Abstract
Background
The aim of this study was to compare long-term oncological, functional outcomes and quality of life (QoL) after transanal total mesorectal excision (TaTME) and laparoscopic total mesorectal excision (LaTME) for rectal cancer.
Methods
A systematic review and meta-analysis based on Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines were conducted on PubMed and Cochrane database. Non-randomized controlled trials (NRCTs) which compared TaTME with LaTME were included.
Results
Ten non-randomized studies were identified, including a total of 638 patients (323 TaTME and 315 LaTME). Age, sex, body mass index, neoadjuvant treatment and American Society of Anesthesiologists (ASA) staging of patients in the two groups were comparable in all included studies. The follow-up period was significantly shorter in the TaTME group than in the LaTME group. No significant differences in local (p = 0.71) and distant (p = 0.23) recurrence rate, 2-year disease-free (p = 0.86) and overall (p = 0.25) survival was found. Also, no significant differences in function outcomes and QoL, including the Wexner score (p = 0.48) or the International Prostate Syndrome Score (IPSS) (p = 0.64) were found. However, the low anterior resection syndrome (LARS) score was significantly higher in the TaTME group (p = 0.04).
Conclusions
TaTME and LaTME have similar long-term oncological and functional outcomes as well as QoL. The only exception is higher LARS scores after TaTME. The current data are based mainly on observational studies and further randomized controlled trials are required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Though total mesorectal excision (TME) is a well-established standard treatment for rectal carcinomas, low lying tumors in obese and/or male patients remain a challenge even for experienced surgeons. Complex surgery in narrow pelvis results in higher rate of positive resection margin either in laparoscopic or open approaches, as well as morbidity and high colostomy rates, poor quality of life (QoL).
To improve the quality of the surgical specimen, a technique of transanal TME (TA TME) was developed in 2010 [1]. The transanal approach has the advantages of more precise dissection around distal rectum and better visualization of adjacent structures, which may potentially increase the rate of specimens with good quality of mesorectal excision and negative resection margins. On the other hand, TaTME makes it possible to stay within the embryological dissection plain, which keeps the pelvic plexus intact and reduces undesirable functional sequealae in turn.
Several randomized control trials (RCTs) comparing laparoscopic TME (LaTME) and TaTME approaches were initiated [2,3,4], but are still in progress. Currently, only a few studies comparing intraoperative, postoperative and pathological outcomes between LaTME and TaTME are available. There are even less studies comparing the quality of life (QoL), functional results and long-term outcomes (locoregional recurrence, distal metastasis, disease-free and overall survival). Moreover, some of the previously published meta-analyses included data from abdominoperineal resections (APRs), which may bias the outcomes, and some included studies with short-term results only. No meta-analysis comparing the QoL and functional outcomes after LaTME and TaTME has been published. Thus, the aim of our systematic review and meta-analysis was to fill these gaps of the knowledge assessing the long-term oncological and functional outcomes as well as QoL after TaTME.
Materials and methods
Search strategy
The systematic review and meta-analysis were conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (http://www.prisma-statement.org/) [5]. A literature search was performed through PubMed and Cochrane Database of Systematic reviews, using the following search strategy: (“total mesorectal excision” OR TME OR “mesorectal excision” OR approach OR proctectomy) AND (perineal OR transanal OR transanal OR “down-to-up” OR “bottom-up” OR “transanal specimen extraction” OR NOSE OR “natural orifice transluminal endoscopic surgery” OR NOTES) AND “rectal cancer”. No restrictions were applied in terms of language, year or status of publication. Reference lists of selected publications, other systematic reviews or meta-analyses were hand-searched for additional relevant studies. The search dates were from February 1, 1973 to February 8, 2020.
Inclusion and exclusion criteria
In accordance with the population, intervention, comparison, outcomes and study design (PICOS) criteria, the following eligibility criteria were selected for inclusion of the publications in the meta-analysis: (a) population: patients were diagnosed with rectal cancer; (b) intervention: surgical treatment; (c) comparison: TaTME versus LaTME; (d) outcomes: long-term outcomes (locoregional recurrence, distant metastases, disease-free (DFS) and overall (OS) survival), functional results and QoL compared between two groups; and (e) study design: RCTs, cohort trials or matched case–control (MCC) trials with sample size greater than 15.
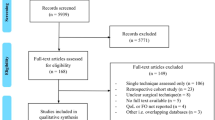
The exclusion criteria were as follows: (a) lack of the sufficient data or outcomes of interest; (b) duplicate publication; (c) APR and (d) non-comparative studies, reviews, meta-analyses, letters, case reports or conference abstracts. The search strategy is illustrated in Fig. 1.
Data extraction and quality assessment
Two authors (I.A. and M.N.) independently reviewed and assessed each included study, according to the inclusion and exclusion criteria. In addition, they extracted and summarized the data from the included studies independently. For each study, the following information was collected: (a) study characteristics: the first author, country, year of publication, number of patients, study type (RCT/cohort trial/MCC trial); (b) patient baseline: tumor site, gender, age, body mass index (BMI), neoadjuvant treatment, American Society of Anesthesiologists (ASA) class, duration of follow-up; (c) study outcomes: long-term outcomes, including local and distant recurrence, overall and disease-free survival, functional results and the QoL. The quality of non-randomized controlled trials (NRCTs) was evaluated using the Newcastle–Ottawa Scale (NOS) criterion [6]. If the mean and standard deviation (S.D.) were not provided, they were calculated using the method described by Wan and colleagues [7].
In accordance with Cochrane guidelines, we did not investigate publication bias as our search considered less than ten studies for each data comparison [8]. All analyses were performed using the Review Manager 5.3 software.
Results
Study characteristics
A total of 1,260 relevant publications were identified in the initial literature search. Finally, 10 studies [9,10,11,12,13,14,15,16,17,18] were included in the meta-analysis (Fig. 1), with a total of 638 patients (323 patients in the TaTME group, 315 patients in the LaTME group). There were 8 MCC studies [9, 10, 12,13,14,15,16,17], 1 prospective cohort studies [18] and 1 retrospective study [11]. The quality assessment of all NRCTs were evaluated using NOS and the results ranged from 7 to 8 stars, which corresponded to good quality.
Patient characteristics
The baseline characteristics of patients are reported in Table 1 and information about available type of long-term outcomes demonstrated in Table 2. Long-term oncological outcomes (Table 3) were assessed in 6 studies [9,10,11,12,13,14], which incorporated the results of the treatment of 405 patients. Age, sex, body mass index (BMI), neoadjuvant treatment and ASA score of patients in the TaTME and the LaTME groups were comparable in those studies. There were 118/171 (69%) males in the TaTME group and 128/200 (64%) in the LaTME group (p = 0.28). The mean difference of age between the two groups (the TaTME and the LaTME) was − 0.16 (95% CI − 2.55–2.23; p = 0.90; I2 = 0%; n = 333). The mean difference for BMI (6 studies) between the two groups was 0.54 (95% CI − 0.19–1.28; p = 0.15; I2 = 0%; n = 405). One hundred forty patients in the TaTME (74%) and 160 in the LaTME (74%) group had neoadjuvant chemoradiation (p = 0.50). Patients with ASA I (II) were prevalent in both groups: 157/171 (92%) and 188/200 (94%) in the TaTME and the LaTME groups, respectively (p = 0.28).
Functional outcome and QoL were reported in 6 studies (Table 4). There were 135/201 (67%) males in the TaTME group and 100/168 (60%) in the LaTME group (p = 0.28). The information about age was available in five studies and the mean difference for age between the TaTME and the LaTME groups was 1.18 (95% CI − 2.17–4.53; p = 0.49; I2 = 53%; n = 297). The mean difference for BMI (six studies) between the two groups was 0.61 (95% CI − 0.28–1.50; p = 0.18; I2 = 0%; n = 369). Neoadjuvant therapy was delivered to 108 (54%) patients in the TaTME group and to 110 (65%) in the LaTME group (p = 0.42). In the six studies with available ASA scores, 174/201 (87%) patients in the TaTME group had ASA I (II) comparing to 157/168 (93%) patients in the LaTME group (p = 0.15).
Long-term oncological outcome
Long-term oncological outcome was reported in 6 studies [9,10,11,12,13,14] including 405 patients: 188 patients in the TaTME group and 217 patients in the LaTME group (Table 3). It is noteworthy that the follow-up (Fig. 2a) was significantly shorter in the TaTME groupthan in the LaTME group (WMD − 17.30; 95% CI − 26.21 to − 8.39; p = 0.0001; I2 = 89%). There were six studies [9,10,11,12,13,14] that reported the local recurrence rate (2.1 vs. 3.2%, OR 0.78, 95% CI 0.22–2.79, p = 0.71; I2 = 0%; n = 405) and three studies [10, 11, 14], which the mentioned distant metastasis rate (7.1 vs. 13.3%, OR 0.53, 95% CI 0.19–1.47, p = 0.23; I2 = 0%; n = 196) (Fig. 2b and c).
Three studies [9, 13, 14] evaluated 2 year overall (RR 1.04, 95% CI 0.97–1.11, p = 0.25; I2 = 27%; n = 239) and disease-free (RR 1.01, 95% CI 0.92–1.11, p = 0.86; I2 = 0%; n = 239) survival (Fig. 3a and b). In summary, no significant difference between the two groups in long-term oncological outcomes was detected by the meta-analysis.
Functional outcome
Functional outcome was reported in 6 studies [13,14,15,16,17,18], including 369 patients (201 patients in the TaTME group, 168 patients in the LaTME group) and presented in Tables 4, 5. There were four studies [15,16,17,18] that reported low anterior resection syndrome (LARS) scores, two studies [14, 15] that assessed Wexner incontinence scores (Table 4), and two studies [17, 18] that evaluated international prostate symptom scores (IPSS) (Table 5).
As for oncological outcomes, the follow-up (Fig. 4a) for functional outcomes and QoL of the included studies was significantly shorter in the TaTME group than in the LaTME group (WMD − 38.23; 95% CI − 48.35 to − 28.12; p = 0.00001; I2 = 93%).
The mean LARS score (Fig. 4b was significantly higher in the TaTME group than in the LaTME group (WMD 2.88; 95% CI 0.15–5.60; p = 0.04; I2 = 0%). There was no significant difference in the mean Wexner score between the two groups for (WMD − 0.79; 95% CI − 3.00 to 1.42; p = 0.48; I2 = 34%) or the mean IPSS (WMD − 1.06; 95% CI − 5.59–3.46; p = 0.64; I2 = 53%) (Fig. 4c and d).
Quality of life
One case matched [17] and one prospective cohort [18] studies addressed QoL. In both of them, the EORTC QLQ C30 (Table 6) and EORTC QLQ C29 (Table 7) were used for comparison between the TaTME and the LaTME groups. In general, QoL was quite similar in both groups, though both studies reportyed a higher score for diarrhea after TaTME according to the EORTC QLQ C30. Also Veltcamp et al. [18] found that TaTME was associated with a higher score for fecal incontinence (p = 0.032) and sore skin (p = 0.023) according to the EORTC QLQ C29.
Discussion
Specimen oriented surgery is a key to successful treatment of rectal cancer. Laparoscopic surgery for rectal cancer has become the standard procedure due to minimal invasiveness and fast recovery. However, large RTCs AlaCaRT, ACOSOG Z6051 and COLOR II [19,20,21] failed to meet the criteria of non-inferiority for pathologic outcomes when compared with the open approach for rectal cancer. This result can be explained by the complexity of surgery in the deep pelvis with a limited view and difficult instrument triangulation, especially in patients with a narrow pelvis, bulky tumors and abdominal obesity. The transanal approach has been suggested to address these issues and improve short- and long-term results. TaTME provides enhanced view of the presacral and perirectal planes and facilitates dissection by tissue distension by the carbon dioxide gas [22,23,24]. The reported rate of positive circumferential resection margins (CRM) after TaTME was significantly lower than after LaTME in many studies, showing that a novel technique can potentially improve local control [25, 26].
Oncological outcomes, functional outcomes and QoL are considered as critical parameters after TME. This systematic review and meta-analysis showed no significant difference in oncological outcomes, including 2-year disease-free and overall survival rate, between the transanal and laparoscopic TME groups. Also, no significant difference between the two groups in locoregional recurrence and distant metastasis rates was found and no obvious heterogeneity was observed between the groups (I2 < 40%). However, the follow-up period in selected studies was significantly shorter for the TaTME group, than for the LaTME group. Rasulov et al. [27] reported similar results in local recurrence, distant metastasis, and 3-year disease-free survival in the two groups; however, this study included only selected “difficult” patients. Our results correspond to those of four meta-analyses published in the last 3 years, comparing long-term oncological results after transanal and laparoscopic TME, and showing no significant difference between the two groups [28,29,30,31]. However, some of them compared results of APRs (transanal access platforms were not used, transanal dissection was performed with transanal retractors etc.), which may bias the outcomes. For these reasons, we excluded the studies by Kanso et al. [32], Denost et al. [33]. Similarly, we excluded the study by Pontallier et al. on the functional outcomes and QoL [34]. In addition, our meta-analysis included the most recent studies (which were not included in the previous analyses) and compared only “pure” TaTME and LaTME procedures.
Currently, the published data on the functional outcomes and QoL after TaTME and LaTME are limited and there are no meta-analyses available. The function results of the included studies were assessed using the Wexner and LARS score via a questionnaire. Fecal incontinence is the element of LARS with the highest impact on QoL in terms of social and professional life [35]. Although TaTME related to a better visualization of sacral nerves and thus could be associated with better functional results [36], Foo et al. showed that in 3 months after stoma reversal the LARS score was significantly higher in the TaTME group than in the conventional TME group (p = 0.045). However, no significant difference was found at 6 and 12 months after surgery [37]. This meta-analysis demonstrated a similar Wexner score after TaTME and LaTME; however, the LARS score was significantly higher in the TaTME group. The difference in LARS score has several potential explanations.
No information about the height of anastomosis above sphincters were found in the studies included in the meta-analysis. In terms of tumor height from the anal verge Rubinkiewicz et al. [15] reported a median height of 4 cm (IQR 3–5 cm) vs. 3 cm (IQR 2–4 cm) in LaTME and TaTME groups, respectively (p = 0.01). The significant difference between the groups in tumor location may lead to a risk of selection bias. In both studies of Bjoern et al. [16, 17], the height of the tumor was comparable: p = 0.509 [16] and p = 0.599 [17] in the two groups Veltcamp [18] reported that tumor height was ≤ 15 cm from the anal verge for TaTME and LaTME without providing any details, though the authors mentioned that it was comparable (p = 0.569).
Another explanation is the discrepancy between times to follow-up between groups.
Only one study, published by Rubinkiewicz et al. [15] reported LARS svores obtained at a cetrtain timepoint. Fecal incontinence was assessed at 6 months after ileostomy reversal and the timepoint of comparison was the same in the two groups. In other studies [16,17,18], a timepoint of assessment of anorectal function was not mentioned [16,17,18]. In these studies Bjoern [16], Bjoern [17], Veltcamp [18] only follow-up period was reported and it was significantly shorter for the TaTME group than for the LaTME group: 23.8 vs. 70.6, 22.6 vs.75.1 and 20.0 vs. 59.5 months, respectively.
The significantly longer time to follow-up in the laparoscopic group can explain less severe symptoms of LARS, which improve over time. Furthermore, anal sphincter function also improves in long-term period.
Prolonged anal dilation due to the use of transanal platform for Ta TME is a possible risk factor for worse functional outcome. Allaix [38] compared anal function before and after local excision of rectal lesions using transanal endoscopic microsurgery (TEM). The authors found that anal function was impaired after TEM, though only temporarily. The insertion of a transanal platform and dilatation of the anal sphincter during TaTME might have a similar adverse effect on anal function and lead to a higher LARS score.
The last but not the least explanation of a worse LARS score is a technical bias towards lower anastomosis after TaTME than after conventional TME, which can affect the anal transitional zone. However, no data on patients after intersphincteric resection (ISR) were found in the studies [15,16,17,18].
In terms of LARS and Wexner scores, no obvious heterogeneity was observed between the groups (I2 < 40%). The presented meta-analysis did not detect significant differences in IPSS between the two groups. Although the heterogeneity between the studies that reported IPSS was substantial (I2 = 53%), it was not statistically significant.
Only two studies included in our meta-analysis, focused on patients’ QoL, reporting the dataobtained with the EORTC QLQ-C30 (Table 6), EORTC QLQ-CR29 (Table 7) and EQ-5D-3L (Table 8) questionnaires [17, 18]. In the study published by Bjoern et al. [17], according to EORTC QLQ-C30, emotional functioning and the symptom of diarrhea were significantly in favor of LaTME (p = 0.041 and p = 0.009, respectively). The remaining functional and symptom scales were comparable. According to EORTC QLQ-CR29, pain in the buttocks and dysgeusia were significantly in disfavor of the TaTME group (p = 0.011 and p = 0.047, respectively). All other functional scales and symptoms were comparable in the two groups. In the study published by Veltcamp et al. [18], according to the EORTC QLQ-CR29, the fecal incontinence score was worse for the TaTME group. According to the EORTC QLQ-CR29, EORTC-QLQ C30 and EQ-5D-3L, all other functional scales and symptoms were comparable in the two groups.
Our systematic review and meta-analysis has some limitations. In the ten included studies, there were no RCTs. They are still in progress and will be published in the next few years [2,3,4]. The lack of RCTs in the analysis can lead to a high risk of bias, such as selection, performance, detection bias, and substantial heterogeneity between studies. Thus, we need to wait until the COLOR III, ETAP-GRECCAR and TaLaR have their final data regarding long-term oncological, functional outcomes and QoL. Moreover, the ten studies included were all published in English, and thus, publication bias cannot be excluded. We did not contact the authors to obtain additional data which were not published, although it would potentially improve the quality of the meta-analysis.
Conclusions
The results of this meta-analysis showed that both TaTME and the LaTME have similar long-term oncological and functional outcomes as well as similar QoL. The only exception is a significantly higher LARS score in the TaTME group. The current data are based mainly on observational studies and further well-designed RCTs are required.
References
Sylla P, Rattner DW, Delgado S, Lacy AM (2010) NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc 24:1205–1210
Deijen CL, Velthuis S, Tsai A, Mavroveli S, de Lange-de Klerk ESM, Sietses C, Tuynman JB, Lacy AM, Hanna GB, Bonjer HJ (2016) COLOR III: a multicentre randomised clinical trial comparing transanal TME versus laparoscopic TME for mid and low rectal cancer. Surg Endosc 30:3210–3215
Lelong B, de Chaisemartin C, Meillat H, Cournier S, Boher JM, Genre D, Karoui M, Tuech JJ, Delpero JR (2017) A multicenter randomised controlled trial to evaluate the efficacy, morbidity and functional outcome of endoscopic transanal proctectomy versus laparoscopic proctectomy for low-lying rectal cancer (ETAP-GRECCAR 11 TRIAL): rationale and design. BMC Cancer 17:1–8
Zeng Z, Luo S, Chen J, Cai Y, Zhang X, Kang L (2019) Comparison of pathological outcomes after transanal versus laparoscopic total mesorectal excision: a prospective study using data from randomized control trial. Surg Endosc. https://doi.org/10.1007/s00464-019-07167-1,October4
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and metaanalyses: the PRISMA statement. PLoS Med 62:1006–1012
Wells GA, Shea BJ, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P (2005) The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analyses. Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa. Available from http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 14:1–13
Clarke M, Oxman AD (1999) Cochrane reviewers’ handbook 4.0. The Cochrane Collaboration, Oxford
Chen YT, Kiu KT, Yen MH, Chang TC (2019) Comparison of the shortterm outcomes in lower rectal cancer using three different surgical techniques: transanal total mesorectal excision (TME), laparoscopic TME, and open TME. Asian J Surg 42:674–680
Mege D, Hain E, Lakkis Z, Maggiori L, Prost a la Denise J, Panis Y, (2018) Is trans-anal total mesorectal excision really safe and better than laparoscopic total mesorectal excision with a perineal approach first in patients with low rectal cancer? A learning curve with case-matched study in 68 patients. Colorectal Dis 20:O143–O151
Veltcamp Helbach M, Koedam TWA, Knol JJ, Diederik A, Spaargaren GJ, Bonjer HJ, Tuynman JB, Sietses C (2019) Residual mesorectum on postoperative magnetic resonance imaging following transanal total mesorectal excision (TaTME) and laparoscopic total mesorectal excision (LapTME) in rectal cancer. Surg Endosc 33:94–102
Marks JH, Montenegro GA, Salem JF, Shields MV, Marks GJ (2016) Transanal TATA/TME: a case-matched study of taTME versus laparoscopic TME surgery for rectal cancer. Tech Coloproctol 20:467–473
Lelong B, Meillat H, Zemmour C, Poizat F, Ewald J, Mege D, Lelong JC, Delpero JR, de Chaisemartin C (2017) Short- and mid-term outcomes after endoscopic transanal or laparoscopictransabdominal total mesorectal excision for low rectal cancer: a single institutional case-control study. J Am Coll Surg 224:917–925
De’Angelis N, Portigliotti L, Azoulay D, Brunetti F (2015) Transanal total mesorectal excision for rectal cancer: a single center experience and systematic review of the literature. Langenbeck’s Arch Surg 400:945–959
Rubinkiewicz M, Zarzycki P, Witowski J, Pisarska M, Gajewska N, Torbicz G, Nowakowski M, Major P, Budzyński A, Pedziwiatr M (2019) Functional outcomes after resections for low rectal tumors: comparison of transanal with laparoscopic total mesorectal excision. BMC Surg 19:1–6
Bjoern MX, Perdawood SK (2020) Manometric assessment of anorectal function after transanal total mesorectal excision. Tech Coloproctol 24:231–236
Bjoern MX, Nielsen S, Perdawood SK (2019) Quality of life after surgery for rectal cancer: a comparison of functional outcomes after transanal and laparoscopic approaches. J Gastrointest Surg 23:1623–1630
Veltcamp Helbach M, Koedam TWA, Knol JJ, Velthuis S, Bonjer HJ, Tuynman JB, Sietses C (2018) Quality of life after rectal cancer surgery: differences between laparoscopic and transanal total mesorectal excision. Surg Endosc 33:79–87
Stevenson ARL, Solomon MJ, Lumley JW, Hewett P, Clouston AD, Gebski VJ, Davies L, Wilson K, Hague W, Simes J (2015) Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: the ALaCaRT randomized clinical trial. JAMA 314:1356–1363
Fleshman J, Branda M, Sargent DJ et al (2015) Effect of laparoscopic assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes: the ACOSOG Z6051 randomized clinical trial. JAMA 314:1346–1355
Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MHGM, de Lange-de Klerk ESM, Lacy AM, Bemelman WA, Andersson J, Angenete E, Rosenberg J, Fuerst A, Haglind E (2015) A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 372:1324–1332
Hompes R, Arnold S, Warusavitarne J (2014) Towards the safe introduction of transanal total mesorectal excision: the role of a clinical registry. Colorectal Dis 16:498–501
Sylla P, Bordeianou LG, Berger D, Han KS, Lauwers GY, Sahani DV, Sbeih MA, Lacy AM, Rattner DW (2013) A pilot study of natural orifice transanal endoscopic total mesorectal excision with laparoscopic assistance for rectal cancer. Surg Endosc 27:3396–3405
Heald RJ (2013) A new solution to some old problems: transanal TME. Tech Coloproctol 17:257–258
Nagtegaal ID, Marijnen CA, Kranenbarg EK, Van de Velde CJ, van Krieken JH, Committee PR, Investigators CC (2002) Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit. Am J Surg Pathol 26:350–357
Adam IJ, Mohamdee MO, Martin IG, Scott N, Finan PJ, Johnston D, Dixon MF, Quirke P (1994) Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet 344:707–711
Rasulov AO, Dzhumabaev KE, Kozlov SYE, Mamedli ZZ, Kulushev VM, Gordeev SS, Kuzmichev DV, Polynovsky AV (2018) Transanal mesorectumectomy for rectal cancer—is it optimal surgery for ‘difficult’ patients? Khirurgiia (Mosk) 6:4–21
Aubert M, Mege D, Panis Y (2019) Total mesorectal excision for low and middle rectal cancer: laparoscopic versus transanal approach-a meta-analysis. Surg Endosc. https://doi.org/10.1007/s00464-019-07160-8,October15
Lin D, Yu Z, Chen W, Hu J, Huang X, He Z, Zou YF, Yu X, Guo X, Wu XJ (2019) Transanal versus laparoscopic total mesorectal excision for mid and low rectal cancer: a meta-analysis of short-term outcomes. Wideochir Inne Tech Maloinwazyjne 14:353–365
Zhang X, Gao Y, Dai X, Zhang H, Shang Z, Cai X, Shen T, Cheng X, Yu K, Li Y (2019) Short- and long-term outcomes of transanal versus laparoscopic total mesorectal excision for mid-to-low rectal cancer: a meta-analysis. Surg Endosc 33:972–985
Simillis C, Lal N, Thoukididou SN, Kontovounisios C, Smith JJ, Hompes R, Adamina M, Tekkis PP (2019) Open versus laparoscopic versus robotic versus transanal mesorectal excision for rectal cancer: a systematic review and network meta-analysis. Ann Surg 270:59–68
Kanso F, Maggiori L, Debove C, Chau A, Ferron M, Panis Y (2015) Perineal or abdominal approach first during intersphincteric resection for low rectal cancer: which is the best strategy? Dis Colon Rectum 58:637–644
Denost Q, Loughlin P, Chevalier R, Celerier B, Didailler R, Rullier E (2018) Transanal versus abdominal low rectal dissection for rectal cancer: long-term results of the Bordeaux’ randomized trial. Surg Endosc 32:1486–1494
Pontallier A, Denost Q, Van Geluwe B, Adam JP, Celerier B, Rullier E (2016) Potential sexual function improvement by using transanal mesorectal approach for laparoscopic low rectal cancer excision. Surg Endosc 30:4924–4933
Trenti L, Galvez A, Biondo S, Solis A, Vallribera-Valls F, Espin-Basany E, Garcia-Granero A, Kreisler E (2018) Quality of life and anterior resection syndrome after surgery for mid to low rectal cancer: a cross-sectional study. Eur J Surg Oncol 44:1031–1039
Chouillard E, Regnier A, Vitte RL, Bonnet BV, Greco V, Chahine E, Daher R, Biagini J (2016) Transanal NOTES total mesorectal excision (TME) in patients with rectal cancer: is anatomy better preserved? Tech Coloproctol 20:537–544
Foo CC, Kin Ng K, Tsang JS, Siu-Hung Lo O, Wei R, Yip J, Lun Law W (2020) Low anterior resection syndrome after transanal total mesorectal excision: a comparison with the conventional top-to-bottom approach. Dis Colon Rectum 63:497–503
Allaix ME, Rebecchi F, Giaccone C, MistrangeloMorino M (2011) Long-term functional results and quality of life after transanal endoscopic microsurgery. Br J Surg 98:1635–1643
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Drs. Iuliia Alimova, Stanislav Chernyshov, Marat Nagudov and Evgeniy Rybakov have no conflicts of interest or financial ties to disclose.
Ethical approval
Ethics approval was not required for this systematic review and meta-analysis.
Informed Consent
Informed consent was not required for this systematic review and meta-analysis.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alimova, I., Chernyshov, S., Nagudov, M. et al. Comparison of oncological and functional outcomes and quality of life after transanal or laparoscopic total mesorectal excision for rectal cancer: a systematic review and meta-analysis. Tech Coloproctol 25, 901–913 (2021). https://doi.org/10.1007/s10151-021-02420-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10151-021-02420-z