.1038/nrn3680
Eyzaguirre C, Kuffler SW (1955) Processes of excitation in the dendrites and in the soma of single isolated sensory nerve cells of the lobster and crayfish. J Gen Physiol 39:87–119
Fedorenko GM, Uzdensky AB (2009) Ultrastructure of neuroglial contacts in crayfish stretch receptor. Cell Tissue Res 337:477–490. doi:10.1007/s00441-009-0825-7
Florey E, Florey E (1955) Microanatomy of the abdominal stretch receptors of the crayfish (Astacus fluviatilis L.). J Gen Physiol 39:69–85
Fomichev NI (1986) Crayfish. Methods of study. Nauka, Leningrad
Giacobini EE (1969) Chemical studies of individual neurons. II. Invertebrate nerve cell. Neurosci Res (NY) 2:111–202
Goldberg JL, Barres BA (2000) The relationship between neuronal survival and regeneration. Annu Rev Neurosci 23:579–612
Ilyinsky OB (1975) Physiology of sensory systems: P. 3. Physiology of mechanoreceptors. Nauka, Leningrad
Komandirov MA, Knyazeva EA, Fedorenko YP, Rudkovskii MV, Stetsurin DA, Uzdensky AB (2011) On the role of phosphatidylinositol 3-kinase, protein kinase B/Akt, and glycogen synthase kinase-3β in photodynamic injury of crayfish neurons and glial cells. J Mol Neurosci 45:229–235. doi:10.1007/s12031-011-9499-1
Kuffler SW, Eyzaguirre C (1955) Synaptic inhibition in an isolated nerve cell. J Gen Physiol 39:155–184
Leksrisawat B, Cooper AS, Gilberts AB, Cooper RL (2010) Muscle receptor organs in the crayfish abdomen: a student laboratory exercise in proprioception. J Vis Exp 45:e2323. doi:10.3791/2323
Lin JH, Rydqvist B (1999) The mechanotransduction of the crayfish stretch receptor neuron can be differentially activated or inactivated by local anaesthetics. Acta Physiol Scand 166:65–74
Lobanov AV, Uzdensky AB (2009) Protection of crayfish glial cells but not neurons from photodynamic injury by nerve growth factor. J Mol Neurosci 39:308–319. doi:10.1007/s12031-009-9199-2
LoPachin RM, Lehning EJ (1997) Mechanism of calcium entry during axon injury and degeneration. Toxicol Appl Pharmacol 143:233–244
McIntyre A, Mehta S, Aubut J, Dijkers M, Teasell RW (2013) Mortality among older adults after a traumatic brain injury: a meta-analysis. Brain Inj 27:31–40
McKay Hart A, Brannstrom T, Wiberg M, Terenghi G (2002) Primary sensory neurons and satellite cells after peripheral axotomy in the adult rat: time course of cell death and elimination. Exp Brain Res 142:308–318
Nicotera P, Leist M, Ferrando-May E (1998) Intracellular ATP, a switch in the decision between apoptosis and necrosis. Toxicol Lett 102:139–142
Pearson WS, Ovalle F Jr, Faul M, Sasser SM (2012) A review of traumatic brain injury trauma center visits meeting physiologic criteria from The American College of Surgeons Committee on Trauma/Centers for Disease Control and Prevention Field Triage Guidelines. Prehosp Emerg Care 16:323–328
Purali N (2005) Structure and function relationship in the abdominal stretch receptor organs of the crayfish. J Comp Neurol 488:369–383
Richardson PM, Miao T, Wu D, Zhang Y, Yeh J, Bo X (2009) Responses of the nerve cell body to axotomy. Neurosurgery 65:A74–A79. doi:10.1227/01.NEU.0000352378.26755.C3
Rishal I, Fainzilber M (2014) Axon–soma communication in neuronal injury. Nat Rev Neurosci 15:32–42. doi:10.1038/nrn3609
Rodríguez-Muela N, Boya P (2012) Axonal damage, autophagy and neuronal survival. Autophagy 8:286–288. doi:10.4161/auto.8.2.18982
Rydqvist B, Lin JH, Sand P, Swerup C (2007) Mechanotransduction and the crayfish stretch receptor. Physiol Behav 92:21–28
Tao-Cheng JH, Hirosawa K, Nakajima Y (1981) Ultrastructure of the crayfish stretch receptor in relation to its function. J Comp Neurol 200:1–21
Trump BF, Berezesky IK (1996) The role of altered [Ca2+]i regulation in apoptosis, oncosis, and necrosis. Biochim Biophys Acta 1313:173–178
Uzdensky AB (1993) Laser microirradiation of a single nerve cell. Proc SPIE 1882:254–267. doi:10.1117/12.147682
Uzdensky AB (2011) Death of neuronal and glial cells induced by photodynamic treatment: ultrastructural changes and signaling mechanisms. In: Schmid CJ, Wolfe JL (eds) Neuronal cell apoptosis. Nova Science Publishers Inc., New York, pp 79–118
Uzdensky AB, Kutko OY (1997) Effect of weak extremely low frequency magnetic field on isolated crayfish stretch receptor neuron: nonlinear dependence on field amplitude and frequency. Electromagn Biol 16:267–279
Uzdensky A, Lobanov A, Bibov M, Petin Y (2007) Involvement of Ca2+ and cyclic adenosine monophosphate-mediated signaling pathways in photodynamic injury of isolated crayfish neuron and satellite glial cells. J Neurosci Res 85:860–870
Acknowledgments
The work was supported by grants Russian Scientific Foundation (Grant 14-15-00068) and Russian Foundation for Basic Research (Grant 14-04-00741).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khaitin, A.M., Rudkovskii, M.V. & Uzdensky, A.B. The method of isolation of the crayfish abdominal stretch receptor maintaining a connection of the sensory neuron to the ventral nerve cord ganglion. Invert Neurosci 15, 176 (2015). https://doi.org/10.1007/s10158-014-0176-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10158-014-0176-2