Abstract
Objective
Using a single-center cohort of Japanese patients with SLE, we attempted to clarify the long-term outcome and factors associated with damage accrual using the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SDI).
Methods
We examined a cohort of 557 patients who had been referred to Niigata University Hospital and diagnosed as having SLE between 1961 and 2013. The patients’ data at the latest visit were collected from their clinical records, and causes of death were defined on the basis of those data. Survival from the time of diagnosis was calculated by the Kaplan–Meier method. The SDI was calculated and analyzed using Spearman’s correlation coefficient and stepwise multiple regression analysis to reveal the factors associated with any organ damage.
Results
Data from 458 of the patients were successfully obtained. The overall 5-year survival rate was 92.2%, and patients diagnosed after 2000 had a significantly high 5-year survival rate of 96.4%. Stepwise multiple regression analysis selected serum creatinine levels (B = 0.6051, p < 0.0001), age (standardized beta = 0.2762, p < 0.001), hypertension (standardized beta = 0.2267, p < 0.001), and antiphospholipid antibody syndrome (standardized beta = 0.1533, p = 0.005) as positive independent variables, whereas administration of bisphosphonate (standardized beta = − 0.1295, p = 0.016) was selected as a negative independent variable.
Conclusion
These results suggest that Japanese patients with SLE have a favorable long-term prognosis, and also indicate that disease control as well as management of chronic complications such as hypertension and osteoporosis has possible effects for prevention of organ damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic lupus erythematosus (SLE) is a chronic, multisystemic autoimmune disease affecting various organs, and principally it can be severe and fatal. However, earlier diagnosis and more appropriate treatment strategies have dramatically improved the 5-year survival rate of patients with SLE from 50% to over 90% over the last several decades, and the 10-year survival rate is also now reported to be nearly 90% [1].
In patients who survive longer than 10 years, the major cause of death has become not simply active SLE itself, and recently the management of SLE has been aimed at not only remission induction and control of the disease activity, but also long-term prevention of chronic organ damage resulting from disease complications and the side effects of treatment. In SLE patients, organ damage has been assessed using the Systemic Lupus International Collaborating Clinics (SLICC)/American College of Rheumatology (ACR) Damage Index (SDI) [2, 3]. The score tends to increase over time [4,5,6], and the majority of SLE patients will accrue damage during their clinical course because of many factors such as older age at onset, high disease activity, and long-term adverse events related to medication [5,6,7,8,9,10,11].
Previous studies have revealed that black, Hispanic, and Asian patients have more severe disease expression and a higher disease burden than Caucasians, and show generally lower long-term survival [12,13,14,15]. Most of those studies were conducted in European countries and the USA, and it was pointed out that the generally lower socioeconomic status of these ethnic minorities in the developed countries might have affected their disease course and prognosis. On the other hand, the domestic system for public sharing of medical expenses in Japan has meant that patients can receive the standard therapy equally, regardless of their socioeconomic status.
In this single-centre observational study, we examined the current long-term outcome, causes of death, and factors associated with damage accrual in Japanese patients with SLE.
Patients and methods
This was a retrospective chart study and cross-sectional study of Japanese SLE patients. Figure 1 shows a consort diagram of patient flow through the study. We examined a cohort of 557 patients who had been referred to Niigata University Hospital and diagnosed as having SLE between 1961 and 2013. In this cohort, 99 patients were lost to follow-up during the study period and data from 458 of the patients were successfully obtained. The criteria for the classification of SLE [16, 17] and anti-phospholipid antibody syndrome (APS) [18, 19] were applied and confirmed in 458 patients at the time of the study. Those patients’ clinical statuses including major complications at the latest visit on April 2014 were taken from their clinical records. The average follow-up time per patient was 20 years (2–65 years). For 50 patients who moved to other hospitals during follow-up period, survey forms were sent to the hospitals concerned and the answers were collected from their primary physicians. The study protocol was approved by the ethics committee of Niigata University Hospital and executed in accordance with the Declaration of Helsinki. Survival or correlation analysis that would have affected data on organ damage was not performed for patients who had dropped out at the time of the study.
In this cohort, long-term survival rate analysis, survival analysis, and the major complications at the time of the study were examined. The complications such as hypertension, dyslipidemia, and diabetes mellitus were defined as patients who took any medication for each disease at the time of the examination, and osteonecrosis was defined as having a diagnosis by orthopedic surgeons for either X-ray or magnetic resonance imaging during the study period.
For 293 patients who were followed at Niigata university hospital at the time of the study, the SDI was calculated and the correlations between SDI and patients’ clinical data and medications were examined.
Among 115 patients who died during the observation period, the causes of death were also defined on the basis of those data. The causes of death were classified as follows: infections, vascular diseases including sudden death, SLE-related complications, and cancers. SLE-related complications included neuro-psychiatric SLE, hemophagocytic syndrome, thrombotic thrombocytopenic purpura, pulmonary arterial hypertension, and pulmonary hemorrhage associated with SLE, in our study.
Statistical analysis
Statistical analysis was performed using Prism 6 for Mac OS X (GraphPad Software, Inc., San Diego, CA, USA), and StatPlus: mac Pro (AnalystSoft Inc., Walnut, CA, USA). The Kaplan–Meier method was used for survival analysis and the log-rank test for trend for comparison of survival rates. The correlations between SDI and patients’ clinical data were analyzed using Spearman’s correlation coefficient and stepwise multiple regression analysis. A p value of < 0.05 was taken to denote statistical significance.
Results
Long-term survival rate and survival analysis
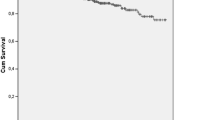
For the study cohort of 458 patients, the overall 5-, 10-, 20-, 30- and 40-year survival rates were 92.2, 88.4, 77.5, 70.8, and 61.8%, respectively (Fig. 2a).
For patients diagnosed as having SLE before 1970 (group 1, n = 10), between 1970 and 1979 (group 2, n = 57), between 1980 and 1989 (group 3, n = 126), between 1990 and 1999 (group 4, n = 125), and after 2000 (group 5, n = 140), the 5-year survival rates were 71.4, 83.1, 94.5, 93.4, and 96.4%, respectively. There was a significant improvement in survival rate between groups 2–5 (p < 0.0001) (Fig. 2b). In this analysis, we omitted patients in group 1 because the number of patients was very small for examination of statistical significance.
Major complications in the study cohort of SLE patients
Of the 343 patients who were alive at the time of this study, common complications including hypertension (40.8%), dyslipidemia (26.8%), diabetes mellitus (15.7%) and osteonecrosis (11.1%) were observed during the study period (Table 1).
Causes of death
Among 115 patients who died during the observation period, the main causes of death were cardiovascular disease (26%), infections (23.4%), SLE-related complications (13%), and cancers (12.1%). In patients who died within 2 years after diagnosis of SLE (n = 34), infections were the predominant cause of death (38.2%), and in those who died between 3 and 10 years after SLE diagnosis (n = 32), vascular disease was the most common cause of death (25%), increasing to 34.6% in patients who died more than 10 years after SLE diagnosis (n = 49). Meanwhile, SLE-related complications still accounted for more than 10% of deaths in patients even at more than 10 years after diagnosis (Fig. 3).
Multivariate and stepwise multiple regression analyses of factors affecting SDI in the study patients
In this study, we examined the correlations between SDI and the characteristics of the patients who visited Niigata University Hospital at the time of the study (n = 293), including sex, age, daily prednisolone dosage, immunosuppressant administration, bisphosphonate administration, vitamin D administration, serum creatinine levels, serum anti ds-DNA antibody levels, serum CH50 levels, association of APS, and hypertension. Table 2 summarizes the background data of the 293 patients at the time of the study. On the basis of Spearman’s correlation coefficient, the SDI score was positively correlated with serum creatinine levels (r = 0.3812, p < 0.0001), age (r = 0.2963, p < 0.0001), hypertension (r = 0.2299, p < 0.0001), disease duration (r = 0.2052, p = 0.0004), and serum CH50 (r = 0.1339, p = 0.0218), whereas it was negatively correlated with administration of bisphosphonate (r = − 0.115, p = 0.049).
Stepwise multiple regression analysis selected serum creatinine levels (B = 0.6051, p < 0.0001), age (B = 0.0246, p < 0.0001), hypertension (B = 0.3201, p = 0.0031), and antiphospholipid antibody syndrome (B = 0.4837, p = 0.0044), as positive independent variables, whereas administration of bisphosphonate (B = − 0.2636, p = 0.0117) was selected as a negative independent variable (Table 3).
Discussion
It has been well recognized that there are significant differences in how the disease process of SLE affects patients of different racial backgrounds, and similar differences have been demonstrated for disease risk and severity, and treatment responses [5, 6, 11,12,13,14,15]. In general, greater disease severity has been observed clinically in several studies of black, Hispanic, and Asian SLE patients, particularly with regard to renal involvement. The probability of long-term survival in these races has also been reported to be generally lower than in Caucasians.
However, these results need to be interpreted with caution because not only ethnicity, but also socioeconomic status and standards of medical therapies in these patients could affect the outcome of SLE. The recent LUMINA (Lupus in Minorities: Nature vs. Nurture) study, involving one of the biggest prospective cohorts of SLE patients from different racial backgrounds, indicated that Hispanic patients in the USA seemed to have a poorer prognosis than their counterparts from Latin America, despite having a comparable genetic background [20]. Moreover, Gomez-Puerta et al. have recently reported that Asian and Hispanic SLE patients had lower mortality than black, white, and Native American patients enrolled in Medicaid, a healthcare insurance system for the low-income population in USA [21].
SLE has been identified as a specific intractable disease by the Ministry of Health, Labour and Welfare, and a system for public sharing of medical expenses has been established for SLE patients in Japan. Therefore, in Japan, it is possible for all SLE patients to receive uniform treatment regardless of their socioeconomic status.
Our present study indicates that the trends in improvement of long-term survival and changes in major causes of death among SLE patients over more than three decades have shown almost the same patterns as those reported from Europe and USA, where Caucasians represent the major ethnic group. These facts indicate the possible effects of socioeconomic background on disease severity and long-term prognosis, rather than racial or regional differences, in patients with SLE.
Since the probability of long-term survival for SLE patients has been greatly improved, as mentioned above, we need to keep focusing on prevention of complications and organ damage through long-term follow-up. Several factors have already been reported to be associated with higher SDI scores, including older age at SLE onset, racial differences, chronic smoldering disease activity and major SLE disease flares, as well as chronic exposure to glucocorticoid, especially with regard to late-onset damage [5,6,7,8,9,10,11]. The latest study of the Hopkins Lupus Cohort has pointed out many factors associated with damage accrual, including older age, African American ethnicity, lower income, antiphospholipid antibodies, and high disease activity, but the most important predictor of damage progression appeared to be corticosteroid use [5]. The SLICC Inception Cohort study also reported that age, African American ethnicity, SLEDAI-2K score, steroid use, and hypertension were associated with transition from no damage to damage [6].
The present study also revealed that serum creatinine level, older age, complications of hypertension and antiphospholipid antibody syndrome were associated with SDI elevation, supporting the results of previous studies, whereas bisphosphonate administration was associated with a decrease of SDI in SLE patients.
Bisphosphonate has been used as a first-line medication since 2004, according to the Japanese Society for Bone and Mineral Research guidelines for management and treatment of glucocorticoid-induced osteoporosis [22], and 54% of patients in our cohort were administered bisphosphonate. In our study, the daily dosage of glucocorticoid was not related to elevation of SDI. It is still controversial whether long-term use of low-dose glucocorticoid is related to progressive damage accumulation in patients with SLE [5,6,7,8,9,10,11, 23], but appropriate prophylaxis or treatments for steroid-induced adverse events such as hypertension, diabetes, dyslipidemia, and osteoporosis, may be beneficial for prevention of further damage.
Meanwhile, this was a single-center cross-sectional study and the number of patients was somewhat underpowered. Hypertension and kidney dysfunction are well-known factors associated with SDI as we have already mentioned. However, these factors might be the result, and not the cause of the elevation of SDI in our study. For example, patients with hypertension was defined as taking medication at the time of examination in our study, but the detailed information such as their blood pressure control or medical history were not obtained. A further large-scale prospective study will be necessary to examine the significance of these factors in terms of damage accrual in Japanese patients with SLE.
In conclusion, we have demonstrated favorable long-term prognosis and patterns of causes of death in Japanese patients with SLE similar to those reported previously for Caucasian patients. Our study results have also indicated the possible role of SLE disease control as well as long-term management of chronic complications such as hypertension and osteoporosis for prevention of further organ damage in patients with SLE.
References
Kasitanon N, Magder LS, Petri M. Predictors of survival in systemic lupus erythematosus. Med (Baltim). 2006;85:147–56.
Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index for systemic lupus erythematosus. Arthritis Rheum. 1996;39:363–9.
Gladman DD, Urowitz MB, Goldsmith CH, Fortin P, Ginzler E, Gordon C, et al. The reliability of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index in patients with systemic lupus erythematosus. Arthritis Rheum. 1997;40:809–13.
Sutton EJ, Davidson JE, Bruce IN. The Systemic Lupus International Collaborating Clinics (SLICC) Damage Index: a systematic literature review. Semin Arthritis Rheum. 2013;43:352–61.
Petri M, Purvey S, Fang H, Magder LS. Predictors of organ damage in systemic lupus erythematosus. The Hopkins Lupus Cohort. Arthritis Rheum. 2012;64:4021–8.
Bruce IN, O’Keeffe AG, Farewell V, Hanly JG, Manzi S, Su L, et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Linics (SLICC) inception cohort. Ann Rheum Dis. 2015;74:1706–13.
Yee CS, Su L, Toescu V, Hickman R, Situnayake D, Bowman S, et al. Birmingham SLE cohort: outcomes of a large inception cohort followed for up to 21 years. Rheumatology. 2015;54:836–43.
Becker-Merok A, Nossent HC. Damage accumulation in systemic lupus erythematosus and its relation to disease activity and mortality. J Rheumatol. 2006;33:1570–7.
Stoll T, SutcliffeN Mach J, et al. Analysis of the relationship between disease activity and damage in patients with systemic lupus erythematosus- a 5-yr prospective study. Rheumatology. 2004;43:1039–44.
Sato H, Miida T, Wada Y, et al. Atherosclerosis is accelerated in patients with long-term well-controlled systemic lupus erythematosus (SLE). Clin Chim Acta. 2007;385:35–42.
Yee CS, Hussein H, Skan J, Bowman S, Situnayake D, Gordon C. Association of damage with autoantibody profile, age, race, sex, and disease duration in systemic lupus erythematosus. Rheumatol (Oxf). 2003;42:276–9.
Chaiamnuay S, Bertoli AM, Roseman JM, McGwin G, Apte M, Duran S, et al. African-American and Hispanic ethnicities, renal involvement and obesity predispose to hypertension in systemic lupus erythematosus: results from LUMINA, a multiethnic cohort (LUMINALV). Ann Rheum Dis. 2007;66:618–22.
Chakravarty EF, Bush TM, Manzi S, et al. Prevalence of adult systemic lupus erythematosus in California and Pennsylvania in 2000: estimates obtained using hospitalization data. Arthritis Rheum. 2007;56:2092–4.
Cooper GS, Parks CG, Treadwell EL, et al. Differences by race, sex and age in the clinical and immunologic features of recently diagnosed systemic lupus erythematosus patients in the Southeastern United States. Lupus. 2002;11:161–7.
Barr RG, Seliger S, Appel GB, et al. Prognosis in proliferative lupus nephritis: the role of socio-economic status and race/ethnicity. Nephrol Dial Transplant. 2003;18:2039–46.
Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7.
Hochberg MC. Updating the American college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725.
Wilson WA, Gharavi AE, Koike T, et al. International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: report of an international workshop. Arthritis Rheum. 1999;42:1309–11.
Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295–306.
Ugarte-Gil MF, Pons-Estel GJ, Molineros J, Wojdyla D, McGwin G, Nath SK, et al. Disease features and outcomes in United States lupus patients of Hispanic origin and their Mestizo counterparts in Latin America: a commentary. Rheumatology. 2016;55:436–40.
Gomez-Puerta JA, Barbhaiya M, Guan H, Feldman CH, Alarcon GS, Costenbader KH. Racial/ethnic variation in all-cause mortality among United States Medicaid recipients with systemic lupus erythematosus. a Hispanic and Asian paradox. Arthritis Rheum. 2015;67:752–60.
Suzuki Y, Nawata H, Soen S, Fujiwara S, Nakayama H, Tanaka I, et al. Guidelines on the management and treatment of glucocorticoid-induced osteoporosis of the Japanese Society for Bone and Mineral Research: 2014 update. J Bone Miner Metab. 2014;32:337–50.
Themaer M, Hernan MA, Zhang Y, Cotter D, Petri M. Relationship between prednisolone, Lupus activity and permanent organ damage. J Rheumatol. 2009;36:560–4.
Acknowledgements
We are grateful to Professor Naohito Tanabe, University of Niigata Prefecture, for his kind advice regarding statistical analyses in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors do not have any conflict of interest regarding to the study.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional research committee at which the studies were conducted (IRB approval number 1754) and with the Declaration of Helsinki and the ethical guidelines for epidemiological studies issued by the Ministry of Health, Labour and Welfare of Japan.
Informed consent
Because data were retrospectively obtained from medical records, informed consent was not obtained in accordance with the above ethical guidelines.
About this article
Cite this article
Wada, Y., Hasegawa, H., Saeki, T. et al. Long-term prognosis and factors associated with damage accrual in Japanese patients with systemic lupus erythematosus. Clin Exp Nephrol 22, 597–602 (2018). https://doi.org/10.1007/s10157-017-1491-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-017-1491-9