Abstract
Background
The effect of posterior tibial nerve stimulation (PTNS) on the mechanisms of anal continence has not been fully demonstrated. The aim of this study was to assess the anal manometric response after percutaneous PTNS in patients with fecal incontinence (FI).
Methods
This was a prospective study in patients with FI undergoing 1 weekly session of percutaneous PTNS for 8 weeks. A clinical assessment (Wexner scale) and a complete study of up to 22 manometric parameters were carried out prior to treatment and 2–4 weeks after the end of treatment.
Results
A total of 32 patients were evaluated. After therapy, there was a decrease in the average Wexner score [12.6 (± 5.2) to 9.5 (± 5.2) (P < 0.005)] and an increase in the “anal canal length at rest” [4.55 (± 0.60) to 4.95 (± 0.21) P = 0.004], without observing variations in other manometric parameters. The decrease in the Wexner score was significantly correlated with an increase in the “pressure at 5 cm at rest” after therapy (r = 0.464 P = 0.030).
Conclusions
In our study, PTNS was associated with a significant decrease in the Wexner score and with an increase in the functional length of the anal canal at rest. The improvement in the Wexner scale was correlated with an increase in pressure at rest in the theoretical area of the anorectal junction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fecal incontinence (FI) is a devastating condition with a relatively high prevalence of 11–17% reported in epidemiological studies [1]. Electrical nerve stimulation has been used as a minimally invasive treatment for functional pathologies of the pelvic floor, both in urology and in coloproctology. Sacral nerve root neuromodulation has been the most widely used of these techniques for the treatment of FI, but in recent years, there has been an increase in the application and use of posterior tibial nerve stimulation (PTNS). The results of PTNS seem to be good, and this treatment method is less aggressive and less costly than sacral neuromodulation [2].
PTNS has shown acceptable results with significant improvement in leakage and quality of life [3]. However, some randomized studies have questioned the true therapeutic effectiveness of the procedure [4, 5].
Evaluation of the results is difficult in FI, with much subjectivity in endpoints. The scores used to measure FI, such as the Jorge–Wexner score [6], quality-of-life surveys such as the Faecal Incontinence Quality of Life Questionnaire (FIQL) questionnaire [7], and defecation diaries, are useful instruments to assess the degree of FI and to evaluate the changes that occur with different treatments. Anorectal manometry (ARM), however, produces objective data and can specify the degree of dysfunction of anal continence in a patient with FI, either due to organic or functional lesions. In addition, ARM can detect objective changes of continence which may be correlated with clinical improvement. Several studies have evaluated the manometric response after PTNS [4, 8,9,10,11,12,13,14].
The aim of the present study was to assess the response to PTNS in patients with FI, determining not only its clinical response but also its impact on anorectal function, studied with ARM.
Materials and methods
A prospective study was conducted in patients with FI who had treatment with PTNS between May 2014 and November 2015. Clinical and functional assessments of the patient were made before the treatment and 2–4 weeks after the end of the treatment. The study was approved by the Clinical Research Ethics Committee of the Hospital of Sagunto.
Inclusion criteria
-
1.
Patients with FI (urgency, passive, or mixed), without sphincter defects or with minor defects determined by endoanal ultrasound. Minor defects were defined as follows: internal anal sphincter (IAS) and/or external anal sphincter (EAS) defects less than 30º.
-
2.
Patients with sphincteric defects that were repaired surgically, with ultrasonographic verification of the integrity of the repair and presenting with residual FI.
-
3.
Patients with FI secondary to low anterior resection syndrome (LARS), without IAS and/or EAS defects greater than 30º, on endoanal ultrasound.
-
4.
Duration of FI symptoms longer than 6 months.
-
5.
No satisfactory response to conservative treatment (dietary measures, antidiarrheal agents, and pelvic floor rehabilitation exercises).
Exclusion criteria
-
1.
Patients with major sphincter injuries: i.e., lesions of the IAS and/or EAS greater than 30º.
-
2.
Patients with FI secondary to active colon or rectal inflammatory disease (inflammatory bowel disease, diverticulitis, other colitis).
-
3.
Patients with unresected digestive system neoplasms.
-
4.
Patients with central nervous system pathology either at the cortical or medullary levels that could be a cause of, or be related to, FI.
-
5.
Patients with pathology of the lower limb that contraindicates tibial puncture including: vascular ulcers, severe venous insufficiency, significant edema, severe cutaneous diseases, etc.
-
6.
Patients with peripheral nerve injuries of the lower extremities.
-
7.
Patients with muscular dystrophy.
Clinical and functional assessment
All patients had a clinical assessment consisting of a complete medical history and physical examination. An endoanal ultrasound was also performed in all patients to detect and assess sphincter injuries. To determine the severity of FI, the Wexner scale [6] was administered to all patients before and after treatment. ARM was performed in all patients before treatment and just after the end of treatment (2–4 weeks). During the manometric study, 22 different parameters were recorded for further analysis, which were divided into several groups: length and distance at rest and contraction (cm), pressure at rest and on contraction (mmHg), asymmetry at rest and on contraction (%), and vector volume at rest and on contraction (mmHg2 × cm).
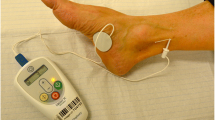
Procedure of percutaneous stimulation of the posterior tibial nerve
The Urgent PC neuromodulation system© (Uroplasty, Wythenshawe, Manchester, UK) was used for the application of the therapy. We performed 1 weekly session consisting of 30 min each, for 8 consecutive weeks in each patient. After locating the anatomical area of the posterior tibial nerve, a test stimulation was performed that started at the minimum intensity level and progressively increased while the response was assessed (paresthesias in the heel, sole of the foot or fingers). Depth, angulation, and even the puncture site were varied for different test stimulations to find the location at which the minimum intensity provided the patient with a sensitive response. After this, the intensity was increased to the maximum tolerable level without causing pain. After several minutes of stimulation, a phenomenon of adaptation occurred in which the patient stopped perceiving the sensory and/or motor response. If this occurred, the intensity was increased again until the response recovered. It was possible that several increases in intensity level were needed to maintain the response throughout the entire session.
Statistical analysis
The sample size was calculated using differences between paired means (repeated in a group), since the main objective was to detect changes in the manometry data at two moments in time before and after the application of the treatment in the same sample. We used data from the manometric results of preliminary studies with the same methodological design in our geographical area, which showed increases of 15 mmHg in the maximum baseline pressure between pre- and post-treatment manometry with a standard deviation of 25 mmHg [13]. Accepting an alpha risk of 0.05 and a beta risk of 0.2 in a bilateral contrast, 25 subjects were required to detect a difference equal to or greater than 15 units. A standard deviation of 25 was assumed. A tracking loss rate of 10% was estimated.
The statistical package IBM SPSS Statistics 19 for Windows was used to analyse the data. The variables are expressed as the mean and standard deviation (SD) or the median and interquartile range (IQR) for variables with non-parametric distribution. For the comparison of variables (before and after the treatment), Student’s t test was used, and Wilcoxon’s test was used in the case of non-parametric distributions. The Pearson correlation coefficient was used as a measure of the linear relationship between two quantitative random variables. The level of statistical significance was 5%. All tests were considered bilateral. The results are expressed as the mean ± standard deviation.
Results
A total of 32 patients, including 28 women (87.5%) and 4 men (12.5%), were evaluated. Their mean age was 60.1 (± 14.1) years. Five patients were diabetic (16.6%). In 9 patients (28.1%), sphincter defects were identified on endoanal ultrasound. Manometry reuslts are presented in Table 1. The etiology and type of FI are presented in Table 2.
All patients received medical and dietary treatment associated with Kegel exercises at home prior to their inclusion in the study. In addition, 4 patients had previously received other specific treatments for their FI: anal sphincteroplasty (3 patients) and sacral neuromodulation test (1 patient).
The mean Wexner score decreased significantly from 12.6 (± 5.2) to 9.5 (± 5.2) (P < 0.005) after the end of treatment. Seven patients (21.9%) experienced a greater than 50% decrease in Wexner score, and a total of 14 patients (43.8%) experienced a greater than 30% decrease.
ARM was completed before and after treatment in 27 patients (84%). After therapy, there was a significant increase in the “anal canal length at rest” [4.55 (± 0.60)–4.95 (± 0.21) P = 0.004]. No significant changes in the remaining manometric parameters were observed after treatment (Table 1). In a more exhaustive analysis of the “anal canal length at rest”, there was an increase in length in 7 patients (26%) after the treatment, and this change was accompanied by a small increase in pressure at 5 cm that exceeded the rectal threshold, so the resting length of the anal canal increased from 4 to 5 cm.
The decrease in the Wexner scores were significantly correlated with the increase in the “pressure at 5 cm at rest” after therapy (r = 0.464 P = 0.030). There were no other significant correlations between the decrease in the Wexner scores and the variations in other manometric parameters after the end of treatment. The group of patients in whom an improvement of more than 30% was observed in the Wexner score after the procedure had a greater increase in the “pressure at 5 cm at rest”, bordering on statistical significance (P = 0.054).
There were no complications secondary to the technique in any patient during the stimulation period.
Discussion
PTNS is a non-invasive technique that seems to provide acceptable results in the treatment of FI.
The number of sessions performed in each patient varies greatly in the literature. Early studies performed several sessions of PTNS per week for a month [12, 15, 16], but this has evolved to a weekly session for 12 weeks [8, 9, 11, 13, 17, 18]. Some authors have performed a second phase of treatment in responding patients [8, 9, 11, 13, 18]. Other groups have worked with a greater number of scheduled sessions [19] and have even carried out maintenance sessions every 6 months [20,21,22]. Our initial scheme was a weekly session for 8 weeks with a new cycle of six sessions offered in those responding patients in whom there was a reduction in the initial effect. With this approach, the duration of the initial therapy was reduced to 2 months, and the interval between the maintenance cycles was extended to 6 months, in an attempt to minimize the socio-occupational problems of the patient, possibly resulting in better treatment completion, and allowing the optimization of health resources.
In the present study, the Wexner scores decreased significantly after therapy, from an average of 12.6–9.5 after treatment. In most series of PTNS published in the literature, there has been a significant improvement in the Wexner scores, with a decrease mostly between 3 and 4 points [8, 9, 13, 17, 20,21,22]. In clinical trials, there have been contradictory data on the variations in the severity scales produced after therapy. In a randomized study [4], there was a greater decrease in Wexner scores after treatment in the transcutaneous stimulation group than in the placebo group. The decrease in scores of more than 30% occurred in 47% of the stimulation group versus 27% of the placebo group, indicating a positive effect of the treatment. Similarly, a recent multicentre randomized study [23] showed a significant improvement in the Wexner scores in both the treatment group and the placebo group, but the improvement was significantly greater in the stimulation group. However, in the Confident trial [5], no significant differences were found in the decrease in the St Marks scores after treatment between the percutaneous stimulation and placebo groups. George et al. [10], in their three-arm trial (percutaneous vs transcutaneous stimulation vs placebo), also found no significant differences in the changes in the St Marks scores after treatment among the three groups.
The ARM method practised in the present study consisted of a complete and exhaustive study in which up to 22 different variables were analysed that could provide information on different physiological aspects of the anal continence function. Of the 22 manometric parameters evaluated in the present study, there was only a significant increase in the functional length at rest of the anal canal after therapy.
Table 3 shows the effects produced by PTNS on the manometric data of different series. Five studies showed significant increases in contraction pressure after treatment [4, 8,9,10,11]. Only one showed a significant improvement in pressures at rest [11]. Regardless, the treatment seemed to produces small pressure increases, with most increases being less than 10 mmHg.
In the literature, there are few data on the possible effects of tibial stimulation on the functional length of the anal canal. George et al. [10] did not show significant differences in the length of the anal canal among three groups (percutaneous, transcutaneous, or placebo) in a randomized study. In our study, there was a significant increase in the length of the functional anal canal at rest, increasing from an average of 4.55 cm pre-treatment to 4.95 cm after therapy in the group as a whole. In a more exhaustive analysis of this finding, there was an increase in length after the treatment in 7 patients (26%), and this change was accompanied by a small increase in pressure 5 cm from the anal margin that exceeded the rectal threshold, forming part of the functional anal sleeve and increasing the length of the anal canal from 4 to 5 cm. Although there was no greater clinical improvement in the group of patients in whom there had been a net increase in the length of the anal canal, a significant correlation was found between the variation in resting pressure at 5 cm and the improvement of the Wexner scores after treatment. Thus, our results suggest that PTNS produces an increase in pressure in the theoretical area of the anorectal junction (5 cm from anal margin), which would entail a relative increase in the length of the anal functional cuff; this could be correlated with an improvement in severity scales after therapy. Similarly, Queralto et al. [12] found in their series a greater increase in pressures at rest after treatment in the upper anal canal (24.03–31.87 mmHg) than in the lower anal canal (29.66–28, 92 mmHg). There is other evidence that has shown the effects of tibial stimulation in deep segments. Bouguen et al. in an experimental randomized study in patients with FI showed an increase in the myogenic response of the rectum to distension after transcutaneous PTNS [24].
In the present study, two patients with LARS were included. A recent study with ten patients showed a significant decrease in the Wexner scores (14–10) and an improvement in the LARS score in half of the patients after PTNS treatment [25].
A limitation of this study is that the clinical and manometric evaluation was performed immediately after the end of therapy. A medium- or long-term assessment has not been carried out. This is because the main objective of the study was to assess the anorectal motor effect produced by PTNS. Other studies have shown the maintenance of some clinical response in the medium term or long term [9], although in many cases with the need for “top up” sessions [22].
Conclusions
Percutaneous PTNS in patients with FI produces clinical improvement with a statistically significant decrease in Wexner scores after treatment and an increase in the functional length at rest of the anal canal without changes in other manometric parameters. There is a significant correlation between the improvement in the Wexner scale and the increase in resting pressures at 5 cm of anal margin.
References
Macmillan A, Merrie AEH (2007) Epidemiology of Faecal Incontinence. In: Ratto C, Doglietto GB (eds) Fecal incontinence. Diagnosis and treatment. Springer-Verlag, Milan
Hotouras A, Murphy J, Allison M, Curry A, Williams NS, Knowles CH, Chan CL (2014) Prospective clinical audit of two neuromodulatory treatments for fecal incontinence: sacral nerve stimulation (SNS) and percutaneous tibial nerve stimulation (PTNS). Surg Today 44(11):2124–2130
Horrocks EJ, Thin N, Thaha MA, Taylor SJ, Norton C, Knowles CH (2014) Systematic review of tibial nerve stimulation to treat faecal incontinence. Br J Surg 101(5):457–468
Leroi AM, Siproudhis L, Etienney I, Damon H, Zerbib F, Amarenco G, Vitton V, Faucheron JL, Thomas C, Mion F, Roumeguère P, Gourcerol G, Bouvier M, Lallouche K, Menard JF, Queralto M (2012) Transcutaneous electrical tibial nerve stimulation in the treatment of fecal incontinence: a randomized trial (CONSORT 1a). Am J Gastroenterol 107(12):1888–1896
Knowles CH, Horrocks EJ, Bremner SA, Stevens N, Norton C, O’Connell PR, Eldridge S, CONFIDeNT study group (2015) s.1. Percutaneous tibial nerve stimulation versus sham electrical stimulation for the treatment of faecal incontinence in adults (CONFIDeNT): a double-blind, multicentre, pragmatic, parallel-group, randomised controlled trial. Lancet. 386(10004):1640–1648
Jorge JM, Wexner SD (1993) Etiology and management of fecal incontinence. Dis Colon Rectum 36(1):77–97
Rockwood TH, Church JM, Fleshman JW et al (2000) Fecal incontinence quality of life scale. Dis Colon Rectum 43:9–17
De la Portilla F, Rada R, Vega J, Gonzalez CA, Cisneros N, Maldonado VH (2009) Evaluation of the use of posterior tibial nerve stimulation for the treatment of fecal incontinence: preliminary results of a prospective study. Dis Colon Rectum 52:1427–1433
De la Portilla F, Laporte M, Maestre MV, Díaz-Pavón JM, Gollonet JL, Palacios C, Vázquez-Monchul JM, García-Cabrera AM, Jiménez-Rodríguez RM, Sánchez Gil JM (2014) Percutaneous neuromodulation of the posterior tibial nerve for the treatment of faecal incontinence—mid-term results: is retreatment required? Colorectal Dis 16(4):304–310
George AT, Kalmar K, Sala S, Kopanakis K, Panarese A, Dudding TC, Hollingshead JR, Nicholls RJ, Vaizey CJ (2013) Randomized controlled trial of percutaneous versus transcutaneous posterior tibial nerve stimulation in faecal incontinence. Br J Surg 100(3):330–338
Arroyo A, Parra P, Lopez A, Peña E, Ruiz-Tovar J, Benavides J, Moya P, Muñoz J, Alcaide MJ, Escamilla C, Calpena R (2014) Percutaneous posterior tibial nerve stimulation (PPTNS) in faecal incontinence associated with an anal sphincter lesion: results of a prospective study. Int J Surg. 12(2):146–149
Queralto M, Portier G, Cabarrot PH, Bonnaud G, Chotard JP, Nadrigny M, Lazorthes F (2006) Preliminary results of peripheral transcutaneous neuromodulation in the treatment of idiopathic fecal incontinence. Int J Colorectal Dis. 21(7):670–672
López-Delgado A, Arroyo A, Ruiz-Tovar J, Alcaide MJ, Diez M, Moya P, Santos J, Calpena R (2014) Effect on anal pressure of percutaneous posterior tibial nerve stimulation for faecal incontinence. Colorectal Dis 16(7):533–537
Marti L, Galata C, Beutner U, Hetzer F, Pipitone N, Wolff K, Borovicka J, Brunner W, Sulz MC, Maurus C (2017) Percutaneous tibial nerve stimulation (pTNS): success rate and the role of rectal capacity. Int J Colorectal Dis. 32(6):789–796
Shafi KA, Ahmed I, El-Sibai O (2003) Mostafa RM (2003) Percutaneous peripheral neuromodulation in the treatment of fecal incontinence. Eur Surg Res. 35(2):103–107
Mentes B, Yuksel O, Aydin A, Tezcaner T, Leventoglu A, Aytac B (2007) Posterior tibial nerve stimulation for faecal incontinence after partial spinal injury: preliminary report. Tech Coloproc 11:115–119
Govaert B, Pares D, Delgado-Aros S, La Torre F, Van Gemert WG, Baeten CG (2010) A prospective multicentre study to investigate percutaneous tibial nerve stimulation for the treatment of faecal incontinence. Colorectal Dis. 12(12):1236–1241
Peña Ros E, Parra Baños PA, Benavides Buleje JA, Muñoz Camarena JM, Escamilla Segade C, Candel Arenas MF, Gonzalez Valverde FM, Albarracín Marín-Blázquez A (2016) Short-term outcome of percutaneous posterior tibial nerve stimulation (PTNS) for the treatment of faecal incontinence. Tech Coloproctol 20(1):19–24
Boyle DJ, Prosser K, Allison ME, Williams NS, Chan CL (2010) Percutaneous tibial nerve stimulation for the treatment of urge faecal incontinence. Dis Colon Rectum 53(4):432–437
Hotouras A, Thaha MA, Allison ME, Currie A, Scott SM, Chan CL (2012) Percutaneous tibial nerve stimulation (PTNS) in females with faecal incontinence: the impact of sphincter morphology and rectal sensation on the clinical outcome. Int J Colorectal Dis. 27:927–930
Hotouras A, Thaha MA, Boyle DJ, Allison ME, Currie A, Knowles CH, Chan CL (2012) Short-term outcome following percutaneous tibial nerve stimulation for faecal incontinence: a single-centre prospective study. Colorectal Dis. 14(9):1101–1105
Hotouras A, Murphy J, Walsh U, Allison M, Curry A, Williams NS, Knowles CH, Chan CL (2014) Outcome of percutaneous tibial nerve stimulation (PTNS) for fecal incontinence a prospective cohort study. Ann Surg. 259(5):939–943
Van der Wilt AA, Giuliani G, Kubis C, van Wunnik BPW, Ferreira I, Breukink SO, Lehur PA, Torre L, Baeten CGMI (2017) Randomized clinical trial of percutaneous tibial nerve stimulation versus sham electrical stimulation in patients with faecal incontinence. Br J Surg. 104(9):1167–1176
Bouguen G, Ropert A, Lainé F, Pequin P, Morcet J, Bretagne JF, Siproudhis L (2014) Effects of transcutaneous tibial nerve stimulation on anorectal physiology in fecal incontinence: a double-blind placebo-controlled cross-over evaluation. Neurogastroenterol Motil 26(2):247–254
Vigorita V, Rausei S, Pereira TP, Trostchansky I, Ruano Poblador A, Moncada Iribarren E, Facal Alvarez C, de San Pereira IA, Casal Núñez E (2017) A pilot study assessing the efficacy of posterior tibial nerve stimulation in the treatment of low anterior resection syndrome. Tech Coloproc 21(4):287–293
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the Clinical Research Ethics Committee of the Hospital of Sagunto.
Informed consent
Informed consent was obtained from all participants included in the study
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rodríguez Carrillo, R., Ruiz Carmona, M.D., Alós Company, R. et al. Evaluation of the anorectal motor response after percutaneous stimulation of the posterior tibial nerve in patients with fecal incontinence. Tech Coloproctol 23, 987–992 (2019). https://doi.org/10.1007/s10151-019-02092-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10151-019-02092-w