Abstract
Background
Hepatocyte nuclear factor 1β (HNF1B), located on chromosome 17q12, causes renal cysts and diabetes syndrome (RCAD). Moreover, various phenotypes related to congenital anomalies of the kidney and urinary tract (CAKUT) or Bartter-like electrolyte abnormalities can be caused by HNF1B variants. In addition, 17q12 deletion syndrome presents with multi-system disorders, as well as RCAD. As HNF1B mutations are associated with different phenotypes and genotype–phenotype relationships remain unclear, here, we extensively studied these mutations in Japan.
Methods
We performed genetic screening of RCAD, CAKUT, and Bartter-like syndrome cases. Heterozygous variants or whole-gene deletions in HNF1B were detected in 33 cases (19 and 14, respectively). All deletion cases were diagnosed as 17q12 deletion syndrome, confirmed by multiplex ligation probe amplification and/or array comparative genomic hybridization. A retrospective review of clinical data was also conducted.
Results
Most cases had morphological abnormalities in the renal–urinary tract system. Diabetes developed in 12 cases (38.7%). Hyperuricemia and hypomagnesemia were associated with six (19.3%) and 13 cases (41.9%), respectively. Pancreatic malformations were detected in seven cases (22.6%). Ten patients (32.3%) had liver abnormalities. Estimated glomerular filtration rates were significantly lower in the patients with heterozygous variants compared to those in patients harboring the deletion (median 37.6 vs 58.8 ml/min/1.73 m2; p = 0.0091).
Conclusion
We present the clinical characteristics of HNF1B-related disorders. To predict renal prognosis and complications, accurate genetic diagnosis is important. Genetic testing for HNF1B mutations should be considered for patients with renal malformations, especially when associated with other organ involvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In humans, the hepatocyte nuclear factor-1 beta (HNF1B) gene is located on chromosome 17q12 and the encoded protein contains an N-terminal dimerization domain, a homeobox and a POU A domain involved in DNA binding, and a transactivation domain at the C-terminus [1]. HNF1B was initially identified as a monogenic diabetes-related gene and the associated first mutation was described in a Japanese family with maturity-onset diabetes of the young (MODY) in 1997 [2]. The encoded protein is a transcription factor involved in the tissue-specific regulation of gene expression and the embryonic development of various organs including the liver, kidney, intestine, pancreas, and genitourinary system [3]. In addition, mutated HNF1B alleles are associated with a variety of disorders in renal development (congenital anomalies of the kidney and urinary tract, CAKUT) including solitary functioning kidney, renal dysplasia, glomerulocystic kidney disease, and oligomeganephronia [1, 4]. Moreover, mutations in HNF1B cause renal cysts and diabetes syndrome (RCAD, OMIM#137920).
HNF1B-associated disease is generally considered to exhibit autosomal-dominant inheritance; however, de novo mutations and whole-gene deletions account for up to 50% of cases [5]. Further, the phenotypes of HNF1B mutation-carriers are extremely variable and there is no clear evidence for a genotype–phenotype relationship [6]. As we believe that these studies are necessary for the treatment and prevention of complications associated with such diseases, we conducted extensive genetic/genotype–phenotype analysis on HNF1B mutations in a Japanese population.
Materials and methods
Subjects
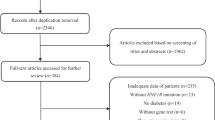
We analyzed HNF1B aberrations in 596 patients with clinically diagnosed CAKUT, Bartter-like syndrome, cystic kidneys, or an unknown cause of renal dysfunction from Sep 2010 to Dec 2018. A total 33 Japanese subjects with HNF1B mutations were recruited. The inclusion criterion was renal disease without a defined etiology. Details regarding renal disease, the presence of diabetes in the probands or a family member, and other clinical features were obtained from the referring clinician or the patient’s hospital records.
Clinical diagnosis
The clinical conditions of the patients in this study were evaluated by their primary doctors according to the following categories. CAKUT, pancreatic and hepatobiliary tract malformations were defined as any abnormalities in the imaging tests including ultrasonographic examination, X-ray fluoroscopic examination, computed tomography, or magnetic resonance imaging. Diabetes was diagnosed as patients with chronic hyperglycemia meeting the following criteria repeatedly: (i) fasting plasma glucose level of ≥ 126 mg/dl; (ii) 2-h value ≥ 200 mg/dl based on 75-g oral glucose tolerance test; (iii) casual plasma glucose level of ≥ 200 mg/dl [7]. Hypomagnesemia was defined as serum magnesium less than 1.4 mEq/l. Hyperuricemia was defined as uric acid greater than 7.0 mg/dl. Genital abnormalities and neurological abnormalities were diagnosed by primary doctors.
Genetic analysis
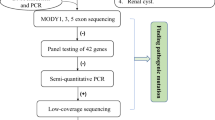
Genomic DNA was isolated from the peripheral blood leukocytes of patients and their family members using the Quick Gene Mini 80 system (Wako Pure Chemical Industries, Ltd., Tokyo, Japan), in accordance with the manufacturer’s instructions. Direct sequencing or targeted sequencing using next-generation sequencing (NGS) was conducted on genes responsible for inherited renal disease. NGS samples were prepared using a HaloPlex target enrichment system kit (Agilent Technologies, Santa Clara, CA, USA), in accordance with the manufacturer’s instructions. Briefly, 225 ng of genomic DNA was used for a restriction reaction and hybridized at 54 °C for 16 h with NGS probes. All indexed DNA samples were amplified by polymerase chain reaction (PCR) and sequenced using the MiSeq platform (Illumina, San Diego, CA). We analyzed the data using SureCall 4.0, which is a desktop application combining algorithms for end-to-end NGS data analysis, from alignment to the categorization of mutations (Agilent Technologies). To determine copy number changes in a sample relative to a reference without copy number alterations, we used pair analysis by SureCall [8].
Multiplex ligation probe amplification
We performed multiplex ligation probe amplification (MLPA) using SALSA P241, P357, or P463 for HNF1B, as suggested by the manufacturer (MRC-Holland, Amsterdam, Netherlands). The MLPA test was performed twice to confirm abnormal changes. Briefly, 50–100 ng of genomic DNA in 5 μl of deionized water was denatured and hybridized overnight with the probe mix. Ligation was performed with the SALSA Ligase 65 enzyme and finally PCR amplification was performed with the SALSA PCR Primer Mix. Amplification products and the Size Standard 600 were mixed thoroughly and subjected to capillary electrophoresis using the Gene Mapper v.3.7 (Thermo Fisher, Waltham, MA, USA).
Array comparative genomic hybridization (aCGH)
The Human Genome CGH Microarray (Agilent Technologies) was utilized and tests were carried out using the SurePrint G3 Human CNV Microarray 400 K Kit or SurePrint G3 Human CGH Microarray 400 K, 180 K, or 60 K Kit (Agilent Technologies), in accordance with the manufacturer’s instructions. Briefly, 1 μg of genomic DNA corresponding to either a human reference control (Promega) or test samples was fragmented by heating it at 95 °C for 10 min. Fragmented DNA was labeled with Cy3 (reference DNA) and Cy5 (test samples) fluorescent dUTPs, using the SureTag Complete Labeling Kit (Agilent Technologies). Purification columns (Agilent) were used to remove the unincorporated nucleotides and dyes. The labeled samples along with human Cot-1 DNA were added together and hybridized on the array slides. Hybridizations of labeled DNA to array slides were performed in a hybridization oven at 65 °C with 20 rpm for 24 or 40 h. The slide was scanned at a resolution of 3 μm on an Agilent SureScan Microarray Scanner (Agilent Technologies). Agilent CytoGenomics software (Agilent Technologies) was used to visualize, detect, and analyze chromosomal patterns within the microarray profiles.
Statistical analysis
Results are presented as the median and intermediate quartile range (IQR). Whenever applicable, the independent samples Mann–Whitney U test was used to compare median differences between two experimental groups. The Kruskal–Wallis test was used to analyze mean differences between more than three groups. Statistical analysis was performed using standard statistical software (JMP version 10 for Windows; SAS Institute, Cary, NC, USA). For all performed hypothesis tests, p < 0.05 was considered statistically significant.
Results (Table 1)
HNF1B mutations
Heterozygous HNF1B alterations, thought to be pathogenic, were identified in 33 patients from 23 families (15 male and 18 female). Among them, 14 cases (42.4%) had a 17q12 deletion and 19 (57.6%) had variants in HNF1B. Thirteen different heterozygous small mutations (six missense (46.2%), three nonsense (23.1%), one frameshift (7.7%), and three splice site mutations (23.1%)) were found in these 19 cases. Except for the frameshift, all missense mutations were localized to the DNA-binding domain (Fig. 1). Among the point mutations, eight of 14 (57.1%) were novel (SC57, SC292, SC323, SC339, SC355, SC392, SC418, and SC487). A family history of a renal, pancreatic, liver, and/or genital tract abnormalities, suggestive of dominant inheritance, was reported in 11/23 cases (47.8%).
Clinicopathological characteristics of the kidneys
Most cases had morphological abnormalities in the renal–urinary tract system. Twenty-four had cystic kidneys, four of which had unilateral multi-cystic dysplastic kidneys. Nine cases had hypoplasia, and three had hydronephrosis and urinary tract abnormalities. One case had renal absence and one case had renal calcification.
One case progressed to stage-5 chronic kidney disease (CKD) during childhood, and renal transplantation commenced at the age of three. The eGFR (estimated glomerular filtration rate) was greater than 90 ml/min/1.73 m2 (CKD stage 1) in four of 28 evaluable patients (13.8%), 60–89 ml/min/1.73 m2 (CKD stage 2) in six (20.7%), 30–59 ml/min/1.73 m2 (CKD stage 3) in 14 (48.3%), and less than 30 ml/min/1.73 m2 (CKD stage 4 or 5) in four (13.7%) (Table 2). Thirteen patients (41.9%) had low serum Mg and six patients (19.4%) had hyperuricemia. Hyperparathyroidism occurred in five of seven patients for whom intact-PTH values were available.
Extra-renal symptoms
Twelve of 31 patients (38.7%) developed diabetes. Pancreatic malformations were detected in seven cases (22.6%), 10 (32.3%) had liver abnormalities, and seven patients (22.6%) had elevated concentrations of transaminase. Two cases had gallstones (the mother of SC448 and B222), and one case had gallbladder morphological abnormalities and cysts (SC226) [9]. Two of eighteen women (11.1%) had confirmed genital abnormalities; one had bicornate uterus and the other had cervical carcinoma. However, abnormal male genitalia were not detected. There was no case of esophageal malformations. Only one case was suspected pigmentary retinal degeneration and this patient had an arachnoid cyst.
Five patients had neurological complications, and all harbored a 17q12 deletion. One patient was reported to have autism and also had facial dysmorphism and joint contracture. Of the remaining patients, one had schizophrenia and three had intellectual disabilities.
HNF1B scores
The HNF1B score is calculated based on 17 items including antenatal discovery, family history, and organ involvement (kidney, pancreas, liver, and genital tract). It is a simple tool to provide a more rational approach to select patients for HNF1B screening [10]. The median HNF1B score was 14 (IQR: 10.5–17). Four individuals had a score below 8 and these individuals all harbored a heterozygous deletion of the entire HNF1B gene.
Genotype–phenotype correlations
When data were classified by mutation, eGFR levels were significantly lower in the patients who carried a heterozygous variant compared to those in patients who harbored a deletion, although the mean age for cases with a deletion was greater than that for patients with a gene variant (median 37.6 ml/min/1.73 m2 vs 58.8 ml/min/1.73 m2, p = 0.0091; Fig. 2). eGFR levels were associated either with deletions, missense mutations, or truncating mutations (median 58.8 ml/min/1.73 m2 vs 35.2 ml/min/1.73 m2 vs 39.45 ml/min/1.73 m2; Fig. 3).
Comparison of age and estimated glomerular filtration rate (eGFR) between groups harboring HNF1B deletions and variants. Patients with heterozygous variants were younger than those with gene deletions (median 11 years vs 32 years, p = 0.005) (a). Difference in eGFR between variant and deletion groups. eGFR levels were significantly lower in the group harboring variants (b)
Comparison of age and estimated glomerular filtration rate (eGFR) among the groups harboring deletions, missense mutations, and truncating variants. Patients with missense and truncating mutations were younger than those with gene deletions (a); deletion: age 32 (19.5–45) years, missense: age 2.5 (0.5–10.75) years, truncating: age 12 (8.5–21) years. Type of HNF1B mutation (deletion, missense, truncating) according to the GFR (b); truncating vs missense: p = 0.0338, missense vs deletion: p = 0.0073, truncating vs deletion: p = 0.0486
Regarding other clinical characteristics (Table 3), patients who carried a deletion had higher frequencies of hypomagnesemia (p = 0.0002) and neurological complications (p = 0.0071) than those who harbored variants.
Discussion
HNF1B mutations are associated with RCAD, which comprises renal cystic dysplasia, MODY, and hepatic, genital, and pancreatic abnormalities, with variabilities in renal and extra-renal defects [11, 12]. The phenotypes of HNF1B mutation-carriers are extremely variable and there is no clear evidence for a genotype–phenotype relationship [11]. Here, we described a large national cohort of 33 phenotyped patients with HNF1B mutations. From the Human Gene Mutation Database (HGMD, https://www.hgmd.cf.ac.uk) based on 240 mutation-positive cases, gross deletions (19.2%), missense or nonsense mutations (47.9%), splice-site mutations (7.1%), small deletions (14.6%), small insertions (7.1%), and indels (0.4%) were reported. In our cohort, we identified gross deletions (42.4%), missense or nonsense mutations (39.4%), small deletions or insertions (3.0%), and splice-site mutations (15.2%), indicating similar trends; however, our groups had a slightly larger number of large deletions and splice site mutations.
HNF1B is expressed in both maturing human collecting ducts and nephrons [13]. An Hnf1b deletion in mouse collecting ducts causes cysts [14, 15]. Furthermore, Hnf1b upregulates the transcription of uromodulin (Umod), polycystic kidney and hepatic disease 1 (Pkhd1), and polycystic kidney disease (Pkd2), the human homologues of which are respectively mutated in medullary cystic kidney disease type 2, autosomal recessive polycystic kidney disease, and a subset of autosomal dominant polycystic kidney disease [15]. Previous reports suggest that renal malformations are the most common manifestation of HNF1B mutations. In fact, most cases in our cohort had renal morphological abnormalities. This is a convincing result from an embryological point of view.
In contrast, progression to end-stage kidney disease (ESKD) in the patients with HNF1B mutations seems rare [4]. In a larger cohort of 71 live births, only one was reported to have reached ESKD (age 3 months) [16]. However, in our report, one patient progressed to CKD stage 5 during childhood with eGFR levels below 90 ml/min/1.73 m2 in 24 (86.2%). These levels were significantly lower in patients who harbored a heterozygous variant compared to those in patients with a deletion (median 37.6 ml/min/1.73 m2 vs 58.8 ml/min/1.73 m2, p = 0.0091). A recent multicenter retrospective cohort study reported that patients with HNF1B mutations have poorer renal prognosis than those with a whole gene deletion, as evidenced by lower eGFR at follow-up and a higher frequency of CKD3–4 or ESKD [17]. Since our facility deals with hereditary renal disease, there is a possibility that patients with poor kidney function are overrepresented. However, it might also demonstrate the dominant-negative effect on kidney function.
Despite its initial identification as a diabetes-related gene, HNF1B variants also comprise a rare cause of MODY, accounting for < 2% of cases, compared to ~ 60% of cases attributed to HNF1A variants [18]. Indeed, in our cohort, nine patients had diabetes. HNF1B mutations are not usually associated with diabetes in childhood. However, diabetes typically manifests in the third or fourth decade of life [19]. In our study, onset occurred in teenagers in two cases, whereas the others were greater than 20 years of age. Previously, in a different cohort of 21 patients, diabetes occurred in approximately one quarter of patients during childhood, with the age at onset between 10 and 14 years [20]. We suggest that these variations depend on the age at the time of diagnosis.
In this study, hyperuricemia, hypomagnesemia and hyperparathyroidism were identified in six, 13, and five patients, respectively. HNF1B regulates the transcription of UMOD, which plays a role in renal urate transport, FXYD2, which plays a role in transcellular magnesium reabsorption, and PTH, which encodes parathyroid hormone [21]. Therefore, these findings are important for patients with HNF1B mutations. However, all patients with hyperuricemia or hyperparathyroidism had CKD higher than stage 3, and thus, renal dysfunction also might affect hyperuricemia and hyperparathyroidism in this study.
Recently, a more complicated score was developed based on 17 parameters, with renal hyper-echogenicity and/or cysts and genital and pancreatic abnormalities scoring highest, followed by other features such as abnormal fetal renal ultrasound, positive family history, renal hypoplasia or dysplasia, and hypomagnesaemia. Using this score, the authors achieved a sensitivity of 98.2% and a specificity of 41.1% [10]. In our cohort, four cases had a score less than 8 and all of these were deletion cases. Thus, we believe that this screening method is useful before conducting genetic analysis, but is not perfect.
Based on phenotype–genotype correlations, patients with deletions were associated with hypomagnesemia and neurological complications. Therefore, in such cases, genetic testing that takes into account large deletions should be performed.
This study had several limitations. First, our study population was small, and it was a retrospective study. Second, the examination of individual patients relied entirely on the clinicians’ decisions, which implies that not all patients were evaluated for structural brain malformations or genital anomalies; lastly, most professional educational evaluations were not accessible.
Conclusion
In this study, we compared the genotypes and clinical findings for HNF1B mutations in Japanese individuals. Although renal symptoms are apparently the most common, we found that it is also necessary to consider other symptoms. Systematic screening for all potential abnormalities should be performed to better determine the frequency and characteristics of these complications. In the routine clinic, pediatrician and clinician awareness of these complications is important. To better understand this rare disease and develop potential interventions, good clinical registries are also needed.
References
Bohn S, Thomas H, Turan G, Ellard S, Bingham C, Hattersley AT, Ryffel GU. Distinct molecular and morphogenetic properties of mutations in the human HNF1beta gene that lead to defective kidney development. J Am Soc Nephrol. 2003;14:2033–41.
Ellard S, Bellanne-Chantelot C, Hattersley AT, European Molecular Genetics Quality Network Mg. Best practice guidelines for the molecular genetic diagnosis of maturity-onset diabetes of the young. Diabetologia. 2008;51:546–53.
Igarashi P, Shao X, McNally BT, Hiesberger T. Roles of HNF-1beta in kidney development and congenital cystic diseases. Kidney Int. 2005;68:1944–7.
Bockenhauer D, Jaureguiberry G. HNF1B-associated clinical phenotypes: the kidney and beyond. Pediatr Nephrol. 2016;31:707–14.
Bellanne-Chantelot C, Chauveau D, Gautier JF, Dubois-Laforgue D, Clauin S, Beaufils S, Wilhelm JM, Boitard C, Noel LH, Velho G, Timsit J. Clinical spectrum associated with hepatocyte nuclear factor-1beta mutations. Ann Intern Med. 2004;140:510–7.
El-Khairi R, Vallier L. The role of hepatocyte nuclear factor 1beta in disease and development. Diabetes Obes Metab. 2016;18(Suppl 1):23–322.
Seino Y, Nanjo K, Tajima N, Kadowaki T, Kashiwagi A, Araki E, Ito C, Inagaki N, Iwamoto Y, Kasuga M, Hanafusa T, Haneda M, Ueki K. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. Diabetol Int. 2010;1:2–20.
Nagano C, Nozu K, Morisada N, Yazawa M, Ichikawa D, Numasawa K, Kourakata H, Matsumura C, Tazoe S, Tanaka R, Yamamura T, Minamikawa S, Horinouchi T, Nakanishi K, Fujimura J, Sakakibara N, Nozu Y, Ye MJ, Kaito H, Iijima K. Detection of copy number variations by pair analysis using next-generation sequencing data in inherited kidney diseases. Clin Exp Nephrol. 2018;22:881–8.
Kanda S, Morisada N, Kaneko N, Yabuuchi T, Nawashiro Y, Tada N, Nishiyama K, Miyai T, Sugawara N, Ishizuka K, Chikamoto H, Akioka Y, Iijima K, Hattori M. New-onset diabetes after renal transplantation in a patient with a novel HNF1B mutation. Pediatr Transplant. 2016;20:467–71.
Faguer S, Chassaing N, Bandin F, Prouheze C, Garnier A, Casemayou A, Huart A, Schanstra JP, Calvas P, Decramer S, Chauveau D. The HNF1B score is a simple tool to select patients for HNF1B gene analysis. Kidney Int. 2014;86:1007–155.
Edghill EL, Bingham C, Ellard S, Hattersley AT. Mutations in hepatocyte nuclear factor-1beta and their related phenotypes. J Med Genet. 2006;43:84–90.
Madariaga L, Moriniere V, Jeanpierre C, Bouvier R, Loget P, Martinovic J, Dechelotte P, Leporrier N, Thauvin-Robinet C, Jensen UB, Gaillard D, Mathieu M, Turlin B, Attie-Bitach T, Salomon R, Gubler MC, Antignac C, Heidet L. Severe prenatal renal anomalies associated with mutations in HNF1B or PAX2 genes. Clin J Am Soc Nephrol. 2013;8:1179–87.
Haumaitre C, Fabre M, Cormier S, Baumann C, Delezoide AL, Cereghini S. Severe pancreas hypoplasia and multicystic renal dysplasia in two human fetuses carrying novel HNF1beta/MODY5 mutations. Hum Mol Genet. 2006;15:2363–75.
Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, Yaniv M, Pontoglio M. Defective planar cell polarity in polycystic kidney disease. Nat Genet. 2006;38:21–3.
Gresh L, Fischer E, Reimann A, Tanguy M, Garbay S, Shao X, Hiesberger T, Fiette L, Igarashi P, Yaniv M, Pontoglio M. A transcriptional network in polycystic kidney disease. EMBO J. 2004;23:1657–68.
Heidet L, Decramer S, Pawtowski A, Moriniere V, Bandin F, Knebelmann B, Lebre AS, Faguer S, Guigonis V, Antignac C, Salomon R. Spectrum of HNF1B mutations in a large cohort of patients who harbor renal diseases. Clin J Am Soc Nephrol. 2010;5:1079–90.
Dubois-Laforgue D, Cornu E, Saint-Martin C, Coste J, Bellanne-Chantelot C, Timsit J. Monogenic Diabetes Study Group of the Societe Francophone du, diabete diabetes, associated clinical spectrum, long-term prognosis, and genotype/phenotype correlations in 201 adult patients with hepatocyte nuclear factor 1B (HNF1B) molecular defects. Diabetes Care. 2017;40:1436–43.
Ryffel GU. Mutations in the human genes encoding the transcription factors of the hepatocyte nuclear factor (HNF)1 and HNF4 families: functional and pathological consequences. J Mol Endocrinol. 2001;27:11–29.
Clissold RL, Hamilton AJ, Hattersley AT, Ellard S, Bingham C. HNF1B-associated renal and extra-renal disease-an expanding clinical spectrum. Nat Rev Nephrol. 2015;11:102–12.
Adalat S, Woolf AS, Johnstone KA, Wirsing A, Harries LW, Long DA, Hennekam RC, Ledermann SE, Rees L, van't Hoff W, Marks SD, Trompeter RS, Tullus K, Winyard PJ, Cansick J, Mushtaq I, Dhillon HK, Bingham C, Edghill EL, Shroff R, Stanescu H, Ryffel GU, Ellard S, Bockenhauer D. HNF1B mutations associate with hypomagnesemia and renal magnesium wasting. J Am Soc Nephrol. 2009;20:1123–31.
Verhave JC, Bech AP, Wetzels JF, Nijenhuis T. Hepatocyte nuclear factor 1β-associated kidney disease: more than renal cysts and diabetes. J Am Soc Nephrol. 2016;27:345–53.
Ishiwa S, Sato M, Morisada N, Nishi K, Kanamori T, Okutsu M, Ogura M, Sako M, Kosuga M, Kamei K, Ito S, Nozu K, Iijima K, Ishikura K. Association between the clinical presentation of congenital anomalies of the kidney and urinary tract (CAKUT) and gene mutations: an analysis of 66 patients at a single institution. Pediatr Nephrol. 2019. https://doi.org/10.1007/s00467-019-04230-w.
Ohara Y, Okada Y, Yamada T, Sugawara K, Kanatani M, Fukuoka H, Hirota Y, Maeda T, Morisada N, Iijima K, Ogawa W. Phenotypic differences and similarities of monozygotic twins with MODY5. J Diabetes Investig. 2019. https://doi.org/10.1111/jdi.13004 (Epub ahead of print).
Acknowledgements
The authors thank all study participants and their families. We are profoundly grateful to Mrs. Tetsuko Yamanouchi, Yoshimi Nozu, and Ming Juan Ye (Kobe University) for their technical assistance. We would like to thank Editage (https://www.editage.jp) for English language editing. Data for SC57, SC226, SC292, SC376, and SC412 patients were published elsewhere [9, 22, 23]. This work was supported by the Health Labor Sciences Research Grant for the Research on Measures for Intractable Diseases (H24-nanchi-ippan-041 to K.I.; H29-nanchi-ippan-039 to N.M.) and Japan Society for the Promotion of Science (KAKENHI Grant numbers JP15K09261 and 18K08243 to N.M.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
K.I has received grant support from Daiichi Sankyo CO., Ltd. and Zenyaku Kogyo Co., Ltd.
Ethical approval
All procedures performed for studies involving human participants were in accordance with the ethical standards of the Institutional Review Board of Kobe University Graduate School of Medicine (IRB approval number 301) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Nagano, C., Morisada, N., Nozu, K. et al. Clinical characteristics of HNF1B-related disorders in a Japanese population. Clin Exp Nephrol 23, 1119–1129 (2019). https://doi.org/10.1007/s10157-019-01747-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-019-01747-0