Abstract
Purpose
The present study aimed to compare the operative and oncologic results of transanal total mesorectum excision (Ta-TME) (“down-to-up”) vs. laparoscopic TME (L-TME, “up-to-down”) for low rectal cancer. Additionally, a systematic review of the literature was performed to assess the quality of the current body of evidence on Ta-TME.
Methods
The study population included 32 consecutive patients who underwent Ta-TME between January 2011 and December 2014 that were compared with a matched group of patients undergoing L-TME between January 2008 and December 2010. The literature search was performed following the PRISMA guidelines for a systematic review.
Results
Ta-TME was associated with significantly shorter operative time (195 vs. 225 min; p = 0.017) and hospital stay (7.8 vs. 9.7 days; p = 0.018) compared to L-TME. No group differences were observed for intra-/postoperative complications and oncologic outcomes. One patient in the Ta-TME and two patients in the L-TME group developed local recurrence. The estimated survival rate at 2 years was 95.5 % for the Ta-TME and 96.6 % for the L-TME group (p = 0.646).
The literature search identified 22 relevant retrospective studies on 423 patients operated on Ta-TME or robotic-assisted transanal TME for rectal cancer. The only two comparative studies found similar short-term oncologic outcomes between Ta-TME and L-TME. A complete mesorectum was observed in 85 % of Ta-TME cases. The conversion rate was estimated at 4.3 % and the postoperative complication rate at 30.4 %.
Conclusions
Ta-TME appears to be safe and feasible. It may find special application in patients with anatomic constraints that could make L-TME highly challenging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Total mesorectal excision (TME) is the key oncological principle in the management of rectal cancer [1]. Indeed, the integrity of mesorectal fascia and the circumferential radial margins are demonstrated as the measure of the quality of the TME specimen and represent the major prognostic factors for rectal carcinoma resection [2–4], influencing the risk of local and overall recurrences as well as the patient’s survival [5].
Recently published randomized clinical trials, such as COLOR II [6], COREAN [7, 8], and CLASICC [9], demonstrated that laparoscopic TME can achieve equivalent short- and long-term (i.e., 3 to 5 years) oncologic outcomes than the conventional approach with better results in terms of patient recovery and hospital stay associated with the minimally invasive surgery [6, 10]. Moreover, the laparoscopic TME appeared to be superior to open surgery in low rectal tumors probably because laparoscopy gets a better view and thus facilitates the mobilization of the rectum in this subset of patients [6]. Still, laparoscopic TME is a demanding technique, and it may be hardly performed in male patients with narrow pelvis or in obese patients. Indeed, approximately 16 % (range 0–30 % according to different studies) of laparoscopic TME are converted to an open approach, mainly due to technical difficulties [6, 9–14].
To overcome these limitations, a few years ago, a “down-to-up” procedure with an endoscopic transanal approach was proposed to give new options in difficult cases and improve the quality of surgery [15]. This technique uses multiport transanal devices with standard laparoscopic instruments to perform TME [16]. Since the first case reported in 2010 [17], few studies evaluated transanal surgery for TME (Ta-TME) in mid and low rectal cancer and concluded that the rectal dissection from below can be much easier than either minimally invasive or open surgery from above [17–19]. More recently, Ta-TME has also been performed using robotic-assisted surgery, which might offer technical advantages over laparoscopy ultimately to lead to a better quality of surgical resection [20, 21]. However, evidence appears to be limited, although promising.
In this clinical and scientific scenario, the present study aimed to compare Ta-TME (down-to-up approach) vs. laparoscopic TME (“up-to-down” approach) in terms of operative and oncologic results in a single center series of patients with rectal cancer. Additionally, a systematic review of the literature was performed to assess the quality of the current body of evidence on Ta-TME.
Materials and methods
Study population
The study population included consecutive patients who underwent transanal surgery for TME (Ta-TME) with coloanal anastomosis (CAA) between January 2011 and December 2014 in the tertiary care center of Henri Mondor Hospital, Créteil (France). All included patients had histologically proven low rectal adenocarcinoma located up to 5 cm from the anal verge. Patients who had undergone Ta-TME were compared with a matched group of patients undergoing laparoscopic TME (L-TME) between January 2008 and December 2010 for the same pathology. Groups were matched based on age, gender, BMI, and type of procedure. The same surgical team performed all L-TME and Ta-TME procedures considered for the analysis. The study was approved by the local Institutional Review Board and performed according to the ethical principles ascertained in the Declaration of Helsinki.
Preoperative assessment
All patients in both groups were preoperatively assessed by the following: magnetic resonance imaging (IMR) and endorectal ultrasonography for local staging, total body computed tomography (CT) for distance metastasis detection, and blood analyses with carcinoembryonic antigen serum concentration. Patients with locally advanced tumors (i.e., T3, T4N0, or T1-T4N1-N2) were treated with neoadjuvant chemoradiation with a total dose of 45–50.4 Gy delivered in daily fractions of 1.8–2 Gy over a 5- to 6-week period associated with 5-fluorouracil infusion.
Surgical techniques
Surgery was performed 6 to 8 weeks after the completion of chemoradiotherapy, if needed. Mechanical bowel cleansing by oral polyethylene glycol solution was systematically performed the day before surgery. All patients received preoperative immunonutrition and intravenous antibiotic prophylaxis with 2 g of cefoxitin (or 400 mg of ciprofloxacin) and 500 mg of metronidazole.
For both Ta-TME and L-TME, patients were placed in the lithotomy position with legs in padded, adjustable stirrups. A postoperative nasogastric tube was not routinely used. Conversely, a urinary catheter was always placed and removed 24 h postoperatively. In all cases, one drain was placed in the pelvic cavity.
The L-TME was performed following the conventional protocol with 5 ports and a 30° scope [22, 23]. Surgical devices used during the procedure included the following: bipolar cautery, Harmonic Scalpel (Ethicon EndoSurgery, Inc., Cincinnati, OH, USA), and Endo GIA stapler (US Surgical Corp., Norwalk, CT, USA). L-TME was carried out up-to-down, according to the key principles of a correct oncologic surgical procedure. After high ligation of the inferior mesenteric vessels, the splenic flexure was mobilized to achieve a tension-free anastomosis, and the colon was divided at the descending sigmoid junction. The rectal excision was completed transanally at the dentate line. Then, after transanal specimen retrieval, a hand-sewn CAA was performed. Diverting ileostomy was always fashioned at the right lower abdomen. Conversion was defined as the shift from L-TME to open surgery.
The Ta-TME started with the placement of the Lone Star Retractor (Lone Star Medical Products Inc., Houston, TX, USA) to expose the anal canal [24, 25]. A full-thickness circumferential rectal transection was performed above the dentate line. For very low-lying tumors, an intersphincteric dissection was also carried out. Then, the distal rectum was closed with a purse string suture as soon as possible to minimize the risk of septic contamination and spillage of tumor cells. After the closure of the rectal stump, the GelPOINT Path Transanal Access Platform (Applied Medical, Rancho Santa Margherita, CA, USA) was introduced trough the anus. A pneumorectum was created with CO2 with a pressure of 10 mmHg using three triangulated ports and a 30° scope. Surgical devices used during the procedure included a bipolar cautery and a Harmonic Scalpel (Ethicon EndoSurgery, Inc., Cincinnati, OH, USA). The avascular presacral plane was developed by gently pushing against the tissue, starting at the dorsal side. The dissection was extended down-to-up first posteriorly, then anteriorly, and finally laterally to achieve a correct oncologic surgical procedure. After the mobilization of the rectum, the peritoneal reflection was exposed and opened, thereby entering the peritoneal cavity. At this point, the procedure was completed by the laparoscopic part, which was performed with four standard laparoscopic ports. The descending colon and sigmoid were mobilized from medial to lateral as during traditional laparoscopic surgery, and the splenic flexure was also mobilized. The fecal stream was diverted with an ileostomy at the right lower abdomen. The rectosigmoid was exteriorized transanally and, after the sigmoid transection and the anal retrieval of the specimen, a hand-sewn CAA was performed. Conversion was defined as a Ta-TME procedure not completed down-to-up transanally.
Study outcomes
Ta-TME and L-TME were compared by means of intraoperative and postoperative variables, including blood loss, operative time, complication rates, conversion rate, postoperative morbidity and mortality (within 90 days), and survival. The Dindo-Clavien classification was used to describe the postoperative complications [26]. Moreover, oncological parameters (e.g., positivity of resection margins, number of lymph nodes harvested) were evaluated and compared between groups. Tumor characteristics and TNM staging were recorded. Pathological examination was performed according to the standardized procedure as described by Quirke et al. [27]. The quality of the mesorectum was scored using three grades, “complete,” “nearly complete,” or “incomplete.” Tumor involvement of the circumferential resection margin of 2 mm or more was considered as positive, as defined by Nagtegaal et al. [3]. The Cleveland Clinic Florid (Wexner) score was used to evaluate fecal incontinence approximately 3 months after ileostomy closure [28].
All patients were followed every 3 months for the first 3 years and every 6 months thereafter (French Guidelines from the Thesaurus National de Cancerologie Digestive, 2011). At the follow-up visits, physical examination, CT, and serum chemistry analysis were performed. Colonoscopy was carried out if abnormalities were detected during any follow-up visit.
Statistical analysis
Statistical analyses were performed with Statistical Package for Social Science (SPSS, IBM SPSS Statistics, Version 22 for Macintosh). For group comparisons, the chi-square test or Fisher’s exact test was used for categorical variables, and, according to the data distribution, the t test or Mann–Whitney U test was applied for continuous variables. Hence, continuous data were expressed as the means (standard deviation, SD) or medians and ranges (minimum to maximum). Overall and disease-free survival rates were analyzed using the Kaplan–Meier method and compared between groups using the log rank (Mantel–Cox) test. A p value <0.05 was considered to be statistically significant.
Systematic review
The literature review was conducted using a systematic approach and following the PRISMA statements checklist [29]. Relevant articles were selected if presenting results about patients treated with transanal TME (Ta-TME), and all its different nomenclatures, including transanal minimally invasive surgery for TME (TAMIS-TME), transanal endoscopic microsurgery (TEM), natural orifice transluminal endoscopic surgery (NOTES), or robotic-assisted transanal surgery for TME (RATS-TME) for rectal cancer. Trials evaluating patients with benign pathology, reports of local excision, or studies on cadaver and swine models were excluded. Case reports were also excluded. All types of TME procedures were considered, including low anterior resection of the rectum (LAR), abdominoperineal resection (APR), partial intersphincteric resection (pISR), and total intersphincteric resection (tISR). The following online databases were searched on May 2015: MEDLINE (through PubMed), EMBASE, Google Scholar, Cochrane and ProQuest Dissertations, and Thesis Database. To increase the probability of identifying all relevant articles, a specific research equation was formulated for each database, using the following keywords and/or MeSH terms: Ta-TME, transanal rectal resection, TAMIS, endoscopic transanal proctectomy (ETAP), transanal proctectomy, NOTES, transanal total mesorectal excision, transanal minimally invasive surgery, transanal TME, and rectal cancer. In addition, reference lists from eligible studies and relevant review articles (not included in the systematic review) were crosschecked to identify additional studies. A grey literature search was also performed by using the OpenGrey database. No time restriction was applied. Studies written in English and meeting the selection criteria were reviewed. Two reviewers (NdeA and LP) independently retrieved, screened, and analyzed the selected studies. Risk of bias and study quality was assessed by using the Newcastle-Ottawa Scale (NOS) [30] and the Grading of Recommendations Assessment Development and Evaluation (GRADE) system [31].
The outcomes of interest included intraoperative variables (e.g., blood loss, operative time, conversion rate, operative time), postoperative results (e.g., postoperative complication, length of hospital stay), oncological criteria (e.g., circumferential and distal margins, number of harvested lymph nodes, quality of mesorectum), feasibility and safety of transanal TME. Data from the studies included in the systematic review were processed for qualitative and possibly quantitative analyses. Outcome measures (mean values, standard deviation, and ranges) were extracted for each treatment approach and estimated as weighted means.
Results
Comparison between Ta-TME and L-TME for rectal cancer
The study population included 32 patients who underwent Ta-TME and 32 patients who underwent L-TME. Demographic and clinical characteristics of the matched groups are shown in Table 1. Overall, the study sample included 42 (65.6 %) males and 22 (34.4 %) females. Only 8 patients (15.6 %) were obese. All patients were diagnosed with rectal adenocarcinoma located in the low rectum, at a median distance from the anal verge of 4 cm (range 2.5–5 cm), and 1 cm from the anal ring (0–3 cm). In both groups, two patients were treated with intersphincteric resection (ISR) to obtain negative distal margins. The majority of the patients (78.1 %) received neoadjuvant therapy. No difference was observed in tumor size, T stage, and N stage between the Ta-TME and L-TME groups.
Operatively, all patients had hand-sewn straight CAA. The Ta-TME procedures were associated with significantly shorter operative time (195 vs. 225 min; p = 0.017) and hospital stay (7.8 vs. 9.7 days; p = 0.018) compared to L-TME (Table 2). No other differences were observed between the two groups about the operative and postoperative variables assessed. No intraoperative complication occurred. However, one Ta-TME and one L-TME were converted due to technical difficulties, which could hamper the achievement of a correct oncologic resection. Specifically, the Ta-TME was converted because of problems with insufflation due to a too early Douglas peritoneum opening, and the L-TME was converted due to difficult exposition during low anterior rectal dissection.
Postoperative mortality was nil. Eight (25 %) patients in the Ta-TME group and 12 (37.5 %) patients in the L-TME group developed postoperative complications (p = 0.419). In the Ta-TME group, postoperative complications included urinary disorder (n = 1), urinary infection (1), wound infection (1), pelvic abscess in the proximity of the anastomosis (anastomotic leakage grade A, according to the International Study Group of Rectal Cancer, 2010) (2), blood cell transfusion (1), anastomotic leakage medically managed (grade B) (1), and anastomotic leakage requiring surgical drainage (grade C) (1). In the L-TME group, postoperative complications included urinary disorders (2), pulmonary infection (1), pelvic abscess in the proximity of the anastomosis (anastomotic leakage grade A) (2), ileus (1), blood cell transfusion (1), anastomotic leakage medically treated (grade B) (4), and anastomotic leakage requiring surgical drainage (grade C) (1). Overall, 5 patients (7.8 %) were readmitted for pelvic collection (2), ileostomy complications (1), acute urinary retention (1), and dehydration (1).
Both procedures were associated with adequate oncologic outcomes; only 4 (6.2 %) patients presented with positive circumferential margin, and 2 (3.1 %) patients with positive distal resection margin. Overall, an average of 18 (SD: 8.97) lymph nodes were harvested, without difference between the Ta-TME and L-TME procedures. The Wexner score did not differ between groups, with a median score of 9 for the Ta-TME group (range 3–15), and a median of 10.5 for the L-TME group (range 4–19) (p = 0.115). Overall, 38 (59.4 %) patients (22 in the Ta-TME group and 16 in the LT-TME group) had a Wexner score <10 (mild incontinence).
The mean follow-up was 32.06 (12.1) months for the Ta-TME group and 62.91 (12.3) for the L-TME group. Two patients (6.2 %) in the Ta-TME group showed tumor recurrence: one patient with R1 resection developed local recurrence at 14 months after Ta-TME and died at 33 months due to an untreatable disease progression; one patient presented with untreatable metachronous lung metastases at 11 months after Ta-TME and died at 27 months. Four patients (12.5 %) in the L-TME group showed tumor recurrence: two patients with R1 resection developed local recurrences at 12 and 13 months after L-TME, respectively, and died at 31 and 37 months, respectively. One patient presented with metachronous liver metastasis diagnosed at 8 months after L-TME and was treated by surgery and chemotherapy; the patient is still alive. The fourth patient in the L-TME group showed metachronous liver and lung metastases at 7 months after L-TME, which were treated by surgery and chemotherapy. He died due to untreatable disease progression at 43 months. For the entire study population, the overall survival rate was 90.6 % over a mean follow-up of 47.5 months. The estimated survival rate at 2 years was 95.5 % for the Ta-TME group and 96.6 % for the L-TME group (p = 0.646). Disease-free survival rate at 2 years was 90.5 % for the Ta-TME group and 85.2 % for the L-TME group (p = 0.395).
Systematic review of the literature
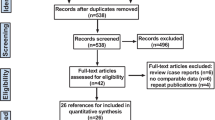
Overall, the literature search identified 270 articles (after removing duplicates); of these, 223 were excluded upon title and abstract evaluation because of not being pertinent to the review question (n = 216) or not pertinent to the study design (n = 7). Out of the remaining 47 articles that underwent a full-text evaluation, 25 were excluded because they did not meet the inclusion criteria. Finally, 22 articles were found eligible for the systematic review and were included in the qualitative synthesis of the current literature (Fig. 1).
Flow chart of study search, selection, and inclusion. Example of search equation: “transanal”[All Fields] AND (“total mesorectal excision”[All Fields] OR “proctectomy”[All Fields] OR “NOTES”[All Fields] OR “hybrid NOTES”[All Fields] OR “robotic”[All Fields] OR “TME”[All Fields] OR “minimally invasive surgery”[All Fields] OR “TaTME”[All Fields]) AND (“2010/01/01”[PDAT]:“3000/12/31”[PDAT])
The selected studies were 20 retrospective case series [18, 20, 21, 24, 25, 32–46] and 2 retrospective case–control comparative studies [47, 48]. They were conducted in 10 different countries including Europe (n = 16), North America (n = 3), South America (n = 1), Asia and the Pacific (n = 2). The study time frame ranged from 2012 to 2015. A total of 423 patients underwent Ta-TME (n = 407) or RATS-TME (n = 16) (range 3–80 patients/study) for rectal cancer most of the time (68.3 % of cases) located in the low rectum (Table 3). Overall, 375 (88.7 %) LAR, 30 (7.1 %) APR, 17 (4 %) pISR, and 1 (0.2 %) tISR were performed by Ta-TME or RATS-TME (Table 4). Only 6/22 (27.2 %) studies used a two-team approach where the abdominal and transanal approaches were carried out simultaneously [18, 31, 38, 39, 43, 47]. The anastomosis was performed by the use of a circular mechanical stapler in 157 cases and as hand-sewn CAA in 152 patients. Stoma diversion was carried out in 74.4 % of the patients. The overall weighted mean operative time was 242.4 min (range 143–375 min). Specifically, the weighted mean operative time of Ta-TME was 240.66 (143–365 min), while the weighted mean operative time of RATS-TME was 283.37 min (range 165–375 min).
Overall, 11 (2.6 % of cases) intraoperative complications were reported, including bleeding (n = 2), perforation of the rectum (4), urethral injuries (2), pneumatosis of small bowel mesentery (1), impossible dissection due to radiotherapy induced fibrosis (1), and air embolism (1). Conversion rate was estimated at 4.3 %. The described 10 cases were converted due to adhesions of previous abdominal surgery (3), technical difficulties due to a narrow pelvis in obese patients (2), tumor features such as high location (1), bulky tumor (1), posterior fixity (2), and bleeding (1).
The overall weighted mean length of hospital stay was 8.31 days (range 4.3–14), with a weighted mean of 8.45 days (range 4.5–14) for Ta-TME and 5.05 days (range 4.3–6) for RATS-TME. Overall, 119 (30.4 %) postoperative complications were reported, including (when specified) persistent postoperative ileus (n = 20), anastomotic leak (17), urinary dysfunction (16), pelvic abscess/pelvic fluid collections (16), dehydration due to high ileostomy output (6), and, more rarely, late strictures at the level of the anastomosis (4). A particular case of a patient readmitted for circular full-thickness ischemia of the mucosa in the anal canal possibly due to transanally placed trocar was reported by Veltcamp Helbach et al. [44]. The overall weighted mean postoperative mortality was 0.51 %. The only two cases of postoperative death reported were due to myocardial infarction [40] and septic complications after reoperation of an anastomotic leakage [44].
Histological findings of the included studies are summarized in Table 5. The quality of the mesorectal specimen was defined as complete (according to Quirke’s classification [2]) in 85.1 % of cases, nearly complete in 8.9 % of cases, and incomplete in 1.9 % of cases. Of note, most clinical series reported only short-term oncological outcomes with a weighted mean follow-up time of 18.3 months (range 2.5–29). Only two comparative studies in the literature evaluated Ta-TME for rectal cancer vs. L-TME [47, 48]. Both studies were in accordance concerning the short-term oncologic outcomes that appeared similar between the two operative techniques. However, Fernandez-Hevia et al. [47] found that significant shorter operative time and hospital stay were associated with Ta-TME compared to L-TME; moreover, early readmissions were more frequent in the laparoscopic group. Finally, two reviewers (NdeA and LP) scored the methodological quality of the included studies according to the criteria described above. All the 22 studies included were assessed at high risk of bias (Supplement Table S1). Overall, the available literature was rate at very low [18, 20, 21, 24, 25, 32–46] or low quality [47, 48] according to the GRADE system.
Discussion
The present matched case–control study shows that Ta-TME combined with abdominal laparoscopic assistance is an oncologically safe approach for low rectal cancer associated with shorter operative time and hospital stay compared to L-TME. These results are in accordance with the previous literature that demonstrated the feasibility of the down-to-up approach for TME [47, 48]. The shorter operative time associated with Ta-TME may reflect the fact that some critical steps of the procedure (e.g., dissection of the distal horizontal part of the rectum) are simplified by this surgical approach, which secures a direct visualization and a safer exposure especially of the low rectum [24]. Indeed, the surgeon’s view in the same axis as the low rectum and the plane of dissection allows overcoming the technical limitations in case of narrow pelvis, bulky tumors, or voluminous prostate. Moreover, the transanal approach could be also started simultaneously with the abdominal approach in a “two-team approach,” and this could further contribute to shorten the related operating time [32]. In the present study, however, as in the majority of the studies reported in the literature, a one-team approach was performed.
In the available comparative studies and in the present one, the conversion rate was low and similar between Ta-TME and L-TME (ranging from 0 to 3 %) [47]. Evidently, these data cannot be directly compared to the results of large randomized controlled trials [14], but they suggest that Ta-TME is a feasible technique. Reasons for conversion include problems with insufflation, bleeding, difficult dissection, and inadequate view, which do not differ between L-TME and Ta-TME. Moreover, when considering the current available literature, the weighted mean conversion rate of Ta-TME is estimated at 4.3 %, which is largely inferior to the 16 % for conversion observed for L-TME in mid and low rectal cancer [6]. Although promising, these results need to be interpreted with caution because they are derived from small retrospective studies only.
Intraoperative and postoperative complication rates appeared to be similar between the two TME techniques, although lower but not significant incidence of postoperative complications was associated with Ta-TME in both the present study (25 % for Ta-TME vs. 37.5 % for L-TME) and in the one published by Fernandez-Hevia et al. [47] (32 % for Ta-TME vs. 51 % for L-TME). Moreover, this latter study found a significant low rate of early readmission supporting a lower incidence of postoperative complications related to Ta-TME [47]. In the literature, the mean postoperative complication rate of Ta-TME is estimated at 30.4 % that is very similar to the one reported in a recent meta-analysis for L-TME in rectal cancer [14]. Also, the Ta-TME related mortality rate, which was nil in the present study, is very low based on the available literature (0.51 %) and similar to L-TME (1 %) and or open TME (2.4 %) [14].
Concerning the quality markers of rectal cancer surgery, Ta-TME was found to achieve complete mesorectum, adequate number of lymph nodes harvested, and negative resection margins in the large majority of the patients. Particularly, 84 % of patients in the present study had a complete mesorectal resection without difference from the L-TME group. This result was also observed by Fernandez-Hevia et al. [47] and Velthuis et al. [48] in their comparative studies. From the literature, the complete mesorectum is observed in an average of 85.1 % of Ta-TME cases, while complete plus nearly complete in 94 % of cases. These estimates are in accordance with the present results and very similar to those reported in the COLOR II trial (84 %), although caution is required because these studies are not directly comparable due to differences in study design, tumor location, and staging. For example, several trials excluded T4 tumors or tumors with distant metastases from their study populations; thus, the generalizability and comparability of the findings are limited. Similarly to the macroscopic quality of the mesorectum, circumferential and distal resection margins were found to be positive only in a small percentage of patients having undergone Ta-TME (3.1 and 6.2 %, respectively, for the presence study and 3.1 and 0.36 %, respectively, for the systematic review of the literature). These data suggest that, despite the direct vision obtained by Ta-TME, the macroscopic distal margin may be underestimated especially when facing highly fibrotic tissues (postradiation). As known, positive margins are the most important predictors of tumor recurrence after surgery [2, 4, 49]; unfortunately, the available literature still lacks long-term studies reliably assessing recurrence rate and survival after Ta-TME. In the present study, the 2-years overall and disease-free survivals were similar between Ta-TME and L-TME, with only 1 patient (3.1 %) in the Ta-TME group and 2 (6.2 %) patients in the L-TME group that developed local recurrence. This finding is in accordance with the 5 % recurrence rate observed in the COLOR II [6] trial, the 2.6 % in the COREAN study [8], and the 5.3 % in the Dutch trial of Kapiteijn et al. [50] for L-TME.
Three studies, including a total of 16 cases, applied robotic surgery to perform TME (RATS-TME) [20, 21, 36]. The very small number of patients treated does not allow drawing any conclusion about the usefulness of this technique. However, based on this preliminary evidence, RATS-TME seems to have satisfactory pathological data and operative results. Its use may be supported by the potential, and still to be proven, advantages of robotic surgery, such as a 3-dimensional view, improved dexterity, reduced tremor, and enhanced ergonomics. Moreover, the stable camera view and the improved visualization of the anatomy including mesorectal plane and plexuses might secure the quality of surgical resection, the oncologic outcomes, and the sexual function far better in the future [21]. Further studies are awaited to assess the clinical and oncologic benefits of robotics in down-to-up TME. Similarly, the use of a 3-dimensional laparoscopic camera may represent an important technical improvement in Ta-TME procedures to obtain a better view, a paramount factor to accomplish a complete cancer resection with sufficient margins [47].
Despite the overall low level of evidence and the lack of randomized controlled trials, Ta-TME could be considered as oncological safe and feasible and a valuable alternative to L-TME for rectal cancer in the hands of experienced laparoscopic colorectal surgeons. This is suggested by the promising oncologic outcomes on the surgical specimen reported in the literature and further supported by the low conversion rate and shorter operative time associated with Ta-TME. Notwithstanding, it is early to conclude that Ta-TME is the option of choice in those selected patients who could be technically demanding by an up-to-down laparoscopy. Moreover, little is known concerning the patient quality of life after Ta-TME, as well as the risk and incidence of nerve damage and functional impairment related to this procedure. Although the Wexner score was acceptable and similar between Ta-TME and L-TME in this study, it remains under investigation whether a prolonged anal dilation due to use of transanal devices could adversely impact on the sphincter functions. Although it appears to be preserved after TEM [51, 52], anorectal function still needs to be assessed following long and complex dissection for rectal cancer by Ta-TME.
Finally, it is advisable that experts in the field make an effort in the near future to establish appropriate Ta-TME training paradigms, standardize the surgical procedure, and define its nomenclature, which appear to be pre-requirements before performing large randomized controlled trials and support the widespread application of this technique.
Conclusion
Ta-TME for mid and low rectal cancer has gained popularity in the last 2 years. The present study and the available literature suggest that Ta-TME is safe and feasible and may find special application in patients with anatomic constraints that could make L-TME highly challenging. The current evidence is promising, although further studies are needed to assess the long-term outcomes of this technique.
References
Heald RJ, Husband EM, Ryall RD (1982) The mesorectum in rectal cancer surgery—the clue to pelvic recurrence? Br J Surg 69:613–616
Quirke P, Steele R, Monson J, Grieve R, Khanna S, Couture J, O'Callaghan C, Myint AS, Bessell E, Thompson LC, Parmar M, Stephens RJ, Sebag-Montefiore D, Investigators MCN-CCT, Group NCCS (2009) Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet 373:821–828
Nagtegaal ID, Marijnen CA, Kranenbarg EK, van de Velde CJ, van Krieken JH, Pathology Review C, Cooperative Clinical I (2002) Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit. Am J Surg Pathol 26:350–357
Lichliter WE (2015) Techniques in total mesorectal excision surgery. Clin Colon Rectal Surg 28:21–27
Kusters M, Marijnen CA, van de Velde CJ, Rutten HJ, Lahaye MJ, Kim JH, Beets-Tan RG, Beets GL (2010) Patterns of local recurrence in rectal cancer; a study of the Dutch TME trial. Eur J Surg Oncol 36:470–476
Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MH, de Lange-de Klerk ES, Lacy AM, Bemelman WA, Andersson J, Angenete E, Rosenberg J, Fuerst A, Haglind E, Group CIS (2015) A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 372:1324–1332
Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim DW, Lim SB, Lee TG, Kim DY, Kim JS, Chang HJ, Lee HS, Kim SY, Jung KH, Hong YS, Kim JH, Sohn DK, Kim DH, Oh JH (2010) Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol 11:637–645
Jeong SY, Park JW, Nam BH, Kim S, Kang SB, Lim SB, Choi HS, Kim DW, Chang HJ, Kim DY, Jung KH, Kim TY, Kang GH, Chie EK, Kim SY, Sohn DK, Kim DH, Kim JS, Lee HS, Kim JH, Oh JH (2014) Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol 15:767–774
Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ (2010) Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg 97:1638–1645
van der Pas MH, Haglind E, Cuesta MA, Furst A, Lacy AM, Hop WC, Bonjer HJ, Group COcLoORIS (2013) Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 14:210–218
Akiyoshi T, Kuroyanagi H, Oya M, Konishi T, Fukuda M, Fujimoto Y, Ueno M, Miyata S, Yamaguchi T (2009) Factors affecting the difficulty of laparoscopic total mesorectal excision with double stapling technique anastomosis for low rectal cancer. Surgery 146:483–489
Ogiso S, Yamaguchi T, Hata H, Fukuda M, Ikai I, Yamato T, Sakai Y (2011) Evaluation of factors affecting the difficulty of laparoscopic anterior resection for rectal cancer: "narrow pelvis" is not a contraindication. Surg Endosc 25:1907–1912
Penninckx F, Kartheuser A, Van de Stadt J, Pattyn P, Mansvelt B, Bertrand C, Van Eycken E, Jegou D, Fieuws S, Procare (2013) Outcome following laparoscopic and open total mesorectal excision for rectal cancer. Br J Surg 100:1368–1375
Arezzo A, Passera R, Scozzari G, Verra M, Morino M (2013) Laparoscopy for rectal cancer reduces short-term mortality and morbidity: results of a systematic review and meta-analysis. Surg Endosc 27:1485–1502
Heald RJ (2013) A new solution to some old problems: transanal TME. Tech Coloproctol 17:257–258
Albert MR, Atallah SB, deBeche-Adams TC, Izfar S, Larach SW (2013) Transanal minimally invasive surgery (TAMIS) for local excision of benign neoplasms and early-stage rectal cancer: efficacy and outcomes in the first 50 patients. Dis Colon Rectum 56:301–307
Sylla P, Rattner DW, Delgado S, Lacy AM (2010) NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc 24:1205–1210
de Lacy AM, Rattner DW, Adelsdorfer C, Tasende MM, Fernandez M, Delgado S, Sylla P, Martinez-Palli G (2013) Transanal natural orifice transluminal endoscopic surgery (NOTES) rectal resection: "down-to-up" total mesorectal excision (TME)—short-term outcomes in the first 20 cases. Surg Endosc 27:3165–3172
Atallah S, Albert M, Larach S (2010) Transanal minimally invasive surgery: a giant leap forward. Surg Endosc 24:2200–2205
Atallah S, Martin-Perez B, Parra-Davila E, deBeche-Adams T, Nassif G, Albert M, Larach S (2015) Robotic transanal surgery for local excision of rectal neoplasia, transanal total mesorectal excision, and repair of complex fistulae: clinical experience with the first 18 cases at a single institution. Tech Coloproctol
Huscher CG, Bretagnol F, Ponzano C (2015) Robotic-assisted transanal total mesorectal excision: the key against the achilles' heel of rectal cancer? Ann Surg 261:e120–e121
Morino M, Parini U, Giraudo G, Salval M, Brachet Contul R, Garrone C (2003) Laparoscopic total mesorectal excision: a consecutive series of 100 patients. Ann Surg 237:335–342
Law WL, Chu KW (2004) Anterior resection for rectal cancer with mesorectal excision: a prospective evaluation of 622 patients. Ann Surg 240:260–268
Tuech JJ, Karoui M, Lelong B, De Chaisemartin C, Bridoux V, Manceau G, Delpero JR, Hanoun L, Michot F (2015) A step toward NOTES total mesorectal excision for rectal cancer: endoscopic transanal proctectomy. Ann Surg 261:228–233
Atallah S, Martin-Perez B, Albert M, deBeche-Adams T, Nassif G, Hunter L, Larach S (2014) Transanal minimally invasive surgery for total mesorectal excision (TAMIS-TME): results and experience with the first 20 patients undergoing curative-intent rectal cancer surgery at a single institution. Tech Coloproctol 18:473–480
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Quirke P, Durdey P, Dixon MF, Williams NS (1986) Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet 2:996–999
Jorge JM, Wexner SD (1993) Etiology and management of fecal incontinence. Dis Colon Rectum 36:77–97
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62:1006–1012
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25:603–605
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ, Group GW (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336:924–926
Chen CC, Lai YL, Jiang JK, Chu CH, Huang IP, Chen WS, Cheng AY, Yang SH (2015) The evolving practice of hybrid natural orifice transluminal endoscopic surgery (NOTES) for rectal cancer. Surg Endosc 29:119–126
Chouillard E, Chahine E, Khoury G, Vinson-Bonnet B, Gumbs A, Azoulay D, Abdalla E (2014) NOTES total mesorectal excision (TME) for patients with rectal neoplasia: a preliminary experience. Surg Endosc 28:3150–3157
Dumont F, Goere D, Honore C, Elias D (2012) Transanal endoscopic total mesorectal excision combined with single-port laparoscopy. Dis Colon Rectum 55:996–1001
Elmore U, Fumagalli Romario U, Vignali A, Sosa MF, Angiolini MR, Rosati R (2015) Laparoscopic anterior resection with transanal total mesorectal excision for rectal cancer: preliminary experience and impact on postoperative bowel function. J Laparoendosc Adv Surg Technol A 25:364–369
Gomez Ruiz M, Parra IM, Palazuelos CM, Martin JA, Fernandez CC, Diego JC, Fleitas MG (2015) Robotic-assisted laparoscopic transanal total mesorectal excision for rectal cancer: a prospective pilot study. Dis Colon Rectum 58:145–153
Knol JJ, D'Hondt M, Souverijns G, Heald B, Vangertruyden G (2015) Transanal endoscopic total mesorectal excision: technical aspects of approaching the mesorectal plane from below-a preliminary report. Tech Coloproctol 19:221–229
Lacy AM, Adelsdorfer C, Delgado S, Sylla P, Rattner DW (2013) Minilaparoscopy-assisted transrectal low anterior resection (LAR): a preliminary study. Surg Endosc 27:339–346
Meng W, Lau K (2014) Synchronous laparoscopic low anterior and transanal endoscopic microsurgery total mesorectal resection. Minim Invasive Ther Allied Technol 23:70–73
Muratore A, Mellano A, Marsanic P, De Simone M (2015) Transanal total mesorectal excision (taTME) for cancer located in the lower rectum: short- and mid-term results. Eur J Surg Oncol 41:478–483
Rouanet P, Mourregot A, Azar CC, Carrere S, Gutowski M, Quenet F, Saint-Aubert B, Colombo PE (2013) Transanal endoscopic proctectomy: an innovative procedure for difficult resection of rectal tumors in men with narrow pelvis. Dis Colon Rectum 56:408–415
Serra-Aracil X, Mora-Lopez L, Casalots A, Pericay C, Guerrero R, Navarro-Soto S (2015) Hybrid NOTES: TEO for transanal total mesorectal excision: intracorporeal resection and anastomosis. Surg Endosc. doi:10.1007/s00464-015-4170-5
Sylla P, Bordeianou LG, Berger D, Han KS, Lauwers GY, Sahani DV, Sbeih MA, Lacy AM, Rattner DW (2013) A pilot study of natural orifice transanal endoscopic total mesorectal excision with laparoscopic assistance for rectal cancer. Surg Endosc 27:3396–3405
Veltcamp Helbach M, Deijen CL, Velthuis S, Bonjer HJ, Tuynman JB, Sietses C (2015) Transanal total mesorectal excision for rectal carcinoma: short-term outcomes and experience after 80 cases. Surg Endosc. doi:10.1007/s00464-015-4221-y
Wolthuis AM, de Buck van Overstraeten A, D'Hoore A (2014) Dynamic article: transanal rectal excision: a pilot study. Dis Colon Rectum 57:105–109
Zorron R, Phillips HN, Wynn G, Neto MP, Coelho D, Vassallo RC (2014) "Down-to-Up" transanal NOTES Total mesorectal excision for rectal cancer: preliminary series of 9 patients. J Minim Access Surg 10:144–150
Fernandez-Hevia M, Delgado S, Castells A, Tasende M, Momblan D, Diaz del Gobbo G, DeLacy B, Balust J, Lacy AM (2015) Transanal total mesorectal excision in rectal cancer: short-term outcomes in comparison with laparoscopic surgery. Ann Surg 261:221–227
Velthuis S, Nieuwenhuis DH, Ruijter TE, Cuesta MA, Bonjer HJ, Sietses C (2014) Transanal versus traditional laparoscopic total mesorectal excision for rectal carcinoma. Surg Endosc 28:3494–3499
Bosch SL, Nagtegaal ID (2012) The importance of the pathologist's role in assessment of the quality of the mesorectum. Curr Colorectal Cancer Rep 8:90–98
Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH, Leer JW, van de Velde CJ, Dutch Colorectal Cancer G (2001) Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 345:638–646
Herman RM, Richter P, Walega P, Popiela T (2001) Anorectal sphincter function and rectal barostat study in patients following transanal endoscopic microsurgery. Int J Colorectal Dis 16:370–376
Kennedy ML, Lubowski DZ, King DW (2002) Transanal endoscopic microsurgery excision: is anorectal function compromised? Dis Colon Rectum 45:601–604
Acknowledgments
The authors would like to thank M. Clotilde Carra, PhD, for her valuable support in the statistical analysis and preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Nil
Conflicts of interest
N. de’Angelis declares that he has no conflict of interest. L. Portigliotti declares that he has no conflict of interest. D. Azoulay declares that he has no conflict of interest. F. Brunetti declares that he has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Authorship
Study conception and design: Nicola de’Angelis and Luca Portigliotti
Data acquisition: Luca Portigliotti and Nicola de’Angelis
Data analysis and interpretation: Nicola de’Angelis, Luca Portigliotti, Francesco Brunetti
Manuscript drafting: Nicola de’Angelis, Luca Portigliotti, Daniel Azoulay, Francesco Brunetti
Manuscript critical revision: Nicola de’Angelis, Luca Portigliotti, Daniel Azoulay, Francesco Brunetti
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 125 kb)
Rights and permissions
About this article
Cite this article
de’Angelis, N., Portigliotti, L., Azoulay, D. et al. Transanal total mesorectal excision for rectal cancer: a single center experience and systematic review of the literature. Langenbecks Arch Surg 400, 945–959 (2015). https://doi.org/10.1007/s00423-015-1350-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-015-1350-7