Abstract
Background
Fungal peritonitis is a serious complication among peritoneal dialysis (PD) patients. The Standardizing Care to Improve Outcomes in Pediatric End Stage Renal Disease (SCOPE) Collaborative is a North American multicenter quality improvement initiative with the primary aim to reduce catheter-related infections in children on chronic dialysis.
Objective
To describe the epidemiology of fungal peritonitis and outcomes of affected patients among pediatric subjects receiving chronic PD and enrolled in SCOPE.
Methods
Data pertaining to PD characteristics, peritonitis episodes and patient outcome were collected between October 2011 and September 2015 from 30 pediatric dialysis centers participating in the SCOPE collaborative. Peritonitis-related data were stratified by etiology, fungal versus bacterial/culture-negative peritonitis. Differences among groups were assessed by Chi-square analysis.
Results
Of 994 patients enrolled in the registry, there were 511 peritonitis episodes of which 41 (8.0%) were fungal. Thirty-six individual patients with 39 unique catheters accounted for the fungal peritonitis episodes. Twenty-three (59%) of the episodes occurred in patients aged < 2 years (p = 0.03). Fungal peritonitis was the initial episode of peritonitis in 48.8% of affected patients, and only 17.1% of these patients had had a previous peritonitis episode within 30 days of the fungal infection. Insertion of the PD catheter at < 2 years of age was associated with an adjusted odds ratio of 2.8 (95% confidence interval 1.24, 6.31) for development of fungal peritonitis compared to older children (p = 0.01). Fungal peritonitis was associated with an increased rate of hospitalization (80.5 vs. 63.4%; p = 0.03), increased length of hospitalization (median of 8 vs. 5 days; p < 0.001) and increased rates of catheter removal (84.6 vs 26.9%; p = 0.001) and technique failure (68.3 vs. 8%; p = 0.001) compared to other causes of peritonitis.
Conclusion
Fungal infections were responsible for 8.0% of peritonitis episodes in the SCOPE collaborative, with the majority of fungal peritonitis episodes occurring in children aged < 2 years. Although no risk factors for infection other than young age were identified, fungal peritonitis was associated with an increased risk of hospitalization, longer hospital stay and an increased frequency of technique failure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fungal peritonitis (FP) is a serious complication among patients receiving chronic peritoneal dialysis (CPD). The reported incidence of FP among pediatric and adult PD populations ranges from 2 to 10.3% [1,2,3,4,5] and from 3 to 15% [6], respectively. FP is associated with increased rates of mortality and technique failure [7].
To date, the largest study describing the epidemiology and risk factors of FP in children on CPD was derived from the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) database by Warady and colleagues [2]. Their report was a retrospective review of 51 FP cases occurring between January 1992 and May 1996 that represented 2.9% of the total peritonitis episodes entered into the NAPRTCS registry during that period of observation. Unfortunately, the study results were somewhat limited by the fact that peritonitis was defined by the treatment of an infection, but without a requirement for the standard cellular criteria defined by the International Society for Peritoneal Dialysis (ISPD) [7, 8]. In addition, and in contrast to contemporary PD care, two-thirds of the patients in that study had a single cuff catheter and one-third of patients were on continuous ambulatory PD [2].
The Children’s Hospital Association’s Standardizing Care to Improve Outcomes in Pediatric End Stage Renal Disease (SCOPE) Collaborative is a multicenter quality transformation effort whose primary aim is to reduce the frequency of catheter-related infections among pediatric patients on chronic dialysis [8]. The collaborative has been prospectively collecting data on pediatric patients receiving CPD since October 2011. A recent publication from the SCOPE collaborative reported that FP accounted for 7.7% [1] of peritonitis episodes recorded by the collaborative, with no additional information pertaining specifically to the fungal infections [4]. We therefore sought to expand on the data generated by the prior NAPRTCS study by analyzing the data pertaining to FP from the SCOPE collaborative. In a previous SCOPE study Sethna et al. [1] compared catheters associated with the development of peritonitis to those that were not, On the present study we chose to evaluate risk factors for the development of FP versus bacterial/culture-negative peritonitis and to describe the respective outcomes of affected catheters and patients from 30 pediatric dialysis centers participating in SCOPE.
Materials and methods
SCOPE collaborative
The design of the SCOPE collaborative has been previously described [8]. All patients with end stage renal disease (ESRD) on chronic PD cared for by the participating pediatric centers (Appendix) were eligible. Patients were enrolled at the time of catheter placement (incident patients) and included patients who initiated chronic dialysis as an inpatient and had not yet performed home dialysis (e.g. infants). Patients with existing catheters were also enrolled (prevalent patients), and information on all prevalent and new catheter insertions during the study period was captured. Therefore, multiple catheter insertions could have been associated with a single patient.
Data collection
Data on demographic, clinical and catheter characteristics, as well as data on peritonitis episodes and outcomes, were collected between 1 October 2011 and 30 September 2015. Peritonitis was defined by a PD effluent white blood cell count of > 100 uL and a differential of > 50% polymorphonuclear cells, with or without a positive culture. Whereas there were some cases of peritonitis that did not meet both criteria, as does occur on occasion with patients on automated PD, all cases of fungal peritonitis met the ISPD criteria. Relapsing peritonitis, defined as a recurrence of peritonitis with the same causative organism within 4 weeks of a previous peritonitis episode, was excluded from the analysis.
Peritonitis was classified as fungal versus bacterial or culture-negative based on the results of peritoneal fluid culture. The treatment of peritonitis was per each center’s practice, but centers were advised to follow ISPD guidelines [7]. Data pertaining to the species of fungi isolated or the specific medication used for treatment of FP or any other cause of peritonitis were not collected by SCOPE.
Centers that contributed cases of FP to the SCOPE database were subsequently sent a survey that asked the following questions: (1) were any antibiotics administered to the patient who experienced FP within 30 days prior to the FP episode? (2) if antibiotics were administered, was fungal prophylaxis prescribed? (3) if fungal prophylaxis was prescribed, what specific medication was used?
Statistical analysis
For the purposes of statistical analysis, the unit of interest was the PD catheter, except for outcome data for which the unit of interest was the peritonitis episode. The time at risk for infection was calculated from the time of catheter insertion for incident patients or entry into the collaborative for prevalent patients, to catheter removal or last follow-up. For patients who contributed multiple catheters to the analysis, patient characteristics at the time of enrollment and clinical characteristics at the time of each catheter insertion were included in the analyses. Descriptive analyses included medians and interquartile ranges (IQRs) of continuous variables, and frequencies and percentages of categorical variables. Univariate comparisons of clinical variables between catheters with fungal peritonitis and those with bacterial or culture-negative peritonitis were made using the Chi-square/Fisher’s test for categorical variables and the Wilcoxon rank sum test for continuous variables.
Multivariable logistic regression was used to assess factors associated with the probability of developing FP and to compare the rates of FP with the rates of bacterial or culture-negative peritonitis. We fit generalized linear mixed models to account for potential correlation in outcomes for patients with multiple catheter insertions. We also fit a hospital-level effect to account for any clustering of outcomes seen in the same institution. Covariates included in the model were age at insertion, touch contamination prior to first FP infection and vesicostomy prior to first FP infection.
All analyses were conducted using SAS version 9.4 (SAS Institute, Inc., Cary, NC). Tests with a p value of < 0.05 were considered to be statistically significant.
Results
Patient characteristics
Among 994 patients on chronic PD contributing 337 PD catheter insertions who were enrolled into SCOPE during the study period (October 2011–September 2015) from 30 pediatric dialysis centers, there were 511 peritonitis episodes of which 41 (8%) were fungal and 470 (92%) were either bacterial or culture negative in origin. FP occurred in 36 individuals (median age at diagnosis of FP 2.5 years; IQR 0.5,11.5) in association with 39 unique PD catheters. Catheters among children aged < 2 years accounted for 59% of the FP episodes, with patients aged 6–12 years representing the next most frequently affected age group (24.9%). Of the 23 FP episodes that occurred in children whose catheters were placed at < 2 years of age, 21 (90%) of those episodes occurred in patients whose catheter was inserted at an age of < 1 year.
When isolating incident newborns who had their PD catheters placed during their initial hospitalization immediately after birth (n = 60), 32 (53.3%) catheters developed peritonitis during follow-up with four (12.5%) episodes of FP and 28 bacterial/culture-negative peritonitis episodes. Of the 32 peritonitis episodes, 18 (56.2%) occurred during the initial hospitalization prior to initiating home dialysis, with only one of those infections due to FP.
There was a fairly even distribution of FP episodes when the data were stratified by gender and race. Congenital anomalies of the kidney and urinary tract (CAKUT) represented the predominant cause of ESRD among those patients who developed FP as well as bacterial/culture-negative peritonitis, followed by focal segmental glomerulosclerosis (Table 1).
Risk factors for developing fungal peritonitis
In the univariate analysis, the FP group had a significantly higher proportion of patients who were < 2 years of age at the time of PD catheter insertion compared to the group with bacterial or culture-negative peritonitis (p = 0.03). However, a comparison of the two cohorts revealed no differences in PD catheter adapter type, orientation of PD catheter exit site, recent touch contamination or presence of a gastrostomy tube, colostomy, vesicostomy or urinary stoma (Table 2).
In the multivariable model, PD catheter insertion at age < 2 years was independently associated with a greater odds of FP [odds ratio (OR) 2.8; 95% confidence inteval (CI) 1.24, 6.31) after adjustment for clustering, touch contamination and vesicostomy (Table 3).
Survey results
Of the 41 surveys that were distributed, 34 (82.9%) were completed. Of the 34 FP episodes about which information was available, antibiotic therapy for any reason preceded 21 (61.8%) of the FP episodes within 30 days prior to diagnosis. Fungal prophylaxis was prescribed in 52.4% of instances in which antibiotics were administered. The most common antifungal agents used were nystatin (45.5%) and fluconazole (45.5%). Gentamicin (55.9%) followed by mupirocin (32.4%) were the most common antibiotic creams used with standard PD catheter dressing changes. When stratified by age (< or > 5 years), there was a higher percentage of patients aged < 5 years who did not receive fungal prophylaxis when antibiotics were administered within 30 days prior to the development of FP (57.1% < 5 years, 28.6% > 5 years; p = 0.22).
Temporal association with previous peritonitis episodes
Twenty Of the 41 FP episodes, 21 (48.8%) were the initial peritonitis episode for the patient. Seven of the remaining 21 (33.3%) FP episodes occurred within 30 days of a prior peritonitis episode. Of the seven FP episodes that were preceded by a previous peritonitis episode, three were secondary to Staphylococcus species, two were Gram negative (Escherichia coli and Haemophilus influenza), and one each was multi-organism and culture negative.
Peritonitis among incident catheter insertions
Of the 337 PD catheter insertions with peritonitis, 208 (61.7%) episodes occurred in incident patients. Of the 208 incident catheter insertions with peritonitis, 22 (10.6%) were fungal and 186 (89.4%) were bacterial/culture negative. Time to infection among incident patients for fungal peritonitis was 261.5 (IQR 134, 494) days compared to 144.5 (IQR 43, 346) among the bacterial/culture-negative peritonitis group (p = 0.064).
Outcomes of fungal peritonitis
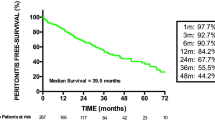
Patients with FP were hospitalized more frequently than patients with bacterial or culture-negative peritonitis (80.5 vs. 63.4%, respectively; p = 0.03) (Fig. 1). In fact, the development of FP was associated with a more than threefold increased risk for hospitalization (OR 3.13; 95% CI 1.08, 9.05; p = 0.04) compared to bacterial/culture-negative peritonitis after adjustment for age, sex and race (Table 4). Among patients who were hospitalized, those with FP had longer lengths of stay (median 8 days IQR 6, 21) than those patients who experienced bacterial/culture-negative peritonitis (median 5 days, IQR 3, 9; p < 0.001). FP was also associated with an increased risk of catheter removal (84.6 vs. 26.9%; p = 0.001) and technique failure requiring change in dialysis modality (68.3 vs. 8%; p = 0.001) (Fig. 1). There was no difference in mortality with one (2.6%) death observed in the FP group and seven (3.2%) deaths observed in the bacterial/culture-negative peritonitis group.
Discussion
Among a large pediatric cohort receiving chronic PD, we sought to identify specific risk factors for the development of FP as compared to the development of bacterial or culture-negative peritonitis. FP accounted for 8% of all peritonitis episodes over a 4-year period. Age of < 2 years was associated with an increased risk of developing FP compared with bacterial and culture-negative peritonitis, and FP was associated with increased rates and length of hospitalization, catheter removal and technique failure.
The frequency of FP in our pediatric PD cohort is second only to the Iranian experience [4] and almost three- to fourfold higher than the rates reported by large registries such as North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) [2] and the International Pediatric Peritonitis Registry (IPPR) [5] and data from the Netherlands [3], Korea [9] and Australia [10]. The reasons for the high incidence of FP in the patients enrolled in SCOPE are unclear. The inclusion of a more vulnerable population by including neonates who initiated PD in the hospital prior to being discharged home did not fully account for the increased incidence. We did find that more than one-half of patients who had their catheter placed during their initial hospitalization eventually developed peritonitis, with half of the peritonitis episodes (1 of which was FP) occurring prior to being discharged home. The high vulnerability of neonates receiving CPD for infection has recently been addressed in a SCOPE analysis by Zaritsky et al. [11] which demonstrated an annualized peritonitis rate of 1.73 for infants (aged< 1 year) during their initial hospitalization versus a rate of 0.46 over the course of the entire first year of dialysis Thus, while neonates who initiate dialysis during the hospitalization in which their PD catheter is placed are at increased risk for peritonitis, the risk is not specific to infections of fungal origin.
Age was an independent risk factor for the development of FP in our pediatric PD cohort. Younger age was also seen as a risk factor for FP in the NAPRTCS database [2] but not in the Iranian experience [4]. In the SCOPE collaborative, as well as in the NAPRTCS, age of < 2 years is also a risk factor for the development of any peritonitis episode, highlighting the fact that this patient population appears to be particularly vulnerable to infection-related complications [1]. In fact, of the 23 catheters inserted in patients aged < 2 years that were associated with an episode of FP, 20 were in children aged < 1 year. It is generally believed that bladder and bowel incontinence increases the risk of infection in the youngest population. Neonates, and especially preterm infants, may also be especially at risk for any infection as their immune system is not fully developed [12], and the performance of PD may be associated with low serum immunoglobulin G levels and an increased susceptibility for infection [13, 14].
Another hypothesis for age being a risk factor is the increased time on dialysis. Younger children are not large enough to receive a kidney transplant and, therefore, they have more exposure time to develop any infections, including FP. Among incident catheter insertions we did find that the time to development of the first fungal peritonitis episode was longer than that of bacterial/culture-negative peritonitis.
Recent antibiotic use, especially for the treatment of bacterial peritonitis, has historically been identified as an important risk factor for the development of FP [6]. Exposure to antibiotics is thought to eradicate normal fecal flora, allowing opportunistic organisms to invade the peritoneal cavity via transmural migration. From the NAPRTCS data, Warady et al. reported that 56% of patients who developed FP had exposure to antibiotics within 1 month prior to its development, with half of the antibiotic exposure due to treatment of bacterial peritonitis [2]. More striking is the Dutch and Iranian experiences. The study from the Netherlands demonstrated that 78% of the FP episodes were preceded by antibiotic exposure during the previous month, with 86% of the episodes of antibiotic exposure associated with bacterial peritonitis [3]. The Iranian study reported that all 16 cases of FP episodes were preceded by antibiotic exposure in the prior month [4]. Results on the adult experience is similar, with FP episodes preceded by antibiotic exposure within 3 months reported in 65–87% of the patients [14,15,16]. Our experience was very similar, with a high frequency of antibiotic exposure prior to FP, although only 17% of cases followed antibiotic treatment of peritonitis within the 30 days preceding FP. All of these data provide important evidence for pediatric dialysis programs that antibiotic exposure for treatment of any infection is an important risk factor for the development of FP. Interestingly, however, it is also imperative to recognize that close to 50% of our patients had FP as their first peritonitis episode, similar to what was previously reported by the NAPRTCS collaborative [2].
Gram-negative peritonitis specifically has also been reported to be associated with an increased risk for the development of fungal peritonitis [17]. Chou et al. reported in a retrospective review that 19% of the FP episodes in adults were preceded by peritonitis, with Gram-negative bacteria being the most common infecting organisms (42.1%). These results were confirmed by the Dutch pediatric experience in which they reported that 46% of the bacterial peritonitis episodes that preceded FP were due to Gram-negative organisms [3]. In contrast, we did not find a significant number of FP episodes that were preceded by another peritonitis episode within 30 days. We also did not find a significant difference in the frequency of Gram-positive vs. Gram-negative organisms for those episodes of bacterial peritonitis that preceded the development of FP. The hypothesis that either Gram-negative organisms or the antimicrobials utilized for its therapy portend an increased risk for development of FP clearly mandates further study.
The recommendation for fungal prophylaxis when antibiotic therapy is administered to patients receiving CPD remains controversial. To date, two randomized studies in adults have evaluated the use of fungal prophylaxis to prevent FP. Lo et al. [18] randomized patients to receive oral nystatin four times daily or no prophylaxis when antibiotics were prescribed for any reason. The nystatin group showed a reduction in the rate of Candida peritonitis (1.9/100 vs. 6.4/100; p < 0.05). However, antibiotics did not precede all FP episodes, and no statistically significant difference was found between the groups with respect to the risk for antibiotic-related Candida peritonitis. In the second randomized control trial [19], patients in the intervention group were given oral fluconazole, 200 mg every other day, during the course of antibiotic therapy for PD catheter-related infections and were prospectively monitored for 30–150 days for the occurrence of FP. A total of 420 bacterial peritonitis and 52 exit site or tunnel infection episodes were randomized to either the intervention or the control arm. Compared with the control group, the intervention group experienced a significantly fewer number of FP episodes (3 vs. 15 episodes; p = 0.005). Based on these and other smaller studies with historical controls, the ISPD recommends that the use of oral nystatin or fluconazole be considered during antibiotic administration to reduce the risk of fungal peritonitis [7]. Although fungal prophylaxis was used 52.4% of the time when FP was preceded by any antibiotic use, based on our experience our study was not powered to provide any recommendation regarding the type or duration of fungal prophylaxis for patients on PD. Additional data on this subject from large databases such as SCOPE or the International Pediatric Peritoneal Dialysis Network (IPPN) are imperative because of the significant impact that FP has on patient outcomes.
Our outcome data is consistent with current knowledge that FP is associated with an increased risk for morbidity, including increased hospitalization, catheter removal and technique failure. This is consistent with the findings by Warady et al. from the NAPRTCS database [2], although Warady et al.’s study did demonstrate that 53% of patients were able to return to PD 6 months after their FP episode. Possible reasons for the high technique failure rate (68.3%) in our experience and the experiences of others may be the scarring of the peritoneum that results from the infection and precludes the ongoing performance of PD, or a resultant patient and/or provider preference to remain on hemodialysis subsequent to the PD catheter removal that took place as part of the treatment for FP. Our study did not delineate any additional specific reasons for technique failure in our cohort.
Fortunately, unlike the experience from adult studies where FP is associated with a high mortality rate (15–50%) [6], we did not find an increased risk of mortality among the FP group. Our experience is similar to that of other pediatric studies, and the difference may be due to increased co-morbidities and/or dialysis vintage of adult patients as compared to pediatric patients.
Our study does have a number of limitations. The reporting of peritonitis episodes in the SCOPE collaborative is voluntary; however, because it is a quality initiative of the participating sites, it is presumed that reporting is complete. We also did not collect specific fungal species data or the antifungal treatment regimen used when FP was diagnosed. These data will be collected by SCOPE for studies on future peritonitis episodes. In contrast, the strength of our study lies in the fact that the SCOPE collaborative provides prospective data collected from a large number of pediatric dialysis centers across North America and from patients immediately following PD catheter insertion and prior to the initiation of home PD. We also used the well-established ISPD definition of peritonitis to identify episodes of peritonitis. This approach permits a better understanding of all PD-related exposures and outcomes.
In conclusion, the frequency of FP in participating sites of the SCOPE collaborative was greater than that in other pediatric experiences, with the majority of the episodes occurring in the youngest age group and often presenting as the initial episode of peritonitis. The fact that the majority of FP episodes occurred after antibiotic exposure for any reason needs to be recognized when considering potential prophylactic strategies for infection prevention. Further prospective study of patients experiencing FP as part of the SCOPE collaborative, as well as other multicenter registries, is also necessary to better identify additional potential mitigating factors that result in the reduction FP peritonitis episodes in children and maintenance of PD as a viable bridge to transplantation.
References
Sethna CB, Bryant K, Munshi R, Warady BA, Richardson T, Lawlor J, Newland JG, Neu A, Investigators S (2016) Risk factors for and outcomes of catheter-associated peritonitis in children: the SCOPE collaborative. Clin J Am Soc Nephrol 11:1590–1596
Warady BA, Bashir M, Donaldson LA (2000) Fungal peritonitis in children receiving peritoneal dialysis: a report of the NAPRTCS. Kidney Int 58:384–389
Raaijmakers R, Schröder C, Monnens L, Cornelissen E, Warris A (2007) Fungal peritonitis in children on peritoneal dialysis. Pediatr Nephrol 22:288–293
Hooman N, Madani A, Sharifian Dorcheh M, Mahdavi A, Derakhshan A, Gheissari A, Esfahani ST, Otukesh H, Mohkam M, Falahzadeh MH, Hosseini Al Hashemi G, Azir A, Merikhi A, Golikhani F, Latif E, Karimi S, Zakavat T, Mohseni P, Ataei N, Nickavar A, Basiratnia M (2007) Fungal peritonitis in Iranian children on continuous ambulatory peritoneal dialysis: a national experience. Iran J Kidney Dis 1:29–33
Schaefer F, Feneberg R, Aksu N, Donmez O, Sadikoglu B, Alexander SR, Mir S, Ha IS, Fischbach M, Simkova E, Watson AR, Möller K, von Baum H, Warady BA (2007) Worldwide variation of dialysis-associated peritonitis in children. Kidney Int 72:1374–1379
Matuszkiewicz-Rowinska J (2009) Update on fungal peritonitis and its treatment. Perit Dial Int 29[Suppl 2]:S161–S165
Warady BA, Bakkaloglu S, Newland J, Cantwell M, Verrina E, Neu A, Chadha V, Yap HK, Schaefer F (2012) Consensus guidelines for the prevention and treatment of catheter-related infections and peritonitis in pediatric patients receiving peritoneal dialysis: 2012 update. Perit Dial Int 32[Suppl 2]:S32–S86
Neu AM, Miller MR, Stuart J, Lawlor J, Richardson T, Martz K, Rosenberg C, Newland J, McAfee N, Begin B, Warady BA, Participants SC (2014) Design of the standardizing care to improve outcomes in pediatric end stage renal disease collaborative. Pediatr Nephrol 29:1477–1484
Lee KO, Park SJ, Kim JH, Lee JS, Kim PK, Shin JI (2013) Outcomes of peritonitis in children on peritoneal dialysis: a 25-year experience at severance hospital. Yonsei Med J 54:983–989
Bordador EB, Johnson DW, Henning P, Kennedy SE, McDonald SP, Burke JR, McTaggart SJ, Registry AaNZDaT (2010) Epidemiology and outcomes of peritonitis in children on peritoneal dialysis in Australasia. Pediatr Nephrol 25:1739–1745
Zaritsky JJ, Hanevold C, Quigley R, Richardson T, Wong C, Ehrlich J, Lawlor J, Rodean J, Neu A, Warady BA, SCOPE Investigators (2017) Epidemiology of peritonitis following maintenance peritoneal dialysis catheter placement during infancy: a report of the SCOPE collaborative. Pediatr Nephrol. https://doi.org/10.1007/s00467-017-3839-5
Ygberg S, Nilsson A (2012) The developing immune system—from foetus to toddler. Acta Paediatr 101:120–127
Lalan S, Dai H, Warady BA (2017) Hypogammaglobulinemia in infants receiving chronic peritoneal dialysis. Pediatr Nephrol 32:503–509
Goldie SJ, Kiernan-Tridle L, Torres C, Gorban-Brennan N, Dunne D, Kliger AS, Finkelstein FO (1996) Fungal peritonitis in a large chronic peritoneal dialysis population: a report of 55 episodes. Am J Kidney Dis 28:86–91
Prasad KN, Prasad N, Gupta A, Sharma RK, Verma AK, Ayyagari A (2004) Fungal peritonitis in patients on continuous ambulatory peritoneal dialysis: a single centre Indian experience. J Inf Secur 48:96–101
Wang AY, Yu AW, Li PK, Lam PK, Leung CB, Lai KN, Lui SF (2000) Factors predicting outcome of fungal peritonitis in peritoneal dialysis: analysis of a 9-year experience of fungal peritonitis in a single center. Am J Kidney Dis 36:1183–1192
Chou CY, Kao MT, Kuo HL, Liu JS, Liu YL, Huang CC (2006) Gram-negative and polymicrobial peritonitis are associated with subsequent fungal peritonitis in CAPD patients. Perit Dial Int 26:607–608
Lo WK, Chan CY, Cheng SW, Poon JF, Chan DT, Cheng IK (1996) A prospective randomized control study of oral nystatin prophylaxis for Candida peritonitis complicating continuous ambulatory peritoneal dialysis. Am J Kidney Dis 28:549–552
Restrepo C, Chacon J, Manjarres G (2010) Fungal peritonitis in peritoneal dialysis patients: successful prophylaxis with fluconazole, as demonstrated by prospective randomized control trial. Perit Dial Int 30:619–625
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The Institutional Review Board (IRB) at each participating center approved the collaborative protocol and informed consent was obtained where required by the institution’s IRB.
Conflict of interest
The authors declare no conflict of interest.
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Munshi, R., Sethna, C.B., Richardson, T. et al. Fungal peritonitis in the Standardizing Care to Improve Outcomes in Pediatric End Stage Renal Disease (SCOPE) Collaborative. Pediatr Nephrol 33, 873–880 (2018). https://doi.org/10.1007/s00467-017-3872-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-017-3872-4