Abstract
Photodynamic treatment that causes intense oxidative stress and cell death is currently used in neurooncology. However, along with tumor cells, it may damage healthy neurons and glia. In order to study photodynamic effect on normal nerve and glial cells, we used crayfish stretch receptor, a simple system consisting of only two identified sensory neurons surrounded by glial cells. Photodynamic treatment induced firing abolition and necrosis of neurons as well as necrosis and apoptosis of glial cells. Nerve growth factor but not brain-derived neurotrophic factor or epidermal growth factor protected glial cells but not neurons from photoinduced necrosis and apoptosis. Inhibitors of tyrosine kinases or protein kinase JNK eliminated anti-apoptotic effect of nerve growth factor in photosensitized glial cells but not neurons. Therefore, these signaling proteins were involved in the anti-apoptotic activity of nerve growth factor. These data indicate the possible presence of receptors capable of recognizing murine nerve growth factor in crayfish glial cells. Thus, intercellular signaling mediated by nerve-growth-factor-like neurotrophin, receptor tyrosine kinase, and JNK may be involved in crayfish glia protection from apoptosis induced by photodynamic treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Photodynamic therapy (PDT) is based on generation of highly toxic singlet oxygen by photosensitizing dye molecules upon light exposure in the presence of oxygen. It induces intense oxidative stress followed by death of stained cells. PDT is currently used for cancer treatment (Almeida et al. 2004; Brown et al. 2004; Castano et al. 2005). It is proposed as a promising adjuvant method for treatment of brain tumors, specifically gliomas resistant to radiation and chemotherapy (Eljamel 2004; Stylli and Kaye 2006). Comprehensive experimental studies should precede its neurooncological application. PDT effect on cultured glioma cells (Jiang et al. 2002; Hu et al. 2007) or spheroids (Madsen et al. 2003) has been reported. However, PDT may also injure normal neurons and glial cells surrounding the tumor. Unlike other tissues, the damage of even a small group of neurons may be critical for brain functions. Enhancement of tumor damage along with simultaneous protection of normal neurons and glia would be ideal for such treatment. Photodynamic injury of normal neurons and glial cells in their natural environment where they interact with other neuronal and glial cells as well as signal transduction processes involved in cell protection and death are not sufficiently studied yet.
It should be taken into account that in stressful situations, neurons and surrounding glial cells tightly interact and support survival of each other. Neuron injury may induce death of surrounding glial cells (Kopp et al. 1997; Kolosov and Uzdensky 2006). In turn, glia destruction may suppress neuronal functions and induce death of neurons. Neuroglial interactions are mediated by neurotrophic factors and neuregulins. Moreover, injured neurons may signal glial cells to increase production of neurotrophins for maintaining their survival (Du and Dreyfus 2002; Pellitteri et al. 2006). Nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) are among the most important neurotrophic factors in the vertebrate nervous system (Sofroniew et al. 2001; Binder and Scharfman 2004; Reichardt 2006).
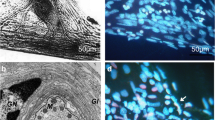
Neuroglial interactions are preferably studied on a simple nervous system, where neurons and glial cells naturally communicate and may be easily identified. We have used the crayfish abdominal stretch receptor, a simple model preparation (Fig. 1), which consists of only two identified sensory neurons (SN) enwrapped by satellite glial cells (SGC), for study of neuroglial interactions under photodynamic injury (Uzdensky et al. 2005, 2007, Uzdensky 2008; Kolosov and Uzdensky 2006). The glial envelope of sensory neurons looks like a multilayer Schwann cell envelope in vertebrates but glial layers are less dense than myelin and contain cytoplasm with organelles. Unlike cell culture, even mixed, in this preparation, the natural neuroglial interactions are preserved. SN has been shown to protect surrounding glial cells from PDT-induced apoptosis (Kolosov and Uzdensky 2006). One can suggest that glia protection was mediated by some unidentified gliatrophic factors secreted by SN and recognized by SGC receptors.
Crayfish stretch receptor. The preparation was double-stained with cell-impermeable propidium iodide that imparts red fluorescence to the nuclei of necrotic cells with the compromised plasma membrane and with Hoechst-33342 that imparts blue-green fluorescence to the chromatin of all cellular nuclei. Inset the bright-field microphotograph. Ax axon, CT connective tissue, D dendrite, gl glial envelope, n nucleus, RM receptor muscle, SN1 and SN2 slowly and rapidly adapting mechanoreceptor neurons. Circle and rectangle the areas, in which necrotic and apoptotic nuclei are counted, respectively. Objective ×10
The important aspect of the present research concerns the evolution of the intercellular signaling in the animal nervous system. The evolutionary emergence of neurotrophic signaling has been hypothesized to play a key role in the significant increase in the number of neurons and formation of the complex brain in higher organisms (Barde 1994; McKay et al. 1999; Jarro et al. 2001; Jarro and Fainzilber 2006). The evolution stage when neurotrophins have emerged is unknown. It is also unknown, whether different invertebrates, in particular crayfish, have neurotrophins. The neurotrophic signaling has not been found yet in the most of invertebrates. For example, the careful genome inspection showed that fly Drosophila and nematode C. elegans lack neurotrophins and their receptors (Hidalgo et al. 2006). On the other hand, analogs of neurotrophic factors or their receptors have been discovered in earthworm Esenia foetida (Davoli et al. 2002) and mollusks Lymnaea stagnalis (Fainzilber et al. 1996; Beck et al. 2004) and Aplysia californica (Ormond et al. 2004). The discovery and identification of neurotrophic signaling in invertebrates is a big and very complicated task. It would be rational to perform such investigation after receiving of indirect indications on the presence of neurotrophic signaling in various invertebrate species. Because of the lack of the information on neurotrophic signaling in the most of invertebrates and the absence of commercially available invertebrate neurotrophins and antibodies to them and their receptors, the information on the effects of vertebrate neurotrophic factors on invertebrate neuronal and glial cells is of importance.
In order to study this problem, we photosensitized isolated crayfish stretch receptor in the presence of nerve growth factor (NGF), or brain-derived neurotrophic factor (BDNF), or epidermal growth factor (EGF). The latter was used as a control for non-specific interactions. Using specific inhibitors, we also explored the possible role of different signaling pathways including tyrosine kinases, JNK and p38 mitogen-activated protein kinases (MAPK) in PDT-induced death of crayfish neurons and glial cells.
Materials and Methods
Chemicals
The following growth factors were used: mouse nerve growth factor NGF 7S (Alomone Labs, Israel), 100 ng/ml; recombinant human-brain-derived neurotrophic factor BDNF (Cytolab/Peprotech Asia, Israel), 1 ng/ml; recombinant human epidermal growth factor EGF, 100 ng/ml (Sigma-Aldrich). Photosensitizer Photosens, a mixture of sulfonated aluminum phthalocyanines, AlPcS n , where n = 3.1, was obtained from NIOPIK (Moscow, Russia). Propidium iodide and Hoechst 33342 were obtained from Chimmed (Moscow, Russia). Genistein, inhibitor of tyrosine kinases; SP600125 and SB202190, specific inhibitors of JNK (Han et al. 2001) and p38 (Davies et al. 2000), respectively, were purchased from SafLab (the Moscow representative of Sigma-Aldrich Co).
Stretch Receptor Preparation and Recording of its Neuronal Activity
Crayfishes, Astacus leptodactylus, both male and female, were purchased at the local market. They were normally two years old. The body size was about 8–10 cm. Their stretch receptors (Fig. 1) were isolated according to Wiersma et al. (1953). Then these were placed into a Plexiglas chamber equipped with a device for receptor muscle extension, and filled with 2 ml of van Harreveld saline (mM: NaCl—205; KCl—5.4; NaHCO3—0.2; CaCl2—13.5; MgCl2—5.4; pH 7.2–7.4). Neuronal spikes were recorded extracellularly from axons by a glass pipette suction electrode, amplified (the amplifier UU-90, Institute of Experimental Medicine, Saint-Petersburg, Russia), digitized by the analog–digital converter L-761 (L-Card, Moscow, Russia), and processed by a personal computer using the home-made software that provided continuous monitoring of a firing frequency level. The irreversibility of firing abolition was estimated at 20–30 min after firing cessation by the loss of neuron responsiveness to receptor muscle stretching. Experiments were carried out at 23 ± 4°C.
Fluorescence-Microscopic Study of Cell Death
In order to reveal death of SN and SGC, 20 μM propidium iodide and 10–20 μM Hoechst 33342 were added to the experimental chamber 8 h after firing abolition, an interval sufficient for development of apoptosis (Uzdensky et al. 2005, 2007). Then, preparations were washed with van Harreveld saline, fixed with 0.2% glutaraldehyde, repeatedly washed, and mounted in glycerol. Fluorescent images were acquired using the fluorescence microscope Lumam-I3 (LOMO, Sank-Petersburg, Russia) equipped with a digital photocamera. Propidium iodide, a membrane impermeable fluorochrome, reveals necrotic cells: it imparts red fluorescence to the nuclei of cells with the compromised plasma membrane. Pink, lilac, or white nuclei seen in Figs. 1 and 2 are also considered to belonged to necrotic cells with different stages of the plasma membrane injury. Hoechst 33342 imparts blue (cyan) fluorescence to the nuclear chromatin. It visualizes intact nuclei of living cells and fragmented nuclei of apoptotic cells. Nucleus fragmentation is the final stage of the apoptotic process where the return point has passed. Blue or red fragmented nuclei in Figs. 1 and 2 belong to apoptotic cells, in which apoptosis has finished and the plasma membrane either remained whole or was damaged (secondary necrosis after primary apoptosis). It should be mentioned that other methods for apoptosis assay, which require cytoplasm or plasma membrane observation (such as caspase activation, cytochrome c release, or annexin V assay), are not suitable for study of glial cells enclosing neurons because of their complex morphology. In fact, the glial envelope consists of ten to 30 layers that enwrap the neuron and form a roulette-like structure (Mashansky et al. 1974), in which various layers may belong to different glial cells: alive, necrotic, or apoptotic. Their optical images overlap, and the cytoplasm or the plasma membranes of these cells cannot be discernible using optical microscopy.
The nuclear morphology of the control (a and b) and photosensitized (c–f) stretch receptor neuron and satellite glial cells in the absence (a, c, and e) or presence (b, d, and f) of 100 ng/ml nerve growth factor. Within 8 h after photodynamic treatment and abolition of neuron activity, the preparation was double-stained with cell-impermeable propidium iodide that imparts the red fluorescence to nuclei of necrotic cells with the compromised plasma membrane and Hoechst 33342 that fluorochromes undamaged alive and fragmented apoptotic nuclei in blue-green. e and f show fragmented apoptotic nuclei of satellite glial cells surrounding the proximal axon regions photosensitized in the absence or in the presence of NGF, respectively. Arrowheads on a–d indicate the neuron nuclei; arrows on c and e fragmented nuclei of apoptotic glial cells. Scale bar on c corresponds to 20 µm for a–d, and that on e to 25 μm for e and f. Objective ×40
Photodynamic Treatment
At the beginning of each experiment, the initial level of SN firing was set at 6–10 Hz by application of the appropriate receptor muscle extension. After a 30-min control recording of SN activity, Photosens (10−7 M) and neurotrophic factors and/or inhibitors were added into the chamber with an interval of 3–5 min. After the 30-min incubation, cells were irradiated with the 670-nm laser diode (“Polus”, Moscow, Russia). The laser beam diameter was 3 mm, so that the neuronal soma and a major part of the axon were irradiated. The saline layer above the cell in the experimental chamber was no more than 1 mm deep, thus, beam attenuation was negligible. The radiant power was measured by the laser dosimeter IMO-2N (‘Etalon’, Volgograd, Russia). The fluence rate was maintained at 0.4 W/cm2 in all experiments. The irradiation exposure was 30 min. It was longer than the duration of bioelectric neuronal response measured from the irradiation start to the moment of firing abolition (typically, 10–20 min), which we called the “neuron lifetime”. Photosens and added reagents were present in the chamber during and after irradiation. After 8 h incubation, sufficient for development of apoptosis, glial cells were stained for fluorescence microscopy.
Application of relatively large concentrations of enzyme inhibitors, SP600125 or SB202190, may quickly disturb neuron activity and kill neurons and glia. On the other hand, too small concentrations may be ineffective. In our experiments, their concentrations were chosen to be two times lower than the predetermined concentrations, which disturbed neuron firing in the darkness for 3–4 h (the isolated control neurons fired in these conditions for 6–10 h). They were added to the experimental chamber 5 min after application of Photosens, i.e., 25 min before irradiation
Data Analysis
The red nuclei of necrotic glial cells stained by propidium iodide were counted in the predetermined standard field (100 × 100 μm2) around the SN soma so that neuron nucleus was situated in its center. Fragmented nuclei of apoptotic glial cells labeled by Hoechst 33342 were counted around the proximal 2-mm axon fragment (Fig. 1). Glial apoptosis was more prominent in this zone than around the neuron body (Kolosov et al. 2005). Their mean number representing the level of glial apoptosis is denoted below as relative units. The one-way ANOVA (analysis of variances) was used for statistical evaluation of the difference between independent experimental groups. Data are presented as mean ± S.E.M.
Results
Control Preparations
In the darkness, isolated and untreated SN fired regularly for 5–8 h with a frequency of about 6–10 Hz. Then, firing frequency gradually decreased and neuronal activity irreversibly ceased. Within 8 h after isolation, the neuronal nuclei and about 80–90% of the glial nuclei remained blue (Fig. 2a), i.e., these cells were alive. SN nuclei were bigger than that of SGC, and fluoresced weaker due to DNA unpacking and, therefore, lesser chromatin density. Chromatin structure with round nucleolus in the nucleus center was more delicate in neuronal nuclei than that in SGC. Unlike neurons, the nucleoli were not seen in the glial nuclei (Fig. 2a). Some SN and SGC nuclei in different control groups became necrotic within 8 h after isolation (0–14% and 5–17%; Figs. 4b–9b and 4c–9c, respectively). Nucleus fragmentation or chromatin clumping into big dense masses characteristic of apoptosis were never observed in isolated, photosensitized, or chemically treated SN like in our previous experiments (Uzdensky et al. 2002, 2005, 2007). Unlike SN, apoptosis of untreated SGC was sometimes registered within 8 h after isolation (Figs. 4d–9d).
Effect of Neurotrophic Factors on Neurons and Glial Cells in the Darkness
In the darkness, NGF 7S (100 ng/ml), BDNF (1 ng/ml) or EGF (100 ng/ml) did not significantly influence neuronal activity, spontaneous necrosis of sensory neurons and satellite glial cells, and apoptosis of glial cells within 8 h after isolation of stretch receptors (Figs. 4–6).
Photodynamic Injury of Neurons and Glial Cells
Neither laser radiation, nor 10−7 M Photosens alone changed neuronal activity significantly. However, their combined action, i.e., PDT treatment, caused gradual inhibition of neuronal firing followed by its irreversible abolishment (Fig. 3). The mean duration of such neuronal response called “neuron lifetime” as in our previous works (Uzdensky et al. 2002, 2005, 2007) varied from 7 to 25 min in different experimental series. At 8 h after PDT, neuronal and glial nuclei shrank. Neuronal nucleoli became indiscernible (Fig. 2c and d). The mean percent of necrotic SN and SGC increased to 30–80% in different experiments depending on animal groups and season (Fig. 2c and Figs. 4–9). Appearance of fragmented nuclei in some glial cells (Fig. 2e) indicated the last stages of apoptosis. The mean level of SGC apoptosis was significantly increased from 3–12 to 13–23 relative units in different experimental series (Figs. 4d–7d, 9d) and even to 62 relative units (Fig. 8d).
Inhibition of firing of crayfish stretch receptor neuron induced by photosensitization with 10−7 M Photosens in the absence (a) or presence (b) of nerve growth factor (100 ng/ml). Thin and double arrows indicate the moments of Photosens and nerve growth factor applying, respectively; thick arrow the irradiation start
Photodynamic effect of 10−7 M Photosens on stretch receptor neuron in the presence of 100 ng/ml NGF 7S. a Neuron lifetime, min; b percent of necrotic neurons within 8 h after PDT; c Percent of necrotic glial cells within 8 h after PDT; d Number of fragmented nuclei of apoptotic glial cells per 2 mm of proximal axon fragment within 8 h after PDT (relative units). The number of the experiments is indicated inside each bar. The numbers above the bars in the histograms refer to which group this bar is being compared. *p < 0.05; **p < 0.01; ***p < 0.001
Effect of Neurotrophic Factors on Photodynamic Injury of SN and SGC
None of the neurotrophins and EGF influenced the PDT-induced abolition of neuronal firing and necrosis (Figs. 4a–6a and 4b–6b, respectively). However, NGF (100 mg/ml) protected glial cells from PDT-induced apoptosis and necrosis, as determined by Hoechst 33342 and propidium iodide staining (Fig. 2f). As shown in Fig. 4c and d, NGF reduced the levels of PDT-induced necrosis and apoptosis by 3.3 and 1.6 times (p < 0.01 and p < 0.05, respectively).
Unlike NGF, BDNF (1 ng/ml) and EGF (100 ng/ml) did not influence significantly PDT-induced damage to glial cells (Figs. 5c,d and 6c,d). Hence, the possible involvement of some signaling proteins in NGF effect on photosensitized glial cells was studied more carefully.
Photodynamic effect of 10−7 M Photosens on stretch receptor neuron in the presence of 1 ng/ml BDNF. a Neuron lifetime, min; b percent of necrotic neurons within 8 h after PDT; c Percent of necrotic glial cells within 8 h after PDT; d Number of fragmented nuclei of apoptotic glial cells per 2 mm of proximal axon fragment within 8 h after PDT (relative units). The number of the experiments is indicated inside each bar. The numbers above the bars in the histograms refer to which group this bar is being compared. *p < 0.05; **p < 0.01
Photodynamic effect of 10−7 M Photosens on stretch receptor neuron in the presence of 100 ng/ml EGF. a Neuron lifetime, min; b percent of necrotic neurons within 8 h after PDT; c percent of necrotic glial cells within 8 h after PDT; d Number of fragmented nuclei of apoptotic glial cells per 2 mm of proximal axon fragment within 8 h after PDT (relative units). The number of the experiments is indicated inside each bar. The numbers above the bars in the histograms refer to which group this bar is being compared. **p < 0.01; ***p < 0.001
Tyrosine Kinase Inhibition
Since receptors of neurotrophins are tyrosine kinases, we used the tyrosine kinase inhibitor genistein for study of their role in NGF effect on photosensitized SN and SGC. As in a previous study (Uzdensky et al. 2005), genistein shortened the bioelectric response of SN to photodynamic treatment independent on the NGF presence (Fig. 7a). It did not influence PDT-induced necrosis of SN and SGC (Fig. 7b and c), and did not change NGF-mediated protection of glial cells against PDT-induced necrosis (Fig. 7c). Genistein also did not influence significantly the level of PDT-induced apoptosis of glial cells (Fig. 7d). However, it abolished NGF-mediated decrease in the level of PDT-induced apoptosis (Fig. 7d) that indicated tyrosine kinase involvement in NGF-mediated protection of glial cells from photoinduced apoptosis.
Photodynamic effect of 10−7 M Photosens on stretch receptor neuron in the presence of 100 ng/ml NGF and /or 50 μM tyrosine kinase inhibitor genistein (Gen). a Neuron lifetime, min; b percent of necrotic neurons within 8 h after PDT; c percent of necrotic glial cells within 8 h after PDT; d number of fragmented nuclei of apoptotic glial cells per 2 mm of proximal axon fragment within 8 h after PDT (relative units). The number of the experiments is indicated inside each bar. The numbers above the bars in the histograms refer to which group this bar is being compared. *p < 0.05; ***p < 0.001
JNK or p38 Inhibition
Binding of neurotrophic factors to receptor tyrosine kinase may initiate three known MAP kinase pathways: ERK, JNK, and p38. JNK and p38 are activated in stress situations (Cobb 1999). Application of SP600125 (10 μM), the specific inhibitor of JNK, or SB202190 (5 μM), the specific inhibitor of p38, did not change significantly neuronal activity and spontaneous necrosis of SN and SGC in the darkness. SP600125 also did not influence spontaneous apoptosis of SGC (the data are not shown). SB202190, however, enhanced glial apoptosis in the darkness but not under PDT treatment (Fig. 8d) indicating the involvement of p38 in SGC protection against apoptosis induced by axotomy and isolation of the stretch receptor but not by its PDT treatment.
PDT effect of 10−7 M Photosens on stretch receptor neuron in the presence of 5 μM p38 inhibitor SB202190 (SB). a Neuron lifetime, min; b percent of necrotic neurons within 8 h after PDT; c Percent of necrotic glial cells within 8 h after PDT; d number of fragmented nuclei of apoptotic glial cells per 2 mm of proximal axon fragment within 8 h after PDT (relative units). The number of the experiments is indicated inside each bar. The numbers above the bars in the histograms refer to which group this bar is being compared. **p < 0.01; ***p < 0.001
SB202190 also significantly shortened the lifetime of photosensitized neurons (Fig. 8a) indicating involvement of p38 in the maintaining of neuronal activity. It did not influence significantly PDT-induced necrosis of neurons (Fig. 8b) but reduced necrosis of glial cells by 30% (p < 0.05; Fig. 8c). This suggests contribution of p38 in photoinduced necrosis of SGC.
SP600125 did not influence PDT-induced changes in neuronal activity, necrosis of SN, and SGC, and apoptosis of glial cells (Fig. 9). However, the NGF-induced protection of glial cells from PDT-induced apoptosis (bar 3 at Fig. 9d) was eliminated in the presence of SP600125 (bar 5). Therefore, JNK could be involved in NGF-mediated prevention of photoinduced apoptosis of glial cells.
PDT effect of 10−7 M Photosens on stretch receptor neuron in the presence of 100 ng/ml NGF and/or 10 μM JNK inhibitor SP600125 (SP). a Neuron lifetime, min; b percent of necrotic neurons within 8 h after PDT; c percent of necrotic glial cells within 8 h after PDT; d Number of fragmented nuclei of apoptotic glial cells per 2 mm of proximal axon fragment within 8 h after PDT (relative units). The number of the experiments is indicated inside each bar. The numbers above the bars in the histograms refer to which group this bar is being compared. *p < 0.05; **p < 0.01; ***p < 0.001
Discussion
The present work shows that murine nerve growth factor NGF 7S but not BDNF or EGF protects crayfish glial cells but not neurons from PDT-induced necrosis and apoptosis. We have mentioned briefly that its active fragment NGF 2.5S have the similar glia-protective activity (Lobanov and Uzdensky 2007). Possibly, crayfish glial cells but not neurons have a receptor capable of recognizing of murine NGF. In stress conditions, a crayfish NGF analog may be involved in the neuron-to-glia signaling. Photodynamic impairment of its production and secretion may decrease the cell protection potential. In this situation, exogenous NGF can maintain survival of glial cells. Such signaling directed from neuron to glia looks atypical since in mammals the neurotrophic signaling pathways lead generally from glial cells to neurons (Sofroniew et al. 2001; Du and Dreyfus 2002; Reichardt 2006), whereas the backward neuron-to-glia signaling is performed by neuregulins (Kopp et al. 1997; Garratt et al. 2000). Although there are not much data, which show directly that NGF released from neurons and recognized by glial TrkA receptors may support survival of injured glial cells, one can suggest the possibility of such NGF/TrkA-mediated pro-survival neuron-to-glia signaling in mammals as well. In fact, TrkA have been found in diverse mammalian glial cells: astrocytes (Wang et al. 1998), oligodendrocytes (Althaus and Richter-Landsberg 2000; Althaus et al. 2008), satellite cells of rat spinal ganglia (Pannese and Procacci 2002), Schwann cells and cells forming human mechanosensory Meissner’s and Pacini’s corpuscles (Vega et al. 1994). NGF expression in adult cortical, hippocampal, and forebrain cholinergic neurons (SN is also a cholinergic neuron) has been well documented (Miller and Pitts 2000; Barker-Gibb et al. 2001; Sofroniew et al. 2001; Berhanu and Rush 2008). NGF effectively influences survival, regeneration, remyelination and other processes in diverse glial cells (Althaus and Richter-Landsberg 2000; Althaus et al. 2008). As shown in rat spinal ganglia, TrkA receptors are involved in the trophic support of satellite cells mediated by NGF released from the associated neurons (Pannese and Procacci 2002).
Although neurotrophins are known to protect mammalian neurons from oxidative stress (Skaper et al. 1998), their involvement in responses of neurons and glia to photodynamic treatment is not well established. It has been only shown that BDNF and CNTF (ciliary neurotrophic factor) protect retinal cells from verteporphyn-mediated photodynamic injury (Paskowitz et al. 2007).
We did not observe apoptosis of crayfish SN treated by PDT or chemical pro-apoptotic agents (Uzdensky et al. 2002, 2005, 2007). It could be intrinsically blocked like in various adult vertebrate neurons (Benn and Woolf 2004). Unlike neurons, apoptosis of glial cells was often induced by PDT. Apoptosis of photosensitized cells is controlled by diverse intracellular signaling pathways (Almeida et al. 2004; Castano et al. 2005; Uzdensky et al. 2008). In the present work, inhibition of tyrosine kinases or JNK eliminated the anti-apoptotic but not anti-necrotic effect of NGF in photosensitized glial cells. Therefore, these enzymes could be involved in the NGF-mediated protection of crayfish glial cells from PDT-induced apoptosis. In mammals, NGF is recognized by receptor tyrosine kinase TrkA, which initiates signaling pathways that supports cell survival. Tyrosine kinase inhibitor genistein is rather non-specific and TrkA is among its targets. Genistein could target a crayfish TrkA-like receptor and thus impair NGF recognition and eliminate its glia-protective effect.
Among signaling pathways initiated by receptor tyrosine kinases, MAP kinase and phosphatidylinositol 3-kinase/protein kinase B pathways are generally anti-apoptotic, whereas phospholipase C/Ca2+ pathway may stimulate apoptosis and necrosis (Cobb 1999; Sofroniew et al. 2001; Reichardt 2006). In the present work, the anti-apoptotic effect of NGF on photosensitized glial cells was prevented by JNK inhibitor SP600125 but not p38 inhibitor SB202190. It is of interest that JNK inhibition did not change the level of PDT-induced apoptosis by itself. Hence, JNK does not directly regulate PDT-induced apoptosis of glial cells but it may be involved in NGF-mediated anti-apoptotic pathway. This is in agreement with the anti-apoptotic role of JNK in HeLa cells photosensitized with hypericin (Assefa et al. 1999). However, other studies showed participation of JNK in PDT-induced apoptosis of cultured cancer cells (Xue et al. 1999; Chan et al. 2000). According to Waetzig and Herdegen (2005), JNK plays a dichotomous role in cell responses to stress: depending on conditions, it may be required either for cell survival of for death. It would be of importance to study the role of upstream and downstream processes in the JNK-related pathway in NGF-mediated protection of glial cells from PDT-induced apoptosis.
Another specific feature of NGF signaling in crayfish stretch receptor is the NGF-mediated protection of SGC from necrosis (Fig. 4c). This shows that necrosis is not an uncontrolled, catastrophic cell death. It may be regulated by the cell signaling systems. This is consistent with the recent data on the involvement of different signaling proteins: phospholipase C, calmodulin, calmodulin-dependent kinase II, protein kinase C, adenylate cyclase, and tyrosine phosphatases in PDT-induced necrosis of these cells (Uzdensky et al. 2005, 2007). The mechanism of anti-necrotic effect of NGF on crayfish glial cells remains unclear.
BDNF is wider distributed in the brain than NGF. It protects very effectively central mammalian neurons from apoptosis induced by oxidative stress (Skaper et al. 1998; Binder and Scharfman 2004). A BDNF concentration of 1 ng/ml used in the present experiments was chosen according to the producer recommendation. It seems much lesser than that of NGF (100 ng/ml) but the molecular weight of NGF 7S (130 kDa) is an order higher than that of BDNF, which is a 27-kDa dimer. So, their molar concentrations differ by one but not two orders. The absence of BDNF effect on neuronal activity or SN and SGC survival after PDT treatment in the present experiments might be due to the lack of its receptors in the crayfish stretch receptor neurons and satellite glial cells, or their low sensitivity compared to that in mammalian brain.
Epidermis growth factor EGF was used in the present work as a control for non-specific interactions, although in some invertebrates such as Drosophila EGF receptor-associated signaling pathway maintains glial survival in response to the ligands Spitz, Vein, and transforming growth factor TGF-α (Hidalgo et al. 2006). However, in the photosensitized crayfish stretch receptor EGF did not influence survival of neurons and glial cells even at a concentration of 100 ng/ml. These data confirm the specificity of NGF effect on survival of glial cells.
The difference in the inter- and intracellular signaling in crayfish neurons and glial cells may allow selective photodynamic injury of neurons with protection of glial cells by NGF. One can hope that differential modulation of the photosensitivity of neurons, normal and malignant glial cells with pharmacological agents, neuro- and gliatrophic factors may, in principal, protect normal neurons and glial cells during selective photodynamic destruction of brain tumors. The human signaling pathways, of course, differ from those in invertebrates, and a similar study should be performed on mammals.
Although NGF rescued crayfish glial cells from PDT-induced death, it did not influence significantly the survival of SN and SGC after isolation and 8-h incubation in the darkness. This seems not to be in agreement with the hypothetical presence of their specific receptors on these cells. However, the level of isolation-induced cell necrosis and apoptosis was only near 10% (bars 1 at Fig. 4b–d) within 8 h after stretch receptor isolation (these preparations comprised the control groups). So, it was difficult to observe any cell protection at this low level. Only when PDT significantly enhanced cell death the protective effects of NGF became evident.
Thus, responses of crayfish satellite glial cells to PDT-induced oxidative stress depend on the neuroglial signal transduction. The crayfish stretch receptor neurons may secrete a neurotrophic factor similar to mammalian NGF that initiate intracellular signaling pathways supporting survival of surrounding glial cells. The crayfish A. leptodactylus as well as the snail L. stagnalis (Ridgway et al. 1991; Wildering et al. 1995; Syed et al. 1996); squid Loligo peallei (Moreno et al. 1998); and silk moth Bombyx mori (Kim et al. 2005) may represent invertebrates with possible neurotrophic signal transduction.
Abbreviations
- BDNF:
-
brain-derived neurotrophic factor
- EGF:
-
epidermis growth factor
- JNK:
-
c-Jun terminal kinase
- MAPK:
-
mitogen-activated protein kinase
- NGF:
-
nerve growth factor
- PDT:
-
photodynamic therapy
- SGC:
-
satellite glial cell
- SN:
-
stretch receptor neuron
References
Almeida, R. D., Manadas, B. J., Carvalho, A. P., & Duarte, C. B. (2004). Intracellular signaling mechanisms in photodynamic therapy. Biochimica et Biophysica Acta, 1704, 59–86.
Althaus, R. D., & Richter-Landsberg, C. (2000). Glial cells as targets and producers of neurotrophins. International Review of Cytology, 197, 203–277. doi:10.1016/S0074-7696(00)97005-0.
Althaus, H. H., Klöppner, S., Klopfleisch, S., & Schmitz, M. (2008). Oligodendroglial cells and neurotrophins: a polyphonic cantata in major and minor. Journal of Molecular Neuroscience, 35, 65–79. doi:10.1007/s12031-008-9053-y.
Assefa, Z., Vantieghem, A., Declercq, W., Vandenabeele, P., Vandenheede, J. R., Merlevede, W., et al. (1999). The activation of the c-Jun N-terminal kinase and p38 mitogen-activated protein kinase signaling pathways protects HeLa cells from apoptosis following photodynamic therapy with hypericin. The Journal of Biological Chemistry, 274, 8788–8796. doi:10.1074/jbc.274.13.8788.
Barde, Y. A. (1994). Neurotrophic factors: an evolutionary perspective. Journal of Neurobiology, 25, 1329–1333. doi:10.1002/neu.480251102.
Barker-Gibb, A. L., Dougherty, K. D., Einheber, S., Drake, C. T., & Milner, T. A. (2001). Hippocampal tyrosine kinase A receptors are restricted primarily to presynaptic vesicle clusters. The Journal of Comparative Neurology, 430, 182–199. doi:10.1002/1096-9861(20010205)430:2<182::AID-CNE1024>3.0.CO;2-Q.
Beck, G., Munno, D. W., Levy, Z., Dissel, H. M., Van-Minnen, J., Syed, N. I., et al. (2004). Neurotrophic activities of Trk receptors conserved over 600 million years of evolution. Journal of Neurobiology, 60, 12–20. doi:10.1002/neu.10329.
Benn, S. C., & Woolf, C. J. (2004). Adult neuron survival strategies—slamming on the brakes. Nature Reviews Neuroscience, 5, 686–700. doi:10.1038/nrn1477.
Berhanu, D. A., & Rush, R. A. (2008). Targeted silencing of TrkA expression in rat forebrain neurons via the p75 receptor. Neuroscience, 153, 1115–1125. doi:10.1016/j.neuroscience.2008.03.025.
Binder, D. K., & Scharfman, H. E. (2004). Brain-derived neurotrophic factor. Growth Factors, 22, 123–131. doi:10.1080/08977190410001723308.
Brown, S. B., Brown, E. A., & Walker, I. (2004). The present and future role of photodynamic therapy in cancer treatment. The Lancet Oncology, 5, 497–508. doi:10.1016/S1470-2045(04)01529-3.
Castano, A. P., Demidova, T. N., & Hamblin, M. R. (2005). Mechanisms in photodynamic therapy: part two—cellular signaling, cell metabolism and modes of cell death. Photodiagnosis and Photodynamic Therapy, 2, 1–23. doi:10.1016/S1572-1000(05)00030-X.
Chan, W. H., Yu, J. S., & Yang, S. D. (2000). Apoptotic signaling cascade in photosensitized human epidermoid carcinoma A431 cells: involvement of singlet oxygen, c-Jun N-terminal kinase, caspase-3 and p21-activated kinase 2. The Biochemical Journal, 351, 221–232. doi:10.1042/0264-6021:3510221.
Cobb, M. H. (1999). MAP kinase pathways. Progress in Biophysics and Molecular Biology, 71, 479–500. doi:10.1016/S0079-6107(98)00056-X.
Davies, S. P., Reddy, H., Caivano, M., & Cohen, P. (2000). Specificity and mechanism of action of some commonly used protein kinase inhibitors. The Biochemical Journal, 351, 95–105. doi:10.1042/0264-6021:3510095.
Davoli, C., Marconi, A., Serafino, A., Iannoni, C., Marcheggiano, A., & Ravagnan, G. (2002). Expression of nerve growth factor-like polypeptides and immunoreactivity related to the two types of neurotrophin receptors in earthworm tissues. Cellular and Molecular Life Sciences, 59, 527–539. doi:10.1007/s00018-002-8444-4.
Du, Y., & Dreyfus, C. F. (2002). Oligodendrocytes as providers of growth factors. Journal of Neuroscience Research, 68, 647–654. doi:10.1002/jnr.10245.
Eljamel, M. S. (2004). Brain PDD and PDT unlocking the mystery of malignant gliomas. Photodiagnosis and Photodynamic Therapy, 1, 303–310. doi:10.1016/S1572-1000(05)00008-6.
Fainzilber, M., Smit, A. B., Syed, N. I., Wildering, W. C., Hermann, P. M., van der Schors, R. C., et al. (1996). CRNF, a molluscan neurotrophic factor that interacts with the p75 neurotrophin receptor. Science, 274, 1540–1543. doi:10.1126/science.274.5292.1540.
Garratt, A. N., Britsch, S., & Birchmeier, C. (2000). Neuregulin, a factor with many functions in the life of a Schwann cell. BioEssays, 22, 987–996. doi:10.1002/1521-1878(200011)22:11<987::AID-BIES5>3.0.CO;2-5.
Han, Z., Boyle, D. L., Chang, L., Bennett, B., Karin, M., Yang, L., et al. (2001). c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. The Journal of Clinical Investigation, 108, 73–81.
Hidalgo, A., Learte, A. R., McQuilton, P., Pennack, J., & Zhu, B. (2006). Neurotrophic and gliatrophic contexts in Drosophila. Brain, Behavior and Evolution, 68, 173–180. doi:10.1159/000094086.
Hu, S. S., Cheng, H. B., Zheng, Y. R., Zhang, R. Y., Yue, W., & Zhang, H. (2007). Effects of photodynamic therapy on the ultrastructure of glioma cells. Biomedical and Environmental Sciences, 20, 269–273.
Jarro, H., & Fainzilber, M. (2006). Building complex brains—missing pieces in an evolutionary puzzle. Brain, Behavior and Evolution, 68, 191–195. doi:10.1159/000094088.
Jarro, H., Beck, G., Conticello, S. G., & Fainzilber, M. (2001). Evolving better brains: a need for neurotrophins? Trends in Neurosciences, 24, 79–85. doi:10.1016/S0166-2236(00)01690-8.
Jiang, F., Chopp, M., Katakowski, M., Cho, K. K., Yang, X., Hochbaum, N., et al. (2002). Photodynamic therapy with photofrin reduces invasiveness of malignant human glioma cells. Lasers in Medical Science, 17, 280–288. doi:10.1007/s101030200041.
Kim, J. H., Sung, D. K., Park, C. W., Park, H. H., Park, C., Jeon, S. H., et al. (2005). Brain-derived neurotrophic factor promotes neurite growth and survival of antennal lobe neurons in brain from the silk moth, Bombyx mori in vitro. Zoological Science, 22, 333–342. doi:10.2108/zsj.22.333.
Kolosov, M., & Uzdensky, A. (2006). Crayfish mechanoreceptor neuron prevents photoinduced apoptosis of satellite glial cells. Brain Research Bulletin, 69, 495–500. doi:10.1016/j.brainresbull.2006.02.018.
Kolosov, M.S., Lobanov, A.V., Aulova, S.A., & Uzdensky, A.B.(2005) Neuroglial interactions during photodynamic injury of the crayfish stretch receptor, in Problems of Neurocybernetics, Vladimirsky B.M., ed., OOO CVVR, Rostov-on-Don, pp.247-250 (In Russian).
Kopp, D. M., Trachtenberg, J. T., & Thompson, W. J. (1997). Glial growth factor rescues Schwann cells of mechanoreceptors from denervation-induced apoptosis. The Journal of Neuroscience, 17, 6697–6706.
Lobanov, A. V., & Uzdensky, A. B. (2007). Neurotrophin NGF protects glial cells, but not neurons, of stretch receptor of the crayfish Astacus astacus from photooxidative stress. Journal of Evolutionary Biochemistry and Physiology, 43, 533–535. doi:10.1134/S002209300705012X.
Madsen, S. J., Sun, C. H., Tromberg, B. J., & Hirschberg, H. (2003). Repetitive 5-aminolevulinic acid-mediated photodynamic therapy on human glioma spheroids. Journal of Neuro-Oncology, 62, 243–250. doi:10.1023/A:1023362011705.
Mashansky, V. F., Zaguskin, S. L., & Fedorenko, G. M. (1974). Histochemical and electron-microscopic study of neuroglial relationships in the crayfish stretch receptor. Tsitologiya, 16, 770–773.
McKay, S., Purcell, A. L., & Carew, T. J. (1999). Regulation of synaptic function by neurotrophic factors in vertebrates and invertebrates: implications for development and learning. Learning & Memory, 6, 193–215.
Miller, M. W., & Pitts, F. A. (2000). Neurotrophin receptors in the somatosensory cortex of the mature rat: co-localization of p75, trk, isoforms and c-neu. Brain Research, 852, 355–366. doi:10.1016/S0006-8993(99)02176-9.
Moreno, H., Nadal, M., Leznik, E., Sugimori, M., Lax, I., Schlessinger, J., et al. (1998). Nerve growth factor acutely reduces chemical transmission by means of postsynaptic TrkA-like receptors in squid giant synapse. Proceedings of the National Academy of Sciences of the United States of America, 95, 14997–15002. doi:10.1073/pnas.95.25.14997.
Ormond, J., Hislop, J., Zhao, Y., Webb, N., Vaillaincourt, F., Dyer, J. R., et al. (2004). ApTrkl, a Trk-like receptor, mediates serotonin-dependent ERK activation and long-term facilitation in Aplysia sensory neurons. Neuron, 44, 715–728. doi:10.1016/j.neuron.2004.11.001.
Pannese, E., & Procacci, P. (2002). Ultrastructural localization of NGF receptors in satellite cells of the rat spinal ganglia. Journal of Neurocytology, 31, 755–763. doi:10.1023/A:1025708132119.
Paskowitz, D. M., Donohue-Rolfe, K. M., Yang, H., Yasumura, D., Matthes, M. T., Hosseini, K., et al. (2007). Neurotrophic factors minimize the retinal toxicity of verteporfin photodynamic therapy. Investigative Ophthalmology & Visual Science, 48, 430–437. doi:10.1167/iovs.06-0690.
Pellitteri, R., Russo, A., & Stanzani, S. (2006). Schwann cell: a source of neurotrophic activity on cortical glutamatergic neurons in culture. Brain Research, 1069, 139–144. doi:10.1016/j.brainres.2005.11.049.
Reichardt, L. F. (2006). Neurotrophin-regulated signalling pathways. Philosophical Transactions of the Royal Society B: Biological Sciences, 361, 1545–1564. doi:10.1098/rstb.2006.1894.
Ridgway, R. L., Syed, N. I., Lukowiak, K., & Bulloch, A. G. (1991). Nerve growth factor (NGF) induces sprouting of specific neurons of the snail, Lymnaea stagnalis. Journal of Neurobiology, 22, 377–390. doi:10.1002/neu.480220406.
Skaper, S. D., Floreani, M., Negro, A., Facci, L., & Giusti, P. (1998). Neurotrophins rescue cerebellar granule neurons from oxidative stress-mediated apoptotic death: selective involvement of phosphatidylinositol 3-kinase and the mitogen-activated protein kinase pathway. Journal of Neurochemistry, 70, 1859–1868.
Sofroniew, M. V., Howe, C. L., & Mobley, W. C. (2001). Nerve growth factor signaling, neuroprotection, and neural repair. Annual Review of Neuroscience, 24, 1217–1281. doi:10.1146/annurev.neuro.24.1.1217.
Stylli, S. S., & Kaye, A. H. (2006). Photodynamic therapy of cerebral glioma—a review. Part I. A biological basis. Journal of Clinical Neuroscience, 13, 615–625. doi:10.1016/j.jocn.2005.11.014.
Syed, N., Richardson, P., & Bulloch, A. (1996). Ciliary neurotrophic factor, unlike nerve growth factor, supports neurite outgrowth but not synapse formation by adult Lymnaea neurons. Journal of Neurobiology, 29, 293–303. doi:10.1002/(SICI)1097-4695(199603)29:3<293::AID-NEU2>3.0.CO;2-4.
Uzdensky, A. B. (2008). Signal transduction and photodynamic therapy. Current Signal Transduction Therapy, 3, 55–74. doi:10.2174/157436208783334277.
Uzdensky, A. B., Bragin, D. E., Kolosov, M. S., Dergacheva, O. Y., Fedorenko, G. M., & Zhavoronkova, A. A. (2002). Photodynamic inactivation of isolated crayfish mechanoreceptor neuron: different death modes under different photosensitizer concentrations. Photochemistry and Photobiology, 76, 431–437. doi:10.1562/0031-8655(2002)076<0431:PIOICM>2.0.CO;2.
Uzdensky, A., Kolosov, M., Bragin, D., Dergacheva, O., Vanzha, O., & Oparina, L. (2005). Involvement of adenylate cyclase and tyrosine kinase signaling pathways in response of crayfish stretch receptor neuron and satellite glia cell to photodynamic treatment. Glia, 49, 339–348. doi:10.1002/glia.20122.
Uzdensky, A., Lobanov, A., Bibov, M., & Petin, Y. (2007). Involvement of Ca2+- and cyclic adenosine monophosphate-mediated signaling pathways in photodynamic injury of isolated crayfish neuron and satellite glial cells. Journal of Neuroscience Research, 85, 860–870. doi:10.1002/jnr.21190.
Uzdensky, A. B., Kolosov, M. S., & Lobanov, A. V. (2008). Neuron and gliocyte death induced by photodynamic treatment: signal processes and neuroglial interactions. Neuroscience and Behavioral Physiology, 38, 727–735. doi:10.1007/s11055-008-9042-1.
Vega, J. A., Vazquez, E., Naves, F. J., Del Valle, M. E., Calzada, B., & Represa, J. J. (1994). Immunohistochemical localization of the high-affinity NGF receptor (gp140-trkA) in the adult human dorsal root and sympathetic ganglia and in the nerves and sensory corpuscles supplying digital skin. The Anatomical Record, 240, 579–588. doi:10.1002/ar.1092400415.
Waetzig, V., & Herdegen, T. (2005). Context-specific inhibition of JNKs: overcoming the dilemma of protection and damage. Trends in Pharmacological Sciences, 26, 455–461.
Wang, Y., Hagel, C., Hamel, W., Müller, S., Kluwe, L., & Westphal, M. (1998). Trk A, B, and C are commonly expressed in human astrocytes and astrocytic gliomas but not by human oligodendrocytes and oligodendroglioma. Acta Neuropathologica, 96, 357–364. doi:10.1007/s004010050906.
Wiersma, C. A. G., Furshpan, E., & Florey, E. (1953). Physiological and pharmacological observations on muscle organ of the crayfish, Cambarus clarkii Girard. The Journal of Experimental Biology, 30, 136–150.
Wildering, W. C., Lodder, J. C., Kits, K. S., & Bulloch, A. G. (1995). Nerve growth factor (NGF) acutely enhances high-voltage-activated calcium currents in molluscan neurons. Journal of Neurophysiology, 74, 2778–2781.
Xue, L., He, J., & Oleinick, N. L. (1999). Promotion of photodynamic therapy induced apoptosis by stress kinases. Cell Death and Differentiation, 6, 855–864. doi:10.1038/sj.cdd.4400558.
Acknowledgment
The authors thank Alomone Labs, Israel for kind providing NGF. The work was supported by Russian Foundation for Basic Researches; grants 05-04-48440, 08-04-01322, and Minobrnauki, grant 2.1.1/6185.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lobanov, A.V., Uzdensky, A.B. Protection of Crayfish Glial Cells but not Neurons from Photodynamic Injury by Nerve Growth Factor. J Mol Neurosci 39, 308–319 (2009). https://doi.org/10.1007/s12031-009-9199-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-009-9199-2