Abstract
Background
Causes of non-resuming peritoneal dialysis (PD) after complicated peritonitis requiring peritoneal catheter (PC) removal remain poorly studied.
Methods
We reviewed all peritonitis episodes in our center between 1997 and 2017. Patients who restarted PD after PC removal (Group 1) were compared to those who did not (Group 2), identifying the causes.
Results
Of 284 peritonitis episodes, PC was removed in 48 patients (16.9%). In 18 (37.5%) patients PC was reinserted, and PD successfully resumed in all, with a median duration of PD afterwards of 14.1 months. In other 30 (62.5%) reinsertion of PC was not attempted. Causes of non-reinsertion were: transfer to hemodialysis 76.6% (n = 23), death 16.7% (n = 5) and transplantation 6.7% (n = 2). Hemodialysis switch was due to non-medical reasons in 47.8% (n = 11) including fear of peritonitis, family decision and social dependence. Group 1 was younger (p = 0.041), with lower Charlson index (p = 0.045) and higher men proportion (p = 0.049). Group 1 had a better patient survival than group 2 (survival at 24 months: 67% and 53%, respectively; log-rank test p: 0.01). There were no differences in survival between groups when adjusted for significant basal characteristics.
Conclusions
Resuming PD after severe peritonitis requiring PC removal is feasible but a high proportion of patients do not restart PD for non-medical reasons, usually older patients with higher Charlson index. A properly structured interview would be a useful tool that could improve return to technique in these patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Despite advances in peritoneal dialysis (PD) aimed at reducing the incidence of infectious complications [1,2,3], peritonitis remains an important cause of morbidity and mortality [4, 5] and a major cause for permanent switch from PD to hemodialysis (HD) [6, 7].
Although antibiotic therapy is usually effective, peritoneal catheter (PC) removal is necessary in up to 16–18% of peritonitis episodes due to complicated peritonitis not responding to antibiotic therapy [8, 9].
According to the International Society for Peritoneal Dialysis (ISPD) committee guidelines, PC removal is indicated in cases of refractory, relapsing and fungal peritonitis, as well as in cases of refractory exit-site and tunnel infection [10, 11]. PC removal may also be considered in cases of repeated peritonitis, mycobacterial and polymicrobial peritonitis. In line with international recommendations, PD can afterwards be resumed after a minimum of 2–3 weeks [12].
Unfortunately, previous studies have demonstrated that very few patients with a PD catheter removed due to peritonitis eventually return to PD and only half of them remain on PD after 2 years [13]. Enormous clinical, psychological, social and economic implications emerge from this fact.
While guidelines provide useful recommendations for PC removal and time of reinsertion, little is known about the outcomes of patients resuming PD under these circumstances. Furthermore, studies specifically aimed at identifying the causes of non-reinitiating PD following PC removal are lacking. The present study was designed to examine these unanswered issues.
Materials and methods
Case selection
Our center is a tertiary care teaching hospital attending a population of approximately 550,000 people in Madrid, Spain. From January 1997 to December 2017, both included, our unit had 332 patients on PD.
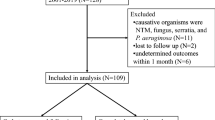
We retrospectively analyzed all PD peritonitis episodes treated in our center from January 1997 to December 2017. All cases in which the PC was removed due to peritonitis that did not respond to standard antimicrobial treatment were identified.
PD catheters removed for other reasons such as ultrafiltration failure, leakages and following successful renal transplantation were not included.
Patients’ demographic features and clinical parameters, including gender, cause of end-stage renal disease, comorbidities, age at the time of peritonitis, time spent on PD preceding peritonitis episode and causative organism, were obtained. All data were collected from our local computerized Renal Registry and from patient´s records.
Patient management
ISPD committee guidelines for diagnostic criteria and management of PD peritonitis, including catheter removal criteria were followed [10].
Peritonitis episodes were treated with our center standard antibiotic protocol, which included intraperitoneal vancomycin combined with aminoglycoside, tailoring to appropriate antimicrobial therapy following culture results. In general, antibiotics were maintained for 14–21 days depending on the organism and antifungal therapy for 4–6 weeks. One patient with tuberculous peritonitis was treated with antituberculous drugs following guidelines [13].
Catheter was removed only in cases of complicated peritonitis failing to respond to medical therapy, which included refractory, relapsing, recurrent and repeated peritonitis or in cases of fungal, mycobacterial and polymicrobial peritonitis.
Refractory peritonitis was considered as failure of peritoneal fluid clearance (dialysis effluent white cell count > 100/μL with > 50% polymorphonuclear) after 5 days of appropriate antibiotic treatment [10].
Relapse was defined as an episode of peritonitis that occurs within 4 weeks after completion of antibiotic therapy for a prior episode by the same organism whereas it was considered as recurrent peritonitis if the organism was different.
Repeated peritonitis was defined as a peritonitis that occurs more than 30 days after completion of therapy by the same organism that caused the prior episode, whereas it was considered as reinfection if the organism was different.
The timing of Tenckhoff catheter removal varied individually depending on the indication for removal. After Tenckhoff catheters were removed, appropriate antibiotic therapy was continued, and patients were switched to temporary hemodialysis.
After PC removal, patients and their families received structured information about renal replacement therapies and the possibility of reinitiating PD in a bespoke interview with a follow-up clinic to sort out patient´s questions and doubts. We implemented a specific questionnaire during the second interview to ask their reasons, as no standardized survey to assess this matter exists nowadays. All doubts and information given were recorded in our Renal Registry. With the only exception of fungal peritonitis, which is a contraindication for PD resumption in our Center, the decision whether to reinitiate PD was entirely up to the patient.
After patients had expressed willingness for PD, reinsertion of catheter was done after a minimum of 3 weeks after its removal, predominantly by laparoscopy. PD exchanges were initiated 14–21 days after placement of the new catheter.
Patients who refused reinitiating PD were enquired about the reasons of their decision in two structured interviews during follow-up and the information was written in patient´s records and our Renal Registry.
Clinical outcome
Patients who restarted PD after PC removal were identified (Group 1) and their demographic characteristics, comorbidities and clinical course were compared with those who did not restart (Group 2). Among the last group, causes of non-reinitiating PD were collected from a specific interview done at the time and written in their records (specific reason questionnaire).
Statistical analysis
Statistical analysis was performed by SPSS 21.0 for Windows (SPSS Inc., Chicago, IL, USA). Results were expressed as median and interquartile range for non-parametric continuous variables, and frequencies and percentage for categorical variables. Comparison of data between groups was performed using the χ2 test for categorical data and U test for continuous non-parametric data. Univariate and multivariate analyses were also done.
All probabilities were two tailed. p values < 0.05 were considered statistically significant. Survival curves were analyzed by the Kaplan–Meier method, compared by log rank test and a Cox proportional hazard model was performed.
Results
Demographics
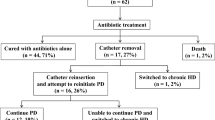
Of 284 peritonitis episodes during the study period, PC was removed in 48 patients due to peritonitis that did not respond to standard antimicrobial treatment, which accounted for 16.9% of all peritonitis episodes in our center. The overall peritonitis rate was 0.393 episodes per patient/year.
Of these, 18 (37.5%) resumed PD (Group 1) and 30 (62.5%) did not (Group 2).
The characteristics of the study cohort are shown in Table 1. The median age of the study cohort was 60 years [inter-quartile range (IQR) 47–70 years], and the median Charlson index was 5 (IQR: 2–7). Most patients were male (70.8%), non-diabetic and non-immunocompromised.
Chronic glomerulonephritis was the most prevalent cause of end-stage renal disease (31.3%), followed by diabetic kidney disease (12.5%), hypertensive disease (8.3%), polycystic kidney disease (8.3%) and unknown causes (21%).
Most patients (64.6%) were on continuous ambulatory PD and the median duration of PD before catheter removal was 24.5 months (IQR 14.2–40). The median number of peritonitis episodes prior to catheter removal was 2 (IQR 1–3).
The microbiologic agents isolated were: S. aureus (20.8%), Candida sp. (18.8%), P. aeruginosa (14.6%), other Gram-negative bacilli (22.9%), mixed growth (8.3%), Mycobacterium sp. (6.3%) and other Gram-positive (2.1%). Negative culture was found in 6.3%. Table 2 lists the causative organisms of peritonitis episodes.
Factors for successfully resuming PD and causes of non-resuming PD
When compared with Group 2, we found that Group 1 was younger (p = 0.041), had a majority of male patients (p = 0.049), and a lower Charlson index (p = 0.045).
There was no significant difference between the two groups in the underlying renal failure diagnosis or diabetic status. There was also no difference in terms of previous peritonitis episodes, initial choice of antibiotic regime, prevalence of associated exit-site infection or duration of PD prior to catheter removal. Except for fungus, organisms causing peritonitis did not predict failure of Tenckhoff reinsertion.
When we analyzed the causes of no reinsertion of PC in Group 2, we found that permanent HD switch was the main reason, involving 76.7% (n = 23) of patients. Permanent HD switch occurred due to non-medical reasons in 47.8% (n = 11), which included: fear of peritonitis (5 patients), family choice (4 patients) and social dependence (2 patients) (Table 3). Other causes of no reinsertion were death during peritonitis episode in 13.3% (4) and transplantation during peritoneal rest in 10% (3).
Patient and technique survival
Patients were followed for a median of 35 months (IQR 12–95) after catheter removal.
After catheter reimplantation, PD was resumed successfully in all patients in Group 1, with a median duration of PD afterwards of 14.1 months (range 4–69). The outcome of patients restarted on PD was: three died while on PD, eight had a kidney transplant, six transferred to HD and one remained on PD. Causes of death were: severe peritonitis, acute mesenteric ischemia and peritoneal sclerosis (Table 4).
On the other hand, outcomes of Group 2 were: 18 died on HD, 9 had a kidney transplant, 2 remained on HD and 1 got lost during follow-up period.
Patient survival is summarized in Fig. 1. Group 1 had a significantly better survival than group 2 (survival at 24 months was 67% and 53%, respectively; log rank test p: 0.01). When adjusted for statistically significant basal characteristic differences between the two groups (Cox proportional hazard model with gender, age and Charlson index), there were no differences in survival between the two groups with age being the only statistically significant factor associated with survival (p < 0.001).
The PD technique survival after PD catheter reinsertion by Kaplan–Meier estimate was 44% at 12 months.
Discussion
The results of the present study confirm that after an episode of peritonitis requiring PC removal, only few patients restart PD and even less remain on PD after two years. In our Center, 16.9% of peritonitis episodes resulted in PD catheter removal, concordant with previous studies [9, 14].
Among these patients, only 37.5% underwent catheter reimplantation. Our rate of catheter reinsertion is lower than that reported in Asian literature [13, 15]. This may be in part explained due to differences in practices in each treating center as well as diverse reimbursement policies between countries [16, 17]. For instance, in Hong Kong, CAPD is the first-line renal replacement therapy for all end-stage renal disease patients and therefore, in some Asian studies catheter reinsertion was attempted in all cases after its removal [9]. Conversely, in our program, the decision to reinitiate PD was almost entirely up to the patient with few exceptions, such as after fungal peritonitis.
Interestingly, our study showed psychological factors and not only medical reasons might play an important role in reinitiating PD and could prevent resumption of technique. Our results demonstrate that a high proportion of patients decide not to restart PD for non-medical reasons, which include fear of peritonitis, family choice and social dependence after this life-threatening complication. These patients were older and had more comorbidities, with patients and their families being more afraid of a repeated episode. Younger patients were more likely to be willing to restart PD, as were those with less comorbidities. We also identified that a higher proportion of males restart PD compared to females. This interesting finding might be related to a more conservative approach in women [18].
Once PD is reinitiated, previous studies identified severe peritonitis and increasing age as predictors of technique failure [19, 20]. Neither demographic nor clinical or dialysis-related data predicted PD resumption failure in our study.
It is also known that monomicrobial peritonitis caused by fungi, Enterobacteriaceae, Pseudomonas sp., and Staphylococcus aureus are associated with poorer outcomes and it has also been suggested that agents with aggressive and/or persistent infections (yeast and surgical enteric peritonitis) might prevent PD restart [21, 22]. In our study, these were the most frequent organisms cultured in patients requiring PC removal. Nevertheless, we did not find any association between the microbial cause of peritonitis and PD resumption in our patients. Although controversial, similar findings were reported by Ram et al. [23]. Our small sample size might have contributed to this surprising result.
In our study technique survival after PD resumption was 44% (8 out of 18) at the end of the first year in Group 1. PD technique survival after reinitiation differs widely in the literature: 42% at 12 months [24] and varying between 56 and 77% at 24 months in other studies [13, 20]. Such a large discrepancy may be the result of limitations in study designs (for instance: small sample size, retrospective design, single center data collection and a lack of adjustment for potential confounding variables).
In our study, patient survival in Group 1 was 67% (6 out of 18) after 2 years and it was better than that of Group 2 (Fig. 1), but when an adjusted model using statistically relevant baseline characteristics was performed that difference disappeared. In keeping with our results, Cho et al. found non-inferior patient, technique and peritonitis-free survival after returning to PD after temporary HD when compared with patients who never transferred to HD or those who remained permanently on HD post-peritonitis [14]. Therefore, we agree that resumption of PD after catheter removal for complicated peritonitis should not be discouraged.
There are numerous clinical implications in the present study.
First, it supports observations made by Szeto, regarding unknown important factors governing peritoneal adhesions in response to peritonitis, that need to be studied [13].
Second, it highlights the need to find predictors of survival and technique success after reinsertion of PD catheter. As mentioned by Sahu et al., until then, PD could successfully be resumed long term based on clinical assessment to select suitable patients [19].
Finally, a major finding is the causes of non-restarting PD, as they have not been described before in the literature. Our study showed that, after PC removal for complicated peritonitis, in almost half of the cases there is no medical reason to prevent PD resumption and non-reinitiation occurs mostly by patient or family choice.
We believe individual training support, improved home assessment and active family involvement are needed to successfully increase PD resumption [25]. Not only patients, but also their families should be counseled about the possibility of reinitiating PD. Advantages and disadvantages of technique should be discussed more than once during HD period, since many patients could change their minds once they have recovered physically and psychologically from peritonitis.
For elderly patients, who are less likely to resume PD because of age-related issues or decisions made by their families, training home-care nurses or family members to provide assistance with PD at home may be a desirable option [26].
This later finding has enormous clinical and economical implications, as it suggests that PD resumption rates could be improved if appropriate measures are taken. A specific clinic visit would clarify patient’s will and situation. Implementing a proper questionnaire to assess patient’s fears, doubts and desire regarding replacement technique to be completed by each patient would be a useful tool that could increase return to technique, reducing dropouts and costs.
There are several limitations to this study. First, the retrospective nature of the cohort, which may have biased results due to missing data, limiting interpretation and generalization of results. Second, there are no standardized questionnaires or scales to assess drop out causes, so we had to perform a bespoke interview with each patient and implement our own. Also, our sample size was relatively small and obtained from a single center, which limits statistical power. Further studies are required in this field before firm recommendations can be made.
Conclusions
To our knowledge, this is the first report examining the causes of non-reinitiating PD after a complicated peritonitis requiring catheter removal.
Our study demonstrated that successfully resuming PD following PC removal for severe peritonitis is feasible with reasonable PD technique survival. Most importantly, it reveals that in a high proportion of patients (mainly older and more comorbid) there is no medical reason to prevent PD resumption and non-reinitiation occurs mostly by patient or family choice. A properly structured interview and index scale would be useful tools that could improve return to technique in these patients.
References
Strippoli GFM, Tong A, Johnson D, Schena FP, Craig JC. Catheter-related interventions to prevent peritonitis in peritoneal dialysis: a systematic review of randomized controlled trials. J Am Soc Nephrol. 2004;15:2735–46.
Bernardini J. Training and retraining: impact on peritonitis. Perit Dial Int. 2010;30:434–6.
Montenegro J, Saracho R, Gallardo I, Martínez I, Muñoz R, Quintanilla N. Use of pure bicarbonate-buffered peritoneal dialysis fluid reduces the incidence of CAPD peritonitis. Nephrol Dial Transplant. 2007;22:1703–8.
Ye H, Zhou Q, Fan L, Guo Q, Mao H, Huang F, Yu X, Yang X. The impact of peritoneal dialysis-related peritonitis on mortality in peritoneal dialysis patients. BMC Nephrol. 2017;18:186.
Fontán MP, Rodríguez-carmona A, García-naveiro R, Rosales M, Villaverde P, Valdés F. Peritonitis-related mortality in patients undergoing chronic peritoneal dialysis. Perit Dial Int. 2005;25:274–84.
Van Biesen W, Veys N, Vanholder R, Lameire N. Peritoneal-dialysis-related peritonitis: the art of rope-dancing. Nephrol Dial Transplant. 2002;17:1878–82.
Digenis GE, Abraham G, Savin E, Blake P, Dombros N, Sombolos K, Vas S, Mathews R, Oreopoulos DG. Peritonitis-related deaths in continuous ambulatory peritoneal dialysis (CAPD) patients. Perit Dial Int. 1990;10:45–7.
Mujais S. Microbiology and outcomes of peritonitis in North America. Kidney Int Suppl. 2006;103:S55–62.
Choi P, Nemati E, Banerjee A, Preston E, Levy J, Brown E. Retrospective analysis of factors associated with catheter removal. Am J Kidney Dis. 2004;43:103–11.
Li PK, Szeto CC, Piraino B, De AJ, Fan S, Figueiredo AE, Fish DN, Goffin E, Kim YL, Salzer W, Struijk DG, Teitelbaum I, Johnson DW. ISPD peritonitis recommendations: 2016 update on prevention and treatment. Perit Dial Int. 2016;36:481–508.
Li PK, Szeto CC, Piraino B, Bernardini J, Figueiredo AE, Gupta A, Johnson DW, Kuijper EJ, Lye WC, Salzer W, Schaefer F, Struijk DG, International Society for Peritoneal Dialysis. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int. 2010;30:393–423.
Georges C. Catheter removal, adjunct therapies and timing of reinsertion of peritoneal dialysis catheter after peritonitis. The CARI Guidelines—caring for Australasians with renal impairment. 2013. www.cari.org.au.
Szeto C-C, Chow K-M, Wong TY-H, Leung C-B, Wang AY-M, Lui S-F, et al. Feasibility of resuming peritoneal dialysis after severe peritonitis and Tenckhoff catheter removal. J Am Soc Nephrol. 2002;13:1040–5.
Cho Y, Badve SV, Hawley CM, Mcdonald SP, Brown FG, Boudville N, Clayton P, Johnson DW. Peritoneal dialysis outcomes after temporary haemodialysis transfer for peritonitis. Nephrol Dial Transplant. 2014;29:1940–7.
Szeto CC, Lai KN, Yu AW, Leung CB, Ho KK, Maw TW, Li PK, Lam CW. Dialysis adequacy of Asian patients receiving small volume continuous ambulatory peritoneal dialysis. Int J Artif Organs. 1997;20:428–35.
Htay H, Cho Y, Pascoe EM, Darssan D, Nadeau-Fredette AC, Hawley C, Clayton PA, Borlace M, Badve SV, Sud K, Boudville N, McDonald SP, Johnson DW. Center effects and peritoneal dialysis peritonitis outcomes: analysis of a National Registry. Am J Kidney Dis. 2018;7:814–21.
Li PK, Szeto C. Success of the peritoneal dialysis programme in Hong Kong. Nephrol Dial Transplant. 2008;23:1475–8.
Ek S. Gender differences in health information behaviour: a Finnish population-based survey. Health Promot Int. 2015;30:736–45.
Cox SD, Walsh SB, Yaqoob MM, Fan SL. Predictors of survival and technique success after reinsertion of peritoneal dialysis catheter following severe peritonitis. Perit Dial Int. 2007;27:67–73.
Sahu KM, Walele A, Liakopoulos V, Bargman JM. Analysis of factors predicting survival of a second peritoneal dialysis catheter. Adv Perit Dial. 2003;19:252–4.
Pérez-Fontán M, Rodríguez-Carmona A. Peritoneal catheter removal for severe peritonitis: landscape after a lost battle. Perit Dial Int. 2007;27:155–8.
Yang C, Chen T, Lin Y, Lin C, Ng Y, Yang W, et al. Determinants of catheter loss following continuous ambulatory peritoneal dialysis peritonitis. Perit Dial Int. 2008;28:361–70.
Ram R, Swarnalatha G, Dakshinamurty KV. Reinitiation of peritoneal dialysis after catheter removal for refractory peritonitis. J Nephrol. 2014;27:445–9.
Troidle L, Gorban-Brennan N, Finkelstein FO. Outcome of patients on chronic peritoneal dialysis undergoing peritoneal catheter removal because of peritonitis. Adv Perit Dial. 2005;21:98–101.
Figueiredo AE, De MTP, Bernardini J, Poli-de-Figueiredo CE, Barretti P, Olandoski M, Pecoits-Filho R, BRAZPD Investigators. Impact of patient training patterns on peritonitis rates in a large national cohort study. Nephrol Dial Transplant. 2015;30:137–42.
Figueiredo AE, Bernardini J, Bowes E, Hiramatsu M, Price V, Su C, Walker R, Brunier G. A syllabus for teaching peritoneal dialysis to patients and caregivers. Perit Dial Int. 2016;36:592–605.
Author information
Authors and Affiliations
Contributions
CC-T data acquisition and analysis; RHS-B: supervision/mentorship and writing; VB-V: statistical analysis; MF-L: supervision; MR-G: research idea, study design, supervision/mentorship and writing. Each author contributed important intellectual content during manuscript drafting or revision, accepts personal accountability for the author’s own contributions, and agrees to ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
We have read and understood the journal policy on disclosing conflicts of interest and declare that we have none. None of the authors have any financial interests.
Human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional review board at which the studies were conducted (IRB approval number 153/19) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
As data were retrospectively collected and derived from routine clinical practice, further consents were waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Chediak Terán, C., Sosa Barrios, R.H., Burguera Vion, V. et al. Resuming peritoneal dialysis after catheter removal due to complicated peritonitis. Clin Exp Nephrol 24, 349–355 (2020). https://doi.org/10.1007/s10157-019-01833-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-019-01833-3