Abstract
Background
We assessed the effectiveness and convenience of a novel semi-automatic kidney volume (KV) measuring high-speed 3D-image analysis system SYNAPSE VINCENT® (Fuji Medical Systems, Tokyo, Japan) for autosomal dominant polycystic kidney disease (ADPKD) patients.
Methods
We developed a novel semi-automated KV measurement software for patients with ADPKD to be included in the imaging analysis software SYNAPSE VINCENT®. The software extracts renal regions using image recognition software and measures KV (VINCENT KV). The algorithm was designed to work with the manual designation of a long axis of a kidney including cysts. After using the software to assess the predictive accuracy of the VINCENT method, we performed an external validation study and compared accurate KV and ellipsoid KV based on geometric modeling by linear regression analysis and Bland–Altman analysis.
Results
Median eGFR was 46.9 ml/min/1.73 m2. Median accurate KV, Vincent KV and ellipsoid KV were 627.7, 619.4 ml (IQR 431.5–947.0) and 694.0 ml (IQR 488.1–1107.4), respectively. Compared with ellipsoid KV (r = 0.9504), Vincent KV correlated strongly with accurate KV (r = 0.9968), without systematic underestimation or overestimation (ellipsoid KV; 14.2 ± 22.0%, Vincent KV; − 0.6 ± 6.0%). There were no significant slice thickness-specific differences (p = 0.2980).
Conclusions
The VINCENT method is an accurate and convenient semi-automatic method to measure KV in patients with ADPKD compared with the conventional ellipsoid method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common type of monogenic renal disease, affecting one in every 500–1000 people [1]. Although the growth of cysts which compress adjacent normal renal parenchyma [2], renal function in early disease stages is preserved through the remnant normal renal parenchymal compensatory mechanism. As a result, approximately half of patients develop end-stage renal disease by their 6th or 7th decade of life [3]. Thus, glomerular filtration rate (GFR) cannot be a useful marker for the progression of ADPKD; and other reliable and convenient surrogate marker is required.
According to the Consortium of Renal Imaging Studies in Polycystic Kidney Disease (CRISP) study, total kidney volume (TKV) is a significant prognostic marker for renal function in ADPKD patients [4]. The same findings were also observed in Japanese ADPKD patients [5]. Based on these outcomes, TKV can be a surrogate marker to predict the progression of renal disease and the risk of developing renal insufficiency [3, 4].
However, TKV measurements of ADPKD patients require high precision [6] and this is difficult to achieve. New KV estimation methods include the manual planimetry segmentation tracing method [7, 8], the estimation from a single middle slice image method [9], stereological approaches [1, 3, 10, 11] and the ellipsoid method [12]. Although the manual planimetry tracing method require manually tracing the border of the kidneys for the full extent of the kidneys with high accuracy, this method is laborious, limiting its use in clinical care. The usefulness of the middle slice method has not yet been established in ADPKD patients with poor renal function. Although the stereological approach is automatic, this method has many restrictions on precisely identifying the kidney boundary with a very irregular and huge cyst [3, 12]. The ellipsoid method involves rough calculation methods, which may cause many inter- and intraobserver errors [12].
In this study, we examined the effectiveness of a novel semi-automatic KV measuring high-speed 3D-image analysis system SYNAPSE VINCENT® (Fuji Medical Systems, Tokyo, Japan) for ADPKD patients.
Materials and methods
Development of a novel software to measure KV in patients with ADPKD
We created a novel semi-automated KV measurement software for patients with ADPKD to be included to the imaging analysis software SYNAPSE VINCENT® (Fuji Medical Systems, Tokyo, Japan). Using image recognition software, the Vincent novel software can extract renal contour including all cysts and measure KV (Vincent KV).
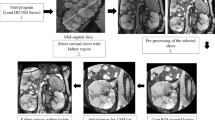
The software algorithm was designed to work with the manual designation of a long axis of a kidney (Fig. 1a). For the designation of the long axis, we rotated the vertical cross-sectional direction of CT images to identify the long axial cross-section. If we could not accurately extract all of the renal regions by the manual designation of the long axis, it was possible to draw a line of the long diameter of the kidney again as many times as needed, and the KV could be automatically measured again each time. In some cases, we repeated this procedure several times (the average number of times: 1.2, maximum number of times: 5). Given the long axis, the algorithm first estimates likelihood values of the kidney for every Hounsfield unit (HU) value from the voxels around the major axis. Next, the algorithm fit an ellipsoidal model to the images, allowing deformation caused by the cysts. Finally, the kidney regions are segmented by maximizing evaluation measures consisting of the sum of the likelihood values, edge strength on the boundaries, and fitness to the ellipsoidal model. Ultimately, this software provided the measurement of KV. Each measurement by this software took almost 10 s. Non-kidney structures (e.g. liver and spleen) were automatically identified and excluded from the measurement area.
a The algorithm was designed to work with the manual designation of a long axis of a kidney including cysts (yellow line). The automated extraction of the renal contour was implemented immediately after the manual designation of the long axis (green range). After the visual confirmation of the exact extraction region, the Vincent software can perform volumetric measure semi-automatically with just one click. b The relationship between Vincent TKV and accurate TKV at the time of development of the novel Vincent software. Vincent TKV was significantly correlated with accurate TKV (r = 0.9989). Circles identify single-patient data. c The relationship between ellipsoid TKV and accurate TKV at the time of the development of the novel Vincent software. Although we could see the correlation between ellipsoid TKV and accurate TKV, the confidence correlation factor (r = 0.9549) was lower than Vincent TKV. Circles identify single-patient data. d The relationship between 5 mm Vincent TKV and accurate TKV at the time of the development of the novel Vincent software. The correlation coefficient between 5 mm Vincent TKV and accurate TKV (r = 0.9963) was almost equal with Vincent TKV, and was higher than ellipsoid TKV. Circles identify single-patient data (color figure online)
To measure accurate kidney volume (accurate KV), we extracted all of the renal regions by manual tracing using a tool for region fill. We also measured KV using the ellipsoid method (ellipsoid KV; renal volume = π/6 × length × width × depth). Length and width measurements were obtained from longitudinal images acquired in planes ranging from sagittal to coronal, while depth measurement was obtained from transverse images of the mid-kidney acquired in the plane perpendicular to the longitudinal plane.
We analyzed the gap between accurate KV and Vincent KV or ellipsoid KV.
External validation study
To assess the predictive accuracy of the Vincent method, we enrolled our ADPKD patients in a validation study. All participants underwent a standardized abdominal CT protocol without the use of intravenous contrast. We confirmed that all DICOM files from the axial direction/coronary CT were taken into workstation and were examined to completely cover both kidney and image quality. We measured KV using the three previously described methods (Vincent KV, ellipsoid KV and accurate KV). We assessed renal function by serum creatinine (sCr), estimated glomerular filtration rate (eGFR), and serum cystatin C. The eGFR was calculated using the simplified MDRD equation modified by the appropriate coefficient for Japanese populations by sex [13]. We evaluated the correlation between renal functions and KV measured by different ways including ellipsoid KV, accurate KV and Vincent KV. Each KV measurements were performed by researchers blinded to the results of other KV measured by other methods and clinical data.
This study was conducted in patients with ADPKD in accordance with ethical principles originating from the Declaration of Helsinki and in compliance with good clinical practice guidelines. Each Universities Institutional Review Boards approved the study (16-033 and 16-188) and all patients provided informed consent. The UMIN Clinical Trials Registry identifier for this prospective clinical trial is R000026758.
Statistical analysis
The means and standard deviations (SDs) of clinical factors were calculated. ANOVA was used to determine the differences between accurate KV, Vincent KV and ellipsoid KV. Comparison was performed using linear regression and Bland–Altman analyses. Paired t test was applied to verify the significance of the bias. We evaluated the correlation between some clinical factors including blood pressure, abdominal circumference and height-adjusted TKV (HtTKV) measured by different ways. Pearson correlation coefficients and their corresponding p values were calculated to assess the relationship between HtTKV measured by accurate TKV, ellipsoid TKV and Vincent TKV, and eGFR. R 2.14.0 was used to determine the outcome measure [14]. Two-tailed p values of less than 0.05 were considered as a statistically significant difference.
Results
Development of the novel software to measure KV in patients with ADPKD
We used data from 22 ADPKD patients (male 9, female 13) to create a novel semi-automated kidney volumetric software. The median age was 46.5 years (IQR 40.25–54). All patients received plain CT scan with varying thickness (median: 0.63 ml) of kidney.
The median accurate KV was 618.7 ml (IQR 448.1–1013.2). There was no significant difference between right (median 566.3 ml, IQR 435.3–928.6) and left (median 621.4 ml, IQR 453.9–1040.3) accurate KV (p = 0.4805). The median accurate TKV was 1207.7 ml (IQR 955.9–1963.6).
With the use of these CT imaging data, we created a novel semi-automated kidney volumetric Vincent software. The median Vincent KV was 615.1 ml (IQR 446.0–952.7). There was no significant difference between rt. (median 564.9 ml, IQR 439.1–882.9) and lt. (median 653.2 ml, IQR 453.2–995.7) Vincent KV (p = 0.5451). The median Vincent TKV was 1254.2 ml (IQR 943.4–1874.5). Vincent TKV was well correlated with accurate TKV (correlation coefficient: r = 0.9989, Fig. 1b).
The median volume of ellipsoid KV was 697.1 ml (IQR 484.0–984.2). There was no significant difference between lt. (median 717.9 ml, IQR 526.4–977.4) and rt. (median 686.1 ml, IQR 480.0–982.6) ellipsoid KV (p = 0.6704). The median ellipsoid TKV was 1207.7 ml (IQR 955.9–1963.6). The correlation coefficient between accurate TKV and ellipsoid TKV was 0.9549 (Fig. 1c) and was lower than Vincent TKV.
For development novel software, we used CT scan with short slice thickness (median 0.63 ml) of the kidney. Therefore, to evaluate the usefulness of CT scan with long slice thickness for this novel software, the CT pictures were reorganized into 5 mm slice thickness (5 mm Vincent KV) and we evaluated the correlation between accurate TKV and 5 mm Vincent TKV. The median volume of 5 mm Vincent KV was 607.2 ml (IQR 441.2–937.4). There was no significant difference between rt. (median 554.3 ml, IQR 445.4–873.8) and lt. (median 657.1 ml, IQR 441.8–984.7) 5 mm Vincent KV (p = 0.6320). The median 5 mm Vincent TKV was 1272.5 ml (IQR 922.9–1883.0). The correlation coefficient between 5 mm Vincent TKV and accurate TKV (r = 0.9963, Fig. 1d) was almost equal with Vincent TKV and was higher than ellipsoid TKV.
We evaluated the variation of error by the different methods of measurement in each case. In 16 patients (72.7%), Vincent TKV was smaller than accurate TKV. The median difference value and rate between Vincent TKV and accurate TKV were 66.2 ml (IQR 19.2–105.7) and 4.3% (IQR 2.5–5.8), respectively. Consequently, Vincent TKV tends to be underestimated. On the other hand, ellipsoid TKV was larger than accurate TKV in 16 cases. The median difference value and rate between ellipsoid TKV and accurate TKV were 183.6 ml (IQR 109.0–414.5) and 18.9% (IQR 6.5–25.3), respectively. The difference value (p = 0.0075) and rate (p = 0.0005) between ellipsoid TKV and accurate TKV were significantly larger than those between Vincent TKV and accurate TKV. In addition, in 18 patients (81.8%), 5 mm Vincent TKV was smaller than accurate TKV. The median difference value and rate between 5 mm Vincent TKV and accurate TKV were 88.4 ml (IQR 31.2–122.0) and 6.0% (IQR 3.7–7.8), respectively. Compared with the ellipsoid TKV, we could not find any significant differences between Vincent TKV—accurate TKV and 5 mm Vincent TKV—accurate TKV in value (p = 0.4754) and rate (p = 0.1111). Therefore, this novel Vincent software is useful for plain CT scan regardless of slice intervals to measure the KV as precise as possible.
External validation study
One hundred twenty-four patients (male 62, female 62) participated in the external validation study. Accurate KV, ellipsoid KV and Vincent KV were measured in all these patients. Patients’ characteristics were shown in Table 1. The mean age was 49.7 years old. The median eGFR was 46.9 ml/min/1.73 m2 (IQR 24.2–62.5). The most common CKD severity is stage 3 (n = 49, 39.5%). All patients received plain CT scan with varying thickness (median 0.63 ml, IQR 0.5–2) of kidney.
Median accurate KV was 627.7 ml (IQR 441.6–1035.0). There was no significant difference between rt. (median 624.6 ml, IQR 435.2–969.8) and lt. (median 651.1 ml, IQR 470.9–1167.7) accurate KV (p = 0.8004). Median Vincent KV and ellipsoid KV were 619.4 ml (IQR 431.5–947.0) and 694.0 ml (IQR 488.1–1107.4), respectively (Fig. 2). There was no significant difference between accurate KV and Vincent KV (p = 0.9214). Similarly, there was no significant difference between accurate KV and ellipsoid KV (p = 0.1159). Linear regression analysis (Fig. 3a) between the ellipsoid KV and the accurate KV resulted in a high correlation coefficient and regression slope near to 1.0 (r = 0.9504, p < 0.001, Fig. 3a). However, Vincent KV (r = 0.9968, p < 0.001, Fig. 3b) exhibited a higher correlation than ellipsoid KV. Figure 3c and d show Bland–Altman plots of accurate KV versus the percentage differences between accurate KV and both ellipsoid KV (Fig. 3c) and Vincent KV (Fig. 3d) methods. Ellipsoid KV systematically under- or overestimate accurate KV, with a mean (± SD) percentage difference of 14.2 ± 22.0% (Fig. 3c). Vincent KV did not systematically under- or overestimate accurate KV, with a mean (± SD) percentage difference of − 0.6 ± 6.0% (Fig. 3d). In 144 of the 226 kidneys (63.7%), the percentage difference between ellipsoid KV and accurate KV exceeded 10%. The difference exceeded 20% in 90 (39.8%) of the kidneys. On the other hand, in 11 of the 246 kidneys (4.4%), the percentage difference between Vincent KV and accurate KV exceeded 10%. The difference exceeded 20% in only 5 (2.0%) of the kidneys.
The linear regression lines between ellipsoid KV (a) or Vincent KV (b) (vertical axis) and accurate KV (horizontal axis). Vincent KV correlates strongly with accurate KV in the external validation study. c, d Shows the Bland–Altman plots. Vincent KV is strongly correlated without systematic under- or overestimation of accurate KV. a: Ellipsoid KV (r = 0.9504, p < 0.001) was significantly correlated with accurate KV. b Vincent KV (r = 0.9968, p < 0.001) was significantly correlated with accurate KV. c % difference between ellipsoid KV and accurate KV X accurate KV. In 144 of the 226 kidneys (63.7%), the percentage difference between ellipsoid KV and accurate KV exceeded 10%. The difference exceeded 20% in 90 (39.8%) of the kidneys. d % difference between Vincent KV and accurate KV X accurate KV. In 11 of the 246 kidneys (4.4%), the percentage difference between Vincent KV and accurate KV exceeded 10%. The difference exceeded 20% in only 5 (2.0%) of the kidneys. Circles identify single-patient data, and horizontal red line denotes the mean value and mean ± 2SD
We compared the Vincent KV CT with a 0.5-mm, a 0.6-mm, a 1–2 mm, and a 5-mm slice thickness. The mean percentage differences between Vincent KV with a 0.5-mm slice thickness (n = 67), a 0.6-mm slice thickness (n = 56), a 1–2 mm slice thickness (n = 62), and a 5-mm slice thickness (n = 61), and accurate KV were − 1.1 ± 5.5, − 1.0 ± 6.3, − 0.9 ± 2.9, 0.7 ± 8.3%, respectively (Fig. 4a). There were no significant slice thickness-specific differences (p = 0.2980).
We compared the Vincent KV CT with a 0.5-mm, a 0.6-mm, a 1–2 mm, and a 5-mm slice thickness (a). The mean percentage differences between Vincent KV with a 0.5-mm slice thickness (n = 67), a 0.6-mm slice thickness (n = 56), a 1–2 mm slice thickness (n = 62), a 5-mm slice thickness (n = 61) and accurate KV were − 1.1 ± 5.5, − 1.0 ± 6.3, − 0.9 ± 2.9, 0.7 ± 8.3%, respectively. There were no significant slice thickness-specific differences (p = 0.2980). The accurate HtTKV (r = −0.4529, p < 0.01, b), ellipsoid HtTKV (r = −0.4255, p < 0.01, c) and Vincent HtTKV (r = −0.4501, p < 0.01, d) were inversely and significantly correlated with eGFR. Compared with ellipsoid HtTKV, correlation coefficient of Vincent HtTKV was closer to that of accurate HtTKV. Circles identify single-patient data
The average number of times needed to measure Vincent KV was 1.4 ± 0.9 times. It took almost 10 s for one measurement by the Vincent method, the median time to measure Vincent KV was 14 s.
Median accurate TKV, Vincent TKV and ellipsoid TKV were 1242.9 ml (IQR 922.3–2047.4), 1248.7 ml (IQR 924.3–2041.7) and 1450.0 ml (IQR 989.5–2167.5), respectively. Although accurate HtTKV (r = −0.4529, p < 0.01, Fig. 4b), ellipsoid HtTKV (r = −0.4255, p < 0.01, Fig. 4c) and Vincent HtTKV (r = −0.4501, p < 0.01, Fig. 4d) were inversely and significantly correlated with eGFR, correlation coefficient of Vincent HtTKV was closer to that of accurate HtTKV compared with ellipsoid HtTKV.
Discussion
Currently, KV measurements used for the management of ADPKD require high precision and have to be convenient [6, 15]. In Japan, the ellipsoid method is commonly used because data on the three longest orthogonally measured dimensions are easily obtainable. However, although renal volume based on the ellipsoid method is simple to use, it was shown to be less accurate in ADPKD kidneys that are irregularly shaped and too large [9, 16]. Hence, we are unable to recommend the use of this method to measure KV.
Therefore, more precise and convenient approaches are actively pursued. Several studies have published on the manual planimetry segmentation tracing method [7, 8]. These manual segmentation methods rely heavily on the physician’s manual input to complete the segmentation process are subject to considerable interobserver variability, and are time-consuming. In an effort to overcome these limitations, the semi-automated [17,18,19] and the fully automated segmentation tracing methods [20] were previously reported. Although these techniques would allow for faster measurement of KV compared with manual contour tracing on image, no study has revealed a fully satisfactory convenient automated technique. Since these methods require particular and specialist technique that depend heavily on the expert user who reviews MR images and identifies the kidney region, they would be very difficult to use in standard clinical practice. In Vincent method, given the long axis of a kidney by manual designation, the automated algorithm can recognize the kidney regions segmented by maximizing evaluation measures consisting of the sum of the likelihood values of the kidney for every HU value from the voxels around the major axis, edge strength on the boundaries, and fitness to the ellipsoidal model.
In the CRISP study, the stereological approaches were performed from MR images with the administration of gadolinium contrast [1, 3, 10, 11]. Volume measurements by MR were time-consuming, expensive, and not available to all patients. In addition, although gadolinium contrast helps detect renal boundary and differentiates renal cysts from the background renal parenchyma, it is indisputable that the administration of gadolinium contrast is unfavorable for patients with renal impairment because of nephrogenic systemic fibrosis. Bae et al. reported that KV and cyst volumes could be closely approximated by multiplying the product of the mid-slice area measurement and the total number of slices in the coronal MR images of ADPKD kidneys [9]. However, the information regarding how these imaging findings relate to progression of the disease in humans may not be available for quite some time.
To make an accurate and simple method available in standard clinical practice, we developed a new technique. The Vincent method can detect various organs by automatic extraction [21]. Our study revealed strong correlations between accurate KV and Vincent KV. We obtained more required analysis of TKV in ADPKD using the Vincent method. No training was necessary to use this method because the processing was nearly automated, and no expertise was required to select the manual designation of the long axis. A manual correction tool is included in this software, and the physicians could use it easily for manual re-designation of the long axis and tracing contours. Furthermore, in this method, image acquisitions for measurements of KV can be obtained in a few minutes. Bae et al. reported that stereology can analyze a typical image volume in 10–20 versus 45–90 min for planimetry [10]. Compared with these previous strategies, the Vincent method can quickly and easily renders the entire kidney area including the cyst by merely drawing a line on the long axis of the kidney. Actually, the economic and institutional burden for introducing the system is not so large. When newly introducing Vincent system, it needs about 5 million yen in Japan. However, if prior Vincent system has already been introduced, it can be implemented by purchasing about 1 million yen of software. In Japan, since prior Vincent system has already been installed in more than 1000 institutions in Japan, it is possible in about 1 million yen in many cases. Although it is desirable to have CT, it is possible to introduce this system in all medical institutions.
Of course, this study has several limitations. Patient population used for analysis in our research has few patients. To improve the understanding of disease prognosis and clinical decision of the ADPKD phenotype, research should be conducted to evaluate automated systems in larger and more diverse patient populations. In particular, Fig. 3d showed that correlation was not accurate in a range of smaller KV volume in this study. Incidentally, there were several cases where it was difficult to identify renal cysts and surrounding organs including such as liver cysts and intestine automatically in cases with small renal volume. Although we consider it a coincidence result, we have to further increase the number of cases and study this method. In this study, some cases needed multiple measurements with the Vincent method. Other atlas-based or automated methods also failed due to the greatly different shape and size of the polycystic kidneys, and due to similar intensity in adjacent tissues. Likewise, from this, there are some real problems to measure accurate KV using the Vincent method at one time. In Vincent method, it is possible to identify the perceived area with images overlapping with CT (Fig. 1a). Therefore, users make a judgmental decision about the need of a repeat measurement in this method with no difficulty. In this study, we used plain CT to measure KV by the Vincent method, but we look forward to the development of this novel measurement technique with MRI. The MRI, although expensive, relatively time-consuming and varies in the quality of images produced, prevents radiation exposure and better defines the cysts.
In the clinical setting, the measurement of KV by traditional approaches such as the ellipsoid method is too time-consuming and inaccurate. Alternatively, the Vincent method is semi-automated and convenient. Further, this method can be used to measure KV in patients with ADPKD accurately.
References
Chapman AB, Guay-Woodford L, Grantham JJ, et al. Renal structure in early autosomal dominant polycystic kidney disease: the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) cohort. Kidney Int. 2003;64:1035–45.
Bae KT, Zhu F, Chapman AB, et al. Magnetic resonance imaging evaluation of hepatic cysts in early autosomal-dominant polycystic kidney disease: the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease cohort. Clin J Am Soc Nephrol. 2006;1:64–9.
Grantham JJ, Torres VE, Chapman AB, et al. Volume progression in polycystic kidney disease. N Engl J Med. 2006;354:2122–30.
Chapman AB, Bost JE, Torres VE, et al. Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2012;7:479–86.
Tokiwa S, Muto S, China T, Horie S. The relationship between renal volume and renal function in autosomal dominant polycystic kidney disease. Clin Exp Nephrol. 2011;15:539–45.
Bae KT, Grantham JJ. Imaging for the prognosis of autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2010;6:96–106.
King BF, Reed JE, Bergstralh EJ, Sheedy PF 2nd, Torres VE. Quantification and longitudinal trends of kidney, renal cyst, and renal parenchyma volumes in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2000;11:1505–11.
Kistler AD, Poster D, Krauer F, et al. Increases in kidney volume in autosomal dominant polycystic kidney disease can be detected within 6 months. Kidney Int. 2009;75:235–41.
Bae KT, Tao C, Wang J, et al. Novel approach to estimate kidney and cyst volumes using mid-slice magnetic resonance images in polycystic kidney disease. Am J Nephrol. 2013;38:333–41.
Bae KT, Commean PK, Lee J. Volumetric measurement of renal cysts and parenchyma using MRI: phantoms and patients with polycystic kidney disease. J Comput Assist Tomogr. 2000;24:614–9.
Bae KT, Tao C, Zhu F, et al. Consortium for Radiologic Imaging Studies Polycystic Kidney Disease: MRI-based kidney volume measurements in ADPKD: reliability and effect of gadolinium enhancement. Clin J Am Soc Nephrol. 2009;4:719–25.
Irazabal MV, Rangel LJ, Bergstralh EJ, et al. A simple model for selecting patients for clinical trials. J Am Soc Nephrol. 2015;26:160–72.
Matsuo S, Imai E, Horio M, et al. Collaborators developing the Japanese equation for estimated GFR: revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
R Development Core Team. R. a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2011.
Spithoven EM, van Gastel MD, Messchendorp AL, et al. DIPAK Consortium: estimation of total kidney volume in autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2015;66:792–801.
Cheong B, Muthupillai R, Rubin MF, Flamm SD. Normal values for renal length and volume as measured by magnetic resonance imaging. Clin J Am Soc Nephrol. 2007;2:38–45.
Mignani R, Corsi C, De Marco M, et al. Assessment of kidney volume in polycystic kidney disease using magnetic resonance imaging without contrast medium. Am J Nephrol. 2011;33:176–84.
Cohen BA, Barash I, Kim DC, Sanger MD, Babb JS, Chandarana H. Intraobserver and interobserver variability of renal volume measurements in polycystic kidney disease using a semiautomated MR segmentation algorithm. Am J Roentgenol. 2012;199:387–93.
Bae K, Park B, Sun H, et al. Segmentation of individual renal cysts from MR images in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2013;8:1089–97.
Kim Y, Ge Y, Tao C, et al. Automated segmentation of kidneys from MR images in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2016;11:576–84.
Mochizuki K, Takatsuki M, Soyama A, Hidaka M, Obatake M, Eguchi S. The usefulness of a high-speed 3D-image analysis system in pediatric living donor liver transplantation. Ann Transplant. 2012;17:31–4.
Acknowledgements
This study was supported in part by Grant-in-Aid for Intractable Renal Diseases Research, Research on rare and intractable diseases, Health and Labour Sciences Research Grants from the Ministry of Health, Labour and Welfare of Japan. We are grateful to Kyoko Suzuki and Jun Masumoto (Fuji Medical Systems, Tokyo, Japan) for developing novel software SYNAPSE VINCENT® to measure KV in patients with ADPKD.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee at which the studies were conducted (IRB Approval number 16-033 and 16-188) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
About this article
Cite this article
Muto, S., Kawano, H., Isotani, S. et al. Novel semi-automated kidney volume measurements in autosomal dominant polycystic kidney disease. Clin Exp Nephrol 22, 583–590 (2018). https://doi.org/10.1007/s10157-017-1486-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-017-1486-6