Abstract
This review outlines the epidemiology, characteristics, risk factors, and prognosis of peritoneal dialysis (PD)-related peritonitis, PD catheter-related infections, and the effects of assisted PD in elderly patients from the Japanese perspective. Based on the literature, the incidence of peritonitis is likely to be higher in elderly patients than in younger patients. The most frequent causative bacteria in elderly patients are Gram-positive bacteria, as in adult PD patients, most commonly due to transcatheter infection. However, elderly patients may have difficulty recognizing cloudy drainage fluid due to decreased visual acuity. Hypokalemia, the use of gastric acid suppressants, prophylactic antibiotic use before endoscopy, biocompatible fluids and hypoalbuminemia considered modifiable risk factors for peritonitis. However, the mechanism by which treatment of hypokalemia prevents peritonitis is unknown. Currently, the relationship between gastric acid suppression therapy and peritonitis in elderly patients is debatable, with no evidence to strongly recommend uniform discontinuation of gastric acid suppression therapy. Exit-site infection (ESI) is a major risk factor for the development of peritonitis, and appropriate prevention and management of ESI may reduce infection-related hospitalizations in PD patients. Currently, no randomized, controlled trials have verified the effectiveness of antibiotic application for ESI in Japan, but results from other countries are awaited. In assisted PD, it is extremely important that family members, caregivers, and nurses who support the procedure receive sufficient education and training from medical professionals familiar with PD. Early detection and treatment of PD-related infections are required because the risk of death increases in elderly patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the data of the Japanese Statistics Bureau, Ministry of Internal Affairs and Communications, in 2023, although the elderly population decreased for the first time since 1950, the elderly population as a proportion of the total population reached a record high of 29.1% [1]. Furthermore, the number of people aged 75 years and over exceeded 20 million for the first time, and one in ten people is aged 80 years or older, making it clear that Japan has the highest proportion of elderly people in the world.
As Japan’s general population ages, dialysis patients are also aging. The average age of patients undergoing dialysis is increasing each year, exceeding 70 years of age in 2019, and the age group with the highest proportion is 70 to 74 years for both men and women [2]. It is not only hemodialysis (HD) patients who are aging, but also peritoneal dialysis (PD) patients. According to a statistical survey by the Japanese Society for Dialysis Therapy (JSDT) at the end of 2009, 55.8% of PD patients were 60 years of age or older, and 15.1% were 75 years of age or older [3]. However, according to a statistical survey at the end of 2018, 65.6% of PD patients were over 60 years of age, and 23.1% were over 75 years of age or older [4] (Fig. 1).
Compared with HD, PD has less effect on hemodynamics, can preserve residual kidney function, and requires fewer regular visits; it may be a better choice for elderly patients with end-stage kidney disease [5]. Furthermore, it has been reported that the quality of life of elderly people is higher on PD than on HD because it has less impact on daily life [6, 7]. However, since PD is home-based medical care, it is necessary for patients to carry out the treatment themselves, and in some cases, assisted PD by family members or medical staff is required [8].
The International Society for Peritoneal Dialysis (ISPD) guidelines for prescribing high-quality, goal-directed PD list frailty in elderly persons as one of the factors that affect patient outcomes [9]. Elderly PD patients must be evaluated for frailty, and the type of support they need must be considered on an individual basis. In addition, countermeasures against infectious diseases are important for maintaining stable, long-term PD. The Standardized Outcomes in Nephrology-PD initiative was conducted with the aim of establishing important outcomes based on priorities among all stakeholders related to PD and lists PD-related infections as one of the core outcomes [10]. However, to date, few reviews have focused on PD-related infections in elderly patients. This review outlines the epidemiology, characteristics, risk factors, and prognosis of PD-related peritonitis, PD catheter-related infections, and the effects of assisted PD in elderly patients.
Peritonitis rate in elderly PD patients
The ISPD guidelines for the prevention and treatment of peritonitis published in 2022 state that the new target for the overall peritonitis rate should be less than 0.40 episodes/patient-year [11]. In contrast, the peritonitis rate in Japanese PD patients in 2022 was 0.20 episodes/patient-year, far below the guideline target [12]. Furthermore, the Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS) showed that the peritonitis rate in Japan is lower than in other developed countries [13]. However, it should be noted that several studies have shown that peritonitis is the most frequent cause of transfer to HD in Japanese PD patients [14, 15].
In Japan, some data regarding peritonitis in elderly PD patients have been reported. In 2011, the JSDT survey reported the relationship between the frequency of peritonitis and age [16] and found that 801 (18.7%) of 4,337 PD patients developed peritonitis. Of these, 209 (25.4%) of 823 patients aged 70 to 79 years and 79 (21.0%) of 377 patients aged 80 years or older developed peritonitis. There was a tendency for the proportion of patients with peritonitis to increase with increasing age (Fig. 2). Another survey of 4406 PD patients investigated the incidence of peritonitis in young and elderly patients in 2016 [17]. Although statistical analysis comparing the two groups was not conducted, the incidence of peritonitis in patients under 45 years of age was 0.21 episodes/patient-year, whereas it was 0.30 episodes/patient-year in patients over 75 years of age. The incidence of peritonitis tends to increase with age. These results suggest that the incidence may be higher in Japanese elderly PD patients than in younger PD patients.
Recently, Jiang et al. conducted a systematic review and meta-analysis of the development of peritonitis in elderly PD patients (defined as 65 years or older) and non-elderly PD patients [18]. The meta-analysis of four studies showed a significantly increased risk of peritonitis in older adults (risk ratio: 1.56, 95% confidence intervals [CI]: 1.18–2.07). The report also presented studies that performed qualitative analyses, with three studies showing no difference between elderly and non-elderly patients, and three studies showing higher risk in elderly patients. Based on the above results, strict management is required, because the incidence of peritonitis is likely to be higher in elderly patients than in younger patients.
Characteristics of PD-related peritonitis in elderly PD patients
PD-related peritonitis can be diagnosed when at least two of the following are present: 1. clinical features consistent with peritonitis, that is, abdominal pain and/or cloudy dialysis effluent; 2. dialysis effluent white cell count > 100/µL or > 0.1 × 109/L (after a dwell time of at least 2 h), with > 50% polymorphonuclear leukocytes; and 3. positive dialysis effluent culture [11]. However, a recent report showed that, though elderly patients experience abdominal pain to the same extent as young patients, they experience fever less frequently and it may be difficult to identify cloudy dialysis effluent [19]. Elderly patients have decreased visual acuity, and they may have difficulty noticing slight clouding in the early stages of peritonitis.
Several studies have investigated the distribution of bacteria causing peritonitis in older and younger adults. Htay et al. reported that there were no differences in the distribution of causative bacteria, except that polymicrobial peritonitis was significantly more common in younger than in elderly patients (20% vs. 10.5%, p = 0.02) [19]. Similarly, De Vecchi et al. also reported that the distribution of bacteria causing peritonitis was similar in older and younger patients [20]. In contrast, Song et al. reported that the incidence of fungal peritonitis, Acinetobacter baumannii, and polymicrobial peritonitis was significantly higher in older patients than in young patients [21]. However, an important finding is that, in these studies, the most frequent causative bacteria in elderly patients were Gram-positive bacteria, similar to previous reports in adult PD patients [22, 23]. Although they did not compare elderly and young patients, Mizuno et al. investigated the microorganisms that cause peritonitis in Japanese PD patients over a three-year period and reported that Gram-positive cocci were the most common, at 42.7% [14].
The most frequent route of infection for peritonitis caused by Gram-positive bacteria is transcatheter infection due to touch contamination, and this risk can be reduced by taking adequate measures. A Japanese registry survey of 3,845 patients showed that approximately 70% of PD patients in Japan use exchange devices, and many of those using them are older [24]. It is thought that elderly patients choose exchange devices to reduce the risk of contact contamination of their hands and fingers. However, this study reported that patients using exchange devices had a 37% higher risk of peritonitis than those using manual exchange (incidence rate ratio: 1.37, 95% CI 1.07–1.75). The reason for this is that approximately 70% of the patients who used the exchange devices were elderly, and it is likely that the device was used for frail patients. In the future, well-designed interventional studies are needed to clarify the effectiveness of exchange devices.
Risk factors for PD-related peritonitis in elderly PD patients
It is also important to be aware of risk factors for peritonitis that are specific to elderly patients. Risk factors can be divided into modifiable and non-modifiable factors, and next we will discuss hypokalemia, the use of gastric acid suppressants, prophylactic antibiotic use before endoscopy, biocompatible fluids and hypoalbuminemia as modifiable risk factors.
Hypokalemia can occur in elderly patients and PD patients when dietary intake is reduced [25]. Several observational studies have previously suggested that hypokalemia is a risk factor for peritonitis [26, 27]. Recently, a randomized, controlled trial (RCT) was conducted to examine the effectiveness of potassium supplementation in preventing the onset of peritonitis [28]. PD patients were divided into a protocol group in which protocol-based potassium supplementation (titratable dose of oral potassium chloride to maintain serum potassium of 4–5 mEq/L) or conventional potassium supplementation (reactive supplementation for serum potassium < 3.5 mEq/L), and they were followed for 52 weeks. The time to the first onset of peritonitis was significantly longer in the protocol group (223 days vs. 133 days [median], P = 0.03), and the hazard ratio (HR) for peritonitis was significant (HR 0.47, 95% CI 0.24–0.93). However, the average age of the patients in this study was 55 years, and questions remain as to whether these results can be applied to elderly patients.
A large prospective observational cohort study from South Korea showed that lower serum potassium levels (< 4.5 mmol/L) were an independent predictor of death in 1,152 PD patients [29]. The association between hypokalemia and death remained even after adjusting for confounding factors such as age, sex, comorbidities, and nutritional status. Based on the above, intervention for hypokalemia in elderly patients may not only reduce the risk of peritonitis, but also contribute to a good patient prognosis. At present, the mechanism by which treatment of hypokalemia prevents peritonitis is unknown, but it may be related to improved intestinal motility, decreased bacterial translocation, and improved cellular immune function [30].
Elderly patients with chronic kidney disease (CKD) are often given antithrombotic therapy because the incidence of cardiovascular disease increases as renal function decreases [31]. Therefore, gastric acid suppressants are often used concomitantly to prevent peptic ulcers. Since histamine H2 receptor antagonists (HRAs), which are excreted by the kidneys, are difficult to use in normal doses in CKD patients, proton pump inhibitors (PPIs) are inevitably used. According to PDOPPS data, of 23,797 PD patients, 6020 (25.3%) were using PPIs, whereas only 1382 (5.8%) were using HRAs [32] (41% of Japanese PD patients were using PPIs and 3% were using HRAs). Further, 47% of patients using PPIs and 48% of patients using HRAs were prescribed antiplatelet drugs.
The use of PPIs was previously reported to be an independent risk factor for the development of idiopathic bacterial peritonitis in cirrhotic patients with ascites [33]. Therefore, Maeda et al. investigated the relationship between the use of PPIs and the onset of initial peritonitis in PD patients. They reported that 86 of 230 patients (37.4%) developed peritonitis, and the use of PPIs was a significant predictor of peritonitis (adjusted HR 1.72, 95% CI 1.11–2.66) [34]. PPIs have been reported to cause collagenous colitis in PD patients, and the effect of the drug must be considered when PD patients develop refractory diarrhea [35].
A meta-analysis of non-RCTs in patients with PD showed that the use of HRAs was associated with increased odds of peritonitis of intestinal origin (odds ratio (OR) 1.4, 95% CI 1.01–1.93) [36]. Based on these results, the ISPD guidelines state that peritonitis of intestinal origin may be prevented by avoiding or limiting the use of HRAs [11]. However, a report investigating the relationship between gastric acid suppression therapy and peritonitis based on PDOPPS data showed no significant association between gastric acid suppression therapy and peritonitis (adjusted HR 1.05, 95% CI 0.98–1.13), and the use of PPIs or HRAs was also not associated with peritonitis [32]. However, a relationship was observed between peritonitis caused by some causative bacteria (particularly streptococci) and gastric acid suppression therapy. Even in this study, the analysis did not include elderly patients, and there is still room for debate regarding the relationship between gastric acid suppression therapy and peritonitis in elderly patients. At present, there is no evidence to strongly recommend uniform discontinuation of gastric acid suppression therapy.
There are few reports that have investigated the relationship between the onset of peritonitis and endoscopic procedure, focusing on elderly PD patients. A report of 408 gastroscopies performed in 216 PD patients (61.7 ± 11.4 years) showed that age (OR 1.08, 95% CI 1.02–1.141) was associated with peritonitis, but prophylactic antibiotic use was associated with a reduced risk of peritonitis [37]. On the other hand, a large Korean study investigating 1,316 endoscopies performed in 570 patients (median age 56 years) reported that age (OR 2.63, 95% CI 1.18–5.90) was significantly associated with an increased risk of peritonitis, but prophylactic antibiotic use was not associated with a reduction in peritonitis [38]. Considering the consistent high incidence of peritonitis after endoscopy in elderly PD patients, prophylactic antibiotic use prior to endoscopy may be advisable according to ISPD guideline [11]. Although prophylactic antibiotic use before endoscopy is recommended to be administered intravenously, Suzuki et al. have demonstrated the effectiveness of oral antibiotics in preventing the onset of peritonitis in elderly PD patients [39].
The balANZ Trial showed that biocompatible fluids may reduce the incidence of peritonitis compared with conventional fluids [40]. However, a meta-analysis of ten RCTs found low quality evidence, the effect of biocompatible fluids on peritonitis rate was unclear [41], and no studies focused on elderly PD patients.
Serum albumin levels decrease with age, and the prevalence of hypoalbuminemia increases [42]. It is also widely known that PD patients often suffer from hypoalbuminemia due to loss of albumin into the peritoneal cavity and fluid overload. Although there have been several reports examining the relationship between hypoalbuminemia and peritonitis, no consensus has been reached [43]. Recently, Hu et al. reported that baseline hypoalbuminemia (HR 0.932, 95% CI 0.896–0.969) was independent risk factors for the occurrence of the first episode of peritonitis [44]. However, the average age of the patients was young, about 50 years old. To our knowledge, there have been no reports examining the relationship between hypoalbuminemia and peritonitis in elderly PD patients. Further studies are needed to evaluate the impact of approaches to malnutrition in elderly PD patients on the incidence of peritonitis.
PD catheter-related infections in elderly PD patients
Exit-site infection (ESI) is a major risk factor for the development of peritonitis, and appropriate prevention and management of ESI may reduce infection-related hospitalizations in PD patients [45]. Infection control is extremely important for elderly people, since their physical functions easily decrease due to hospitalization.
The ISPD guidelines for catheter-related infections were revised in 2023 [46]. The guidelines proposed a new goal for overall ESI incidence to be controlled to no more than 0.40 episodes/patient-year. According to a report investigating the incidence of ESI in Japan in 2016, 19.7% of 4,391 PD patients had at least one ESI, and the incidence was 0.36 episodes/patient-year [17]. In contrast, a recent review showed that the reported incidence of ESI worldwide ranges from 0.06 to 0.42 episodes/patient-year [30]. Judging from these results, it can be said that the incidence of ESI in Japanese PD patients is by no means low. A prospective, observational study of outcomes after PD catheter placement conducted at 49 institutions in Japan reported that the incidence of ESI/tunnel infection within 30 days after surgery was 8.5% in 401 PD patients [47]. This result was outside the audit criteria (ESI/tunnel infection within 30 days of catheter insertion: < 5%) in the ISPD guidelines for creating and maintaining optimal peritoneal dialysis access in adult PD patients [48]. Although the ISPD guidelines have downgraded their recommendations, they still recommend daily topical application of an antibiotic cream or ointment to the catheter exit site [46]. However, the Japanese PD guidelines do not recommend the prophylactic application of antibiotics for catheter-related infections [49], and their application is not covered by insurance. Obata et al. conducted a meta-analysis that included six RCTs and found that mupirocin ointment was not significantly effective in preventing ESI or peritonitis (ESI: rate ratio (RR), 0.36, 95% CI, 0.13–1.05); peritonitis: RR 0.78, 95% CI 0.50–1.21) [50].
Currently, no RCTs have verified the effectiveness of antibiotic application for ESI in Japan, but in Thailand, exit-site care was performed in three groups (chlorhexidine gluconate-impregnated patch group, mupirocin ointment group, and physiological saline treatment group) [51]. It is unclear whether the results can be extrapolated to Japanese medical care, since patients’ economic situations and environmental factors differ greatly, but the results are awaited.
There is a paucity of studies comparing the incidence of ESI in elderly and non-elderly people. An Italian study compared the incidence of ESI in non-diabetic PD patients aged 70 years and older (63 patients) and non-diabetic PD patients aged 40 to 60 years (86 patients) [18]. According to this report, the incidence of ESI was similar in older and younger patients (0.30 episodes/patient-year vs. 0.29 episodes/patient-year; no significant difference). In addition, a study from Chile reported that the incidence of catheter-related infections in 36 patients aged 65 years or older was 0.22 episodes/patient-year [52]. Furthermore, in a study of 31 Canadian elderly PD patients aged 80 years or older, the incidence of ESI was reported to be 0.013 times/patient-month (0.16 episodes/patient-year) [53]. These results suggest that the incidence of ESI may not be higher in older than in younger patients.
Association between assisted PD and PD-related infections
Dementia and dialysis therapy in dialysis patients in Japan were reported in a 2009 statistical survey [3]. According to this study, 9.8% of the dialysis population had dementia, and 321 (5.5%) of 5,856 PD patients were reported to have dementia. It is difficult to perform procedures such as bag exchange in PD patients with dementia, suggesting that some type of support is provided to a certain number of patients. In fact, a study of 4889 PD patients showed that 474 (9.7%) were dependent on others for PD-related procedures [54]. Non-self-management PD is called assisted PD, which is defined as PD that is performed in the patient’s home with the support of a family member, spouse, or healthcare professional such as a nurse [55].
Yabe et al. divided elderly PD patients into four groups based on the presence or absence of cognitive impairment and presence or absence of the exit-site care procedure with assistance and compared the time to first ESI onset for each group [56]. The results showed that the prognosis of the group of patients who received the exit-site care procedure with assistance, regardless of the presence or absence of cognitive impairment, was better than that of the group of patients who did not receive the exit-site care procedure with assistance. Although this study involved a small number of patients, it is considered to be valuable data suggesting the importance of the exit-site care procedure with assistance in the elderly.
Since assisted PD was first reported in 2006 [57], many studies have compared the incidence of peritonitis between self-care PD and assisted PD [58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74] (Table 1). Four of these studies did not perform statistical comparisons [58, 62, 64, 66]. Whereas Verger et al. reported that family-assisted PD was associated with a lower incidence of peritonitis than nurse-assisted PD [59], Duquennoy et al. reported that nurse-assisted PD was superior to family-assisted PD [67]. However, many studies have shown no difference in the incidence of peritonitis between self-care and assisted PD. Wu et al. examined risk factors for peritonitis in 111 elderly people aged 65 years or older who had at least one episode of peritonitis and found that older age (HR 1.06, 95% CI 1.01–1.11), assisted PD (HR 2.64, 95% CI 1.23–5.64), high body mass index (HR 1.11, 95% CI 1.02–1.20), and low serum albumin level (HR 0.94, 95% CI 0.90–0.98) were associated with increased risks of peritonitis [70]. For assisted PD to be successful, it is extremely important that family members, caregivers, and nurses who support the procedure receive sufficient education and training from medical professionals who are familiar with PD [75].
Prognosis of PD-related peritonitis in elderly PD patients
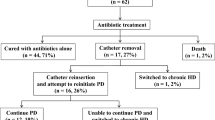
Several studies have shown that PD-related peritonitis has a worse prognosis in elderly PD patients than in young PD patients. In a study using dialysis registry data from Australia and New Zealand, Lim et al. reported that peritonitis-related mortality was significantly higher in elderly patients aged 65 years and older than in younger patients (HR: 2.31; 95% CI: 1.68–3.18) [76]. In addition, Song et al. compared peritonitis-related deaths between 88 elderly patients aged 65 years or older and 306 patients younger than 65 years with peritonitis [21]. They reported that 6 (6.8%) of the elderly and 2 (0.7%) of the young patients died, indicating that the peritonitis-related mortality rate was significantly higher in the elderly patients. Furthermore, there was no significant difference in catheter removal between the two groups, indicating that technique survival was equivalent. Wu et al. also showed that the risk of peritonitis-related death was approximately four times higher in older patients than in younger patients with peritonitis (odds ratio = 3.57, 95% CI 1.38–9.28), but peritonitis in elderly patients was not a risk for technique failure [70]. These results suggest that, although peritonitis in elderly patients is associated with mortality, once cured, it can be managed similarly to that in younger patients and does not require discontinuation of PD or transfer to HD. In fact, Htay et al. reported that antibiotic cure rates were similar in younger and older adults, and interestingly, the odds of peritonitis-related catheter removal were significantly lower in older adults than in younger adults [19]. This suggests that a less invasive, conservative approach may have been chosen in elderly patients.
There have also been several studies of the timing of onset and the prognosis of peritonitis in elderly patients. Guo et al. conducted a study to compare the prognosis of people aged 65 years or older, who were divided into two groups based on the time of onset of peritonitis: an early-onset group (those who developed peritonitis within 12 months after induction), and a late-onset group (those who developed peritonitis more than 12 months after induction) [77]. The results showed that the incidences of multiple peritonitis episodes, technique failure, and all-cause mortality were higher in the early-onset group. Wang et al. targeted people aged 65 years or older and defined patients who developed peritonitis within 6 months of starting PD as the early-onset group, and those who developed peritonitis after that as the late-onset group [78]. The results showed that the mortality rate was lower in the late-onset group than in the early-onset group (HR 0.37, 95% CI 0.16–0.75), and early onset of peritonitis was associated with lower mortality in elderly PD patients. Early onset of peritonitis in elderly patients is thought to be associated with poor visual acuity and poor physical and mental health [79, 80].
In conclusion, elderly PD patients may have a higher incidence of peritonitis than young PD patients, and it is important to assess frailty to determine whether support is needed. In addition, many of the causative bacteria are Gram-positive bacteria, and caution is required because it is difficult for patients to recognize cloudy drainage fluid due to decreased visual acuity. Interventions to address hypokalemia, which may occur due to loss of appetite in elderly persons, the use of acid suppressants, and the introduction of assisted PD may reduce the risk of peritonitis. However, evidence supporting the effectiveness of these preventive measures is still lacking. Further, although the risk of PD withdrawal in elderly patients with peritonitis is similar to that in younger patients, early detection and treatment are required because the risk of death increases.
Elderly patients in Japan are often considered to be late-stage elderly aged 75 years or older, and unfortunately, data on this patient group are lacking worldwide. In addition, the characteristics of the causative bacteria and patient outcomes in elderly Japanese PD patients are not clear. We hope that future large-scale registry research will clarify these issues.
Abbreviations
- HD:
-
Hemodialysis
- PD:
-
Peritoneal dialysis
- JSDT:
-
Japanese Society for Dialysis Therapy
- ISPD:
-
International Society for Peritoneal Dialysis
- PDOPPS:
-
Peritoneal Dialysis Outcomes and Practice Patterns Study
- CI:
-
Confidence interval
- RCT:
-
Randomized, controlled trial
- HR:
-
Hazard ratio
- CKD:
-
Chronic kidney disease
- HRA:
-
Histamine H2 receptor antagonist
- PPI:
-
Proton pump inhibitor
- ESI:
-
Exit-site infection
- RR:
-
Rate ratio
- OR:
-
Odds ratio
References
Ministry of Internal Affairs and Communications. Japan's elderly seen from statistics. https://www.stat.go.jp/data/topics/pdf/topics138.pdf. Accessed 23 February 2024.
Hanafusa N, Abe M, Joki N, et al. Annual dialysis data report 2019, JSDT Renal Data Registry. Ren Replace Ther. 2023;9:47.
Nakai S, Iseki K, Itami N, et al. Overview of regular dialysis treatment in Japan (as of 31 December 2009). Ther Apher Dial. 2012;16:11–53.
Nitta K, Abe M, Masakane I, et al. Annual dialysis data report 2018, JSDT Renal Data Registry: dialysis fluid quality, hemodialysis and hemodiafiltration, peritoneal dialysis, and diabetes. Ren Replace Ther. 2020;6:51.
Brown EA, Johansson L. Epidemiology and management of end-stage renal disease in the elderly. Nat Rev Nephrol. 2011;7:591–8.
Iyasere OU, Brown EA, Johansson L, et al. Quality of life and physical function in older patients on dialysis: a comparison of assisted peritoneal dialysis with hemodialysis. Clin J Am Soc. 2016;11:423–30.
Brown EA, Johansson L, Farrington K, et al. Broadening options for long-term dialysis in the elderly (BOLDE): differences in quality of life on peritoneal dialysis compared to haemodialysis for older patients. Nephrol Dial Transplant. 2010;25:3755–63.
Brown EA, Dratwa M, Povlsen J. Assisted peritoneal dialysis an evolving dialysis modality. Nephrol Dial Transpl. 2007;22:3091–2.
Brown EA, Blake PG, Boudville N, et al. International Society for Peritoneal Dialysis practice recommendations: prescribing high-quality goal-directed peritoneal dialysis. Perit Dial Int. 2020;40:244–53.
Manera KE, Johnson DW, Craig JC, et al. Establishing a core outcome set for peritoneal dialysis: report of the SONG-PD (Standardized Outcomes in Nephrology-Peritoneal Dialysis) Consensus Workshop. Am J Kidney Dis. 2020;75:404–12.
Li PKT, Chow KM, Cho Y, et al. ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment. Perit Dial Int. 2022;42:110–53.
Japanese Society for Dialysis Therapy (JSDT) Renal Data Registry. Chapter 7 Peritoneal Dialysis. https://docs.jsdt.or.jp/overview/file/2022/pdf/07.pdf. Accessed 23 Feb 2024.
Perl J, Fuller DS, Bieber BA, et al. Peritoneal dialysis-related infection rates and outcomes: results from the Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS). Am J Kidney Dis. 2020;76:42–53.
Mizuno M, Ito Y, Tanaka A, et al. Peritonitis is still an important factor for withdrawal from peritoneal dialysis therapy in the Tokai area of Japan. Clin Exp Nephrol. 2011;15:727–37.
Kawanishi H, Marshall MR, Zhao J, et al. Mortality, hospitalization and transfer to haemodialysis and hybrid therapy, in Japanese peritoneal dialysis patients. Perit Dial Int. 2022;42:305–13.
Nakai S, Watanabe Y, Masakane I, et al. An overview of regular dialysis treatment in Japan (As of December 31, 2011) (in Japanese). J Jpn Soc Dial Ther. 2013;46:1–76.
Masakane I, Taniguchi M, Nakai S, et al. Annual dialysis data report 2016, JSDT Renal Data Registry. Ren Replace Ther. 2018;4:45.
Jiang C, Zheng Q. Outcomes of peritoneal dialysis in elderly vs non-elderly patients: a systemic review and meta-analysis. PLoS ONE. 2022;17:e0263534.
Htay H, Seng JJB, Yong MHA, et al. Comparison of clinical presentation and outcomes of peritonitis in the elderly and younger peritoneal dialysis patients. Perit Dial Int. 2019;39:163–8.
De Vecchi AF, Maccario M, Braga M, et al. Peritoneal dialysis in nondiabetic patients older than 70 years: comparison with patients aged 40 to 60 years. Am J Kidney Dis. 1998;31:479–90.
Song P, Yang D, Li J, et al. Microbiology and outcome of peritoneal dialysis-related peritonitis in elderly patients: a retrospective study in China. Front Med (Lausanne). 2022;9: 799110.
Zelenitsky SA, Howarth J, Lagacé-Wiens P, et al. Microbiological trends and antimicrobial resistance in peritoneal dialysis-related peritonitis, 2005 to 2014. Perit Dial Int. 2017;37:170–6.
Ma TK, Chow KM, Kwan BC, et al. peritonitis before peritoneal dialysis training: analysis of causative organisms, clinical outcomes, risk factors, and long-term consequences. Clin J Am Soc Nephrol. 2016;11:1219–26.
Hasegawa T, Noma H, Hamano T, et al. Association between the use of exchange devices for peritoneal dialysis fluids and peritonitis incidence: a nationwide cohort study. Perit Dial Int. 2022;42:177–84.
Yamada S, Inaba M. Potassium metabolism and management in patients with CKD. Nutrients. 2021;13:1751.
Davies SJ, Zhao J, Morgenstern H, et al. Low serum potassium levels and clinical outcomes in peritoneal dialysis-international results from PDOPPS. Kidney Int Rep. 2020;6:313–24.
Ribeiro SC, Figueiredo AE, Barretti P, et al. Low serum potassium levels increase the infectious-caused mortality in peritoneal dialysis patients: a propensity-matched score study. PLoS ONE. 2015;10:e0127453.
Pichitporn W, Kanjanabuch T, Phannajit J, et al. Efficacy of potassium supplementation in hypokalemic patients receiving peritoneal dialysis: a randomized controlled trial. Am J Kidney Dis. 2022;80:580-8.e1.
Lee S, Kang E, Yoo KD, et al. Lower serum potassium associated with increased mortality in dialysis patients: A nationwide prospective observational cohort study in Korea. PLoS ONE. 2017;12:e0171842.
Cho Y, Chow KM, Li PKT, et al. Peritoneal dialysis-related infections. Clin J Am Soc Nephrol. 2023. https://doi.org/10.2215/CJN.0000000000000280.
Ninomiya T, Kiyohara Y, Kubo M, et al. Chronic kidney disease and cardiovascular disease in a general Japanese population: the Hisayama Study. Kidney Int. 2005;68:228–36.
Goldman S, Zhao J, Bieber B, et al. Gastric acid suppression therapy and its association with peritoneal dialysis-associated peritonitis in the Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS). Kidney. 2023;5:370. https://doi.org/10.34067/KID.0000000000000325.
Choi EJ, Lee HJ, Kim KO, et al. Association between acid suppressive therapy and spontaneous bacterial peritonitis in cirrhotic patients with ascites. Scand J Gastroenterol. 2011;46:616–20.
Maeda S, Yamaguchi M, Maeda K, et al. Proton pump inhibitor use increases the risk of peritonitis in peritoneal dialysis patients. PLoS ONE. 2019;14:e0224859.
Murasawa M, Sakurada T, Oishi D, et al. Collagenous colitis associated with rabeprazole in a peritoneal dialysis patient. Perit Dial Int. 2015;35:588–90.
Zhong HJ, Lin D, Lu ZY, et al. Use of gastric-acid suppressants may be a risk factor for enteric peritonitis in patients undergoing peritoneal dialysis: a meta-analysis. J Clin Pharm Ther. 2019;44:209–15.
Chan GC, Wong SH, Ng JK, et al. Risk of peritonitis after gastroscopy in peritoneal dialysis patients. Perit Dial Int. 2022;42:162–70.
Kim JS, Jung E, Kang SH, et al. Safety of endoscopy in peritoneal dialysis patients. Clin Transl Gastroenterol. 2021;12(7):e00379.
Suzuki Y, Mizuno M, Kojima H, et al. Oral antibiotics are effective for preventing colonoscopy-associated peritonitis as a preemptive therapy in patients on peritoneal dialysis. Intern Med. 2021;60:353–6.
Johnson DW, Brown FG, Clarke M, et al. Effects of biocompatible versus standard fluid on peritoneal dialysis outcomes. J Am Soc Nephrol. 2012;23:1097–107.
Htay H, Johnson DW, Wiggins KJ, et al. Biocompatible dialysis fluids for peritoneal dialysis. Cochrane Database Syst Rev. 2018;10(10):CD007554.
Shibata H, Haga H, Ueno M, et al. Longitudinal changes of serum albumin in elderly people living in the community. Age Ageing. 1991;20(6):417–20.
Kerschbaum J, König P, Rudnicki M. Risk factors associated with peritoneal-dialysis-related peritonitis. Int J Nephrol. 2012;2012:483250.
Hu J, Zhang H, Yi B. Peritoneal transport status and first episode of peritonitis: a large cohort study. Ren Fail. 2021;43(1):1094–103.
Chavers BM, Solid CA, Gilbertson DT, et al. Infection-related hospitalization rates in pediatric versus adult patients with end-stage renal disease in the United States. J Am Soc Nephrol. 2007;18:952–9.
Chow KM, Li PKT, Cho Y, et al. ISPD catheter-related infection recommendations: 2023 update. Perit Dial Int. 2023;43:201–19.
Sakurada T, Kojima S, Yamada S, et al. A multi-institutional, observational study of outcomes after catheter placement for peritoneal dialysis in Japan. Perit Dial Int. 2023;43:457–66.
Crabtree JH, Shrestha BM, Chow KM, et al. Creating and maintaining optimal peritoneal dialysis access in the adult patient: 2019 update. Perit Dial Int. 2019;39:414–36.
Ryuzaki M, Ito Y, Nakamoto H, et al. Peritoneal dialysis guidelines 2019 Part 2: main text (Position paper of the Japanese Society for Dialysis Therapy). Ren Replace Ther. 2021;7:46.
Obata Y, Murashima M, Toda N. Topical application of mupirocin to exit sites in patients on peritoneal dialysis: a systematic review and meta-analysis of randomized controlled trials. Ren Replace Ther. 2020;6:12.
Nochaiwong S, Ruengorn C, Noppakun K, et al. Comparative effectiveness of local application of chlorhexidine gluconate, mupirocin ointment, and normal saline for the prevention of peritoneal dialysis-related infections (COSMO-PD Trial): a multicenter randomized, double-blind, controlled protocol. Trials. 2019;20:754.
Ferńandez MA, Ortiz AM, Valenzuela M, et al. Peritoneal dialysis in chronic renal failure patients over 65 years of age. Adv Perit Dial. 2004;20:128–31.
Dimkovic NB, Prakash S, Roscoe J, et al. Chronic peritoneal dialysis in octogenarians. Nephrol Dial Transplant. 2001;16:2034–40.
Hinoshita F, Akiba T, Takashi Katsuki T, et al. Survey on the current situation of peritoneal dialysis facilities for aged patients. J Jpn Soc Dial Ther. 2017;50:139–46.
Covic A, Bammens B, Lobbedez T, et al. Educating end-stage renal disease patients on dialysis modality selection: clinical advice from the European Renal Best Practice (ERBP) Advisory Board. Nephrol Dial Transplant. 2010;25:1757–9.
Yabe H, Okada K, Kono K, et al. Effects of cognitive impairment and assisted peritoneal dialysis on exit-site infection in older patients. Clin Exp Nephrol. 2022;26:593–600.
Lobbedez T, Moldovan R, Lecame M, et al. Assisted peritoneal dialysis experience in a French renal department. Perit Dial Int. 2006;26:671–6.
Oliver MJ, Quinn RR, Richardson EP, et al. Home care assistance and the utilization of peritoneal dialysis. Kidney Int. 2007;71:673–8.
Verger C, Duman M, Durand PY, et al. Influence of autonomy and type of home assistance on the prevention of peritonitis in assisted automated peritoneal dialysis patients An analysis of data from the French Language Peritoneal Dialysis Registry. Nephrol Dial Transplant. 2007;22:1218–23.
Povlsen JV, Ivarsen P. Assisted peritoneal dialysis: also for the late referred elderly patient. Perit Dial Int. 2008;28:461–7.
Castrale C, Evans D, Verger C, et al. Peritoneal dialysis in elderly patients: report from the French Peritoneal Dialysis Registry (RDPLF). Nephrol Dial Transplant. 2010;25:255–62.
Hsieh CY, Fang JT, Yang CW, et al. The impact of type of assistance on characteristics of peritonitis in elderly peritoneal dialysis patients. Int Urol Nephrol. 2010;42:1117–24.
Xu R, Zhuo M, Yang Z, Dong J. Experiences with assisted peritoneal dialysis in China. Perit Dial Int. 2012;32:94–101.
Lobbedez T, Verger C, Ryckelynck JP, et al. Is assisted peritoneal dialysis associated with technique survival when competing events are considered? Clin J Am Soc Nephrol. 2012;7:612–8.
Cheng CH, Shu KH, Chuang YW, et al. Clinical outcome of elderly peritoneal dialysis patients with assisted care in a single medical centre: a 25 year experience. Nephrology (Carlton). 2013;18:468–73.
Querido S, Branco PQ, Elisabete Costa E, et al. Results in assisted peritoneal dialysis: a ten-year experience. Int J Nephrol. 2015;2015:712539. https://doi.org/10.1155/2015/712539.
Duquennoy S, Béchade C, Verger C, et al. Is peritonitis risk increased in elderly patients on peritoneal dialysis? Report from the French Language Peritoneal Dialysis Registry (RDPLF). Perit Dial Int. 2016;36:291–6.
Al Wakeel JS, Al Ghonaim MA, Aldohayan A, et al. Appraising the outcome and complications of peritoneal dialysis patients in self-care peritoneal dialysis and assisted peritoneal dialysis: a 5-year review of a single Saudi center. Saudi J Kidney Dis Transpl. 2018;29:71–80.
Guilloteau S, Lobbedez T, Guillouët S, et al. Impact of assisted peritoneal dialysis modality on outcomes: a cohort study of the French Language Peritoneal Dialysis Registry. Am J Nephrol. 2018;48:425–33.
Wu H, Ye H, Huang R, et al. Incidence and risk factors of peritoneal dialysis-related peritonitis in elderly patients: a retrospective clinical study. Perit Dial Int. 2020;40:26–33.
Ng JK, Chan GC, Chow KM, et al. Helper-assisted continuous ambulatory peritoneal dialysis: does the choice of helper matter? Perit Dial Int. 2020;40:34–40.
Song Q, Yan H, Yu Z, et al. Assisted peritoneal dialysis: a feasible KRT modality for frail older patients with end-stage kidney disease (ESKD). Sci Rep. 2021;11:14928.
Puapatanakul P, Kanjanabuch T, Tungsanga K, et al. Assisted peritoneal dialysis performed by caregivers and its association with patient outcomes. Perit Dial Int. 2022;42:602–14.
Melanson J, Kachmar J, Laurin LP, et al. Assisted peritoneal dialysis implementation: a pilot program from a large dialysis unit in Quebec. Can J Kidney Health Dis. 2022;9:20543581221113388.
Oliver MJ, Abra G, Béchade C, et al. Assisted peritoneal dialysis: Position paper for the ISPD. Perit Dial Int. 2024;7:8968608241246447.
Lim WH, Dogra GK, McDonald SP, et al. Compared with younger peritoneal dialysis patients, elderly patients have similar peritonitis-free survival and lower risk of technique failure, but higher risk of peritonitis-related mortality. Perit Dial Int. 2011;31:663–71.
Guo Q, Chen Y, Wu R, et al. Poorer clinical outcomes of early-onset peritonitis in elderly peritoneal dialysis patients: a longitudinal and multicenter study. Ther Apher Dial. 2022;26:815–21.
Wang Z, Jiang L, Feng S, et al. Early peritonitis is an independent risk factor for mortality in elderly peritoneal dialysis patients. Kidney Blood Press Res. 2015;40:298–305.
Ng X, Liu CL, Liu TP, et al. Surgical outcome of peritoneal dialysis in elderly patients. Int J Gerontol. 2009;3:143–8.
Brown EA, Finkelstein FO, Iyasere OU, et al. Peritoneal or hemodialysis for the frail elderly patient, the choice of 2 evils? Kidney Int. 2017;91:294–303.
Acknowledgements
None.
Funding
No funding has been received.
Author information
Authors and Affiliations
Contributions
TS, MM, MN, and YI participated in the conception of the review. All authors interpreted previous studies. Drafting of the article was done by TS, MM, MN, and YI.
Corresponding author
Ethics declarations
Conflict of interests
The authors have declared that no conflict of interest exists.
Data Availability
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Sakurada, T., Miyazaki, M., Nakayama, M. et al. Peritoneal dialysis-related infections in elderly patients. Clin Exp Nephrol (2024). https://doi.org/10.1007/s10157-024-02531-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10157-024-02531-5