Abstract
Background
There is no level 1a evidence regarding the best technique for skin closure at loop ileostomy reversal. The aim of this study was to evaluate whether purse-string skin closure (PSC) is associated with lower surgical site infection (SSI) rates as compared to linear skin closure (LC).
Methods
EMBASE, MEDLINE, Pubmed, Cochrane Library, Web of Science, and CINAHL databases were systematically searched. PSC was defined as a circumferential subcuticular suture leaving a small circular skin defect allowing for free drainage, granulation, and epithelialization. In LC, the wound edges were approximated side to side with or without drainage. The primary endpoint was SSI rate. Secondary endpoints included operating time, length of hospital stay, wound healing time, and incisional hernia rates.
Study selection
Inclusion criterion was any observational or experimental study comparing PSC to LC in patients undergoing ostomy reversal.
Results
Twenty studies (6 experimental and 14 observational) totaling 1812 patients (826 PSC and 986 LC) were included. SSI rates were significantly lower statistically and clinically in patients with PSC [OR (95% CI) = 0.14 (0.09, 0.21); p < 0.0001; NNT = 6] in the meta-analysis of all studies. The subgroup analysis of randomized trials [OR (95% CI) = 0.10 (0.04, 0.21); p < 0.0001; NNT = 6] as well as the analysis of randomized trials including patients with loop ileostomy only [OR (95% CI) = 0.12 (0.05, 0.28); p < 0.0001; NNT = 5] confirmed this finding.
Conclusions
This meta-analysis found that PSC was associated with significantly decreased rates of SSI in patients undergoing loop ileostomy reversal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diverting the gastroenteric flow with a loop ileostomy has been shown to decrease complications from sepsis after colocolonic or colorectal anastomosis [1]. However, ileostomies are associated with complications including (but not limited to) physiologic adverse events such as dehydration and acute renal failure [2]. Moreover, the closure of the loop ileostomy can be associated with additional short-term postoperative morbidity in 2–33% of the patients [3, 4]. Surgical site infection (SSI) is the most prominent complication after ileostomy reversal with incidence rates of 0–41% [5,6,7]. Techniques already described and used for wound closure at ileostomy reversal include conventional linear skin closure (LC), primary skin closure with subcutaneous vacuum drain [8], and negative pressure wound therapy [9]. Nonetheless, the optimal technique is still a subject of debate. Purse-string skin closure (PSC) was first introduced as an alternative method to decrease the rate of incisional SSI by Banerjee in 1998 [10]. In PSC, the skin is partially closed in a circular fashion placing a purse-string suture in the dermal layer. PSC allows the wound to drain in its center throughout the healing process. Since the introduction of PSC, several studies have reported decreased incisional SSI rates and improved cosmetic appearance with PSC as compared to LC. Two previous systematic reviews showed that PSC led to lower rates of incisional SSI compared to other types of closure methods [11, 12]. However, the studies included in these systematic reviews suffered from a low level of evidence with a high risk of bias rendering a definitive conclusion difficult. In this systematic review and meta-analysis, we reviewed the most up-to-date literature to evaluate the outcomes of PSC compared to LC following ostomy reversal.
Materials and methods
The Cochrane Handbook for Systematic Reviews of Interventions was used to perform this systematic review [13]. The study complies with Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) as well as Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines [14, 15]. The protocol of this meta-analysis was prospectively developed and registered in the International prospective register of systematic reviews PROSPERO: CRD42017069354. The literature search, quality assessment, extraction and analysis of the data, and critical appraisal were performed by two researchers independently (GM and LH). Any methodological discrepancies were revised and corrected by the senior author. The research question was developed within the patient/problem, intervention, comparison, outcome, time, and setting (PICOTS) framework as follows:
-
(P) Population: adults older than 18 years undergoing ostomy reversal.
-
(I) Intervention: PSC.
-
(C) Comparator intervention: LC.
-
(O) Outcomes: SSI rate, operating time, length of stay, incisional hernia rate.
-
(T) Time: both short- and long terms.
-
(S) Setting: in- and outpatient.
Eligibility criteria, definitions, and endpoints
The inclusion criterion was any observational (case–control, retrospective or prospective cohort) or experimental study comparing PSC to LC in patients with temporary ileostomy or colostomy undergoing ostomy reversal. The exclusion criteria were non-comparative descriptive studies; studies comparing either PSC or LC to an irrelevant intervention, systematic reviews, technical notes, and correspondence papers.
PSC was defined as a circumferential subcuticular suture leaving a small circular skin defect allowing for free drainage, granulation, and epithelialization. InLC, the wound edges were approximated side to side with or without drainage. SSI was defined according to the Center for Disease Control (CDC) National Nosocomial Infections Surveillance System [16]. Wound infection and wound sepsis were categorized as SSI. Incisional hernia was defined as either a radiological or clinical defect at the ostomy reversal site.
The primary endpoint was:
-
SSI
Secondary endpoints were:
-
Operating time
-
Length of hospital stay
-
Wound healing time
-
Incisional hernia rate
Search strategy and study selection
EMBASE, MEDLINE via Ovid, Pubmed, Cochrane Library, Web of Science, and CINAHL databases were searched for the following MeSH terms: ‘purse-string’, ‘purse-string’, and ‘closure’. Existence of any ongoing relevant clinical studies was assessed by searching ClinicalTrials.gov. Records of interest were identified and screened through the title, abstract, or full-text article. The references of included articles were screened for additional publications to test the sensitivity of the search strategy.
Quality assessment and data extraction
The quality of the included studies was assessed by two independent researchers according to the Cochrane Handbook for Systematic Reviews of Interventions [13]. Extracted data from the included records were inserted into a pre-defined Microsoft Excel table and included article identifiers (author, journal, and year of publication), study design, number of patients, type of and indication for ostomy, time to ostomy reversal, follow-up, details of study interventions, and pre-defined primary and secondary endpoints.
Statistical analysis
RevMan (version 5.3; Nordic Cochrane Center, Cochrane Collaboration, Copenhagen, Denmark), Microsoft Excel (Version 2016; Microsoft Corporation, WA, USA), and CMA Software (Version 3; Biostat, NJ, USA) were used for statistical analysis. The Mantel–Haenszel method using odds ratios with 95% confidence intervals [OR (95% CI)] as an effect measure was employed to compare dichotomous variables. Continuous variables were compared using the inverse variance method with mean difference and standard error as an effect measure. In cases when the median and range were reported to express continuous variables, Hozo’s formula was utilized to estimate mean and standard deviation (SD) [17]. Statistical heterogeneity among effect estimates was evaluated using I2 and Cochran Chi2, and between-study variance was evaluated using Tau2 statistic when the I2 was 50% or greater [18]. The random-effects model for meta-analysis was utilized in case of high heterogeneity. The findings of the meta-analysis were illustrated on forest plots. Relative risk reduction (RRR), absolute risk reduction (ARR), and number needed to treat/harm (NNT) were calculated for evaluation of clinical significance of the results [19]. Considering that above-mentioned metrics also have variability, 95% CI for NNT were calculated by taking reciprocals of the values defining the 95% CI for the ARR to demonstrate whether there was uncertainty in the NNT [20]. Publication bias was assessed through visual assessment of funnel plots, Egger’s, and Begg and Mazumdar rank correlation tests. A p value < 0.05 was considered statistically significant.
Results
Literature search and study selection
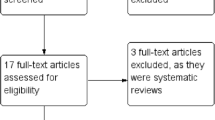
The detailed search strategy is shown in Supplement 1. The PRISMA flow diagram of study selection is illustrated in Fig. 1. Systematically searched databases had 884 records. Screening of the references of eligible studies revealed seven additional records and three records were found at clinicaltrials.gov. A hundred and one records were screened after excluding duplicates and irrelevant articles. Fifty-eight articles included the medical subject headings (MeSH) terms in their titles, but were not related to skin closure techniques. Hence, they were excluded. After assessment of 43 full-text articles and exclusion of reviews, descriptive studies, studies comparing either intervention of interest to an irrelevant comparator, technical notes, and correspondence articles, 21 studies were included in the qualitative synthesis. One randomized controlled trial (RCT) was not included in the quantitative synthesis due to a different study population (patients older than 12 years were included in the study) [21].
Description of included studies
Among 43 potentially eligible studies, 20 were included in the meta-analysis [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41] totaling 1812 patients (826 PSC and 986 LC). A description of the included studies is provided in Table 1. Six studies were RCTs with 1b level of evidence [22,23,24, 29, 34] and 14 (12 retrospective and 2 prospective cohort studies) were observational studies with 2b level of evidence [25,26,27,28, 30,31,32,33, 35,36,37,38,39,40]. Nineteen of 20 records were full-text articles. One record was an abstract published in a surgical journal supplement [40]. In 18 studies, the primary endpoint was incisional SSI [22,23,24,25, 27,28,29,30,31,32,33,34,35,36,37,38,39,40]. In one observational study, the primary endpoint was incisional hernia rates [26]. In another study, multiple comparisons and endpoints were reported [41]. Studies stratified by endpoints are presented in Supplement 2. Thirteen studies included patients with loop ileostomies only [22, 24,25,26,27,28, 32, 34,35,36, 38, 40, 41]. Seven studies included patients with ileostomy or colostomy [23, 29,30,31, 33, 37, 39]. Two of the 20 studies included patients undergoing surgery for colorectal cancer [36, 37].
Description of study populations and interventions
Patients were adults from ten countries: USA, Germany, South Korea, Australia, Japan, UK, Ireland, Mexico, Switzerland, and Iran. The breakdown of patient by country is shown in Fig. 2. The RCTs involved 446 patients (230 PSC vs. 216 LC) from 7 countries: USA, Germany, Australia, Ireland, Mexico, Switzerland, and Iran [22,23,24, 29, 34]. In all studies, patients with PSC were comparable to their counterparts in terms of demographics. The definition of study interventions was heterogeneous (Supplement 3). Eleven studies reported having used absorbable suture for PSC [22, 28, 29, 31, 32, 34, 36,37,38,39, 41]. In six studies, non-absorbable sutures were used [23,24,25,26, 33, 35]. Three studies did not report the type of suture used for PSC [27, 30, 40]. The diameter of the circular skin defect after PSC varied from 3 mm to 20 mm. Drains were routinely used in seven studies [24, 29, 31, 32, 36, 37, 39].
Quality assessment
The summary and graph of the risk of bias in included studies are presented in Fig. 3. Oxford Centre for Evidence-Based Medicine (CEBM) classification was utilized to assess levels of evidence provided by the included studies (Table 1). The quality of cohort studies was evaluated using the Newcastle–Ottawa score (Table 1). There was low risk of selection bias in six RCTs only [22,23,24, 29, 34, 35]. The risks of performance and detection biases were high in observational studies as well as in RCTs. Blinding surgeons to the intervention and the outcome for preventing performance and detection bias is impracticable and unethical. The risks of attrition, reporting, and other biases were low in most studies.
Meta-analysis of the data from all studies
All 20 studies regardless of the evidence level were included.
SSI rate
Surgical site infection was defined as stoma site superficial or deep incisional SSI and was reported in all included studies (826 PSC vs. 986 LC) [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41]. The fixed effects model was utilized as among-study heterogeneity was low (I2 = 1%). SSI rate was 3.1% (26/826) in PSC vs. 20.2% (199/986) in LC. This difference was statistically and clinically significant [OR (95% CI) = 0.14 (0.09, 0.21); p < 0.0001] [NNT (95% CI) = 6 (5, 7)] (Fig. 4; Table 2). It was impossible to correlate the incidence of SSI with any details of intervention, such as aperture size or packing of the wound as the studies did not report such correlations.
Secondary endpoints
Operating time was reported in 13 studies (510 PSC vs. 656 LC) [22,23,24,25, 27,28,29, 31, 34,35,36,37,38]. The fixed effects model was utilized as statistical among-study heterogeneity was low (I2 = 14%). The mean difference in operating time between PSC and LC was not statistically significant [MD (95% CI) = 1.17 (− 2.00, 4.34); p = 0.47] (Fig. 5a).
Twelve studies reported the length of hospital stay (479 PSC vs. 626 LC) [22, 24, 25, 27,28,29, 31, 34,35,36,37,38]. The random-effects model was utilized. The length of hospital stay was significantly shorter in patients with PSC as compared to those with LC [MD (95% CI) = − 2.07 (− 3.72, − 0.42); p = 0.01] with high statistical among-study heterogeneity (I2 = 85%; Tau2 = 6.06) (Fig. 5b).
Incisional hernia rates were reported in 7 studies (396 PSC vs. 333 LC) [23, 24, 26, 30, 36, 38, 41]. The fixed effects model was utilized as among-study heterogeneity was low (I2 = 30%). Incisional hernia rates were 10.3% (41/396) after PSC and 15.3% (51/333) after LC with no statistically significant difference [OR (95% CI) = 0.64 (0.40, 1.02); p = 0.06] (Fig. 5c). It was not possible to test any correlations between SSI and incisional hernia as the authors did not report such correlation.
Outcomes with no temporal relationship to skin closure technique such as anastomotic leak and ileus rates are given in Supplements 4 and 5.
Subgroup meta-analysis of the data from randomized controlled trials
Six RCTs with low risk of bias and evidence level 1b were included.
SSI rate
The SSI rate was reported in all included RCTs including 230 PSC patients and 216 LC patients [22,23,24, 29, 34, 35]. The fixed effects model was utilized given the low statistical among-study heterogeneity (I2 = 0%). The SSI rate was 3% (7/230) in PSC vs. 25.9% (56/216) in LC. This difference was both statistically and clinically significant [OR (95% CI) = 0.10 (0.04, 0.21); p < 0.0001] [NNT (95% CI) = 6 (3.4, 6)] (Fig. 6a) (Table 2).
Two of the six RCTs included patients with ileostomies or colostomies. Another subgroup analysis of RCTs including patients with loop ileostomy only was performed to determine whether colostomy was a confounding factor. Four RCTs (including 141 PSC patients and 131 LC patients) reported incisional SSI rates in patients with loop ileostomy only [22, 24, 34, 35]. The fixed effects model was utilized as the heterogeneity was low (I2 = 0%). SSI rate was 4.3% (6/141) in PSC vs. 28.2% (37/131) in LC. This difference was both statistically and clinically significant [OR (95% CI) = 0.12 (0.05, 0.28); p < 0.0001] [NNT (95% CI) = 5 (3.1, 6.4)] (Fig. 6b; Table 2).
Secondary outcomes
Operating time was reported in all RCTs (230 PSC vs. 216 LC) [22,23,24, 29, 34, 35]. The fixed effects model was utilized as heterogeneity was low (I2 = 16%). The difference in operating time between PSC and LC was not statistically significant [MD (95% CI) = 0.48 (− 3.28, 4.24); p = 0.80] (Fig. 6c).
Five RCTs reported length of hospital stay (199 PSC vs. 186 LC) [22, 24, 29, 34, 35]. The fixed effects model was utilized as the statistical among-study heterogeneity was low (I2 = 0%). No statistical significance was found in the mean difference in length of stay between PSC and LC [MD (95% CI) = − 0.26 (− 0.82, 0.30); p = 0.37] (Fig. 6d).
Three RCTs reported wound healing time (119 PSC vs. 116 LC) [23, 29, 35]. Statistical among-study heterogeneity was high (I2 = 90%; Tau2 = 117.5). Hence, the random-effects model was utilized.
The mean difference in healing time between PSC and LC was not statistically significant [MD (95% CI) = − 1.94 (− 14.98, 11.09); p = 0.77] (Fig. 6e).
Clinical significance
Relative risk reduction (RRR), absolute risk reduction (ARR), and number needed to treat/harm (NNT) for primary endpoint in meta-analysis of all studies, subgroup meta-analysis of RCTs, and subgroup meta-analysis of RCTs including patients with loop ileostomy only are shown in Table 2. The difference in the primary endpoint, namely, SSI rate, was clinically significant favoring PSC over its counterpart.
Sensitivity analysis and publication bias
A sequential exclusion of the abstract with no full text and studies with the highest risk of bias was performed for sensitivity analysis. The findings were not affected by such exclusions. Publication bias was found in the meta-analysis of the data from all studies (asymmetry in the funnel plot of precision; Egger’s p = 0.002; Begg and Mazumdar p = 0.021) (Fig. 7a) (Supplements 6 and 7) and in the subgroup meta-analysis of RCTs (asymmetry in the funnel plot of precision; Egger’s p = 0.007; Begg and Mazumdar p = 0.024) (Fig. 7b) (Supplements 8 and 9). No publication bias was found in the subgroup meta-analysis of RCTs including patients with loop ileostomy only (no asymmetry in the funnel plot of precision; Egger’s p = 0.103; Begg and Mazumdar p = 0.308) (Fig. 7c) (Supplements 10 and 11).
Discussion
Interpretation of the results
The main finding of this meta-analysis was that PSC was associated with decreased SSI rates. Clinically sound conclusions can, however, be drawn only in patients undergoing loop ileostomy reversal. Due to publication bias, the impact of skin closure techniques on SSI rates following colostomy reversal is unclear and should be studied separately. Another potential confounder was the use of biologic mesh, which has become increasingly common practice. In the studies published so far, the role of biologic meshes in the development of SSI was not addressed.
Most secondary endpoints have no temporal relationship to skin closure techniques. Operating time varies depending on a number of factors such as adhesions, mobilization, and anastomosing technique. Skin closure techniques contribute the least to operating time. The same applies to length of hospital stay, wound healing time, and incisional hernia rates. There might be a correlation between SSI and incisional hernia, even though it was impossible to establish in this meta-analysis. The timing and metrics of patient-reported outcomes (PRO) were heterogeneous and did not allow for external validity. Some studies reported Patient and Observer Scar Assessment Scale (POSAS) or Body Image Questionnaire (BIQ) scores [24], whereas others reported 10-point visual analog scales [22, 23, 35], or non-validated 4- or 5-point Likert scales [23, 29, 32, 34].
Existing evidence
The finding that PSC yielded lower SSI rates compared to the LC is consistent with the previous systematic reviews. A meta-analysis found that PSC yielded a 3% SSI rate which compared favorably to other closure techniques such as loose primary closure, secondary closure, delayed primary closure, and primary closure with drain [11]. When compared to LC, PSC yielded statistically significant lower SSI rates [OR (95% CI) = 0.12 (0.02, 0.40)]. This finding is remarkably close to our result of SSI rate of 3.1% [OR (95% CI) = 0.14 (0.09, 0.21); p < 0.0001]. Another meta-analysis showed decreased SSI rates after PSC as compared to LC [2.4% vs. 29.6%; OR (95% CI) = 0.083 (0.03, 0.21); p < 0.001] [12]. A more recent meta-analysis of four RCTs reported significantly decreased SSI rates after PSC as compared to LC [6.79% vs. 25.67%, ARR (95% CI) = 0.25 (0.15, 0.36); p < 0.00001, NNT = 4] [42]. There was no statistically significant difference between the two groups in terms of length of stay, operative time, and wound healing time. Both aforementioned meta-analyses found improved patient cosmetic satisfaction after PSC, although among-study heterogeneity was very high. However, none of the previously published meta-analyses censored studies with colostomy, thereby introducing a confounding effect of colostomy factor.
Strengths and limitations
The strength of this meta-analysis was a scrupulous literature search of several databases, which allowed the inclusion of more clinical studies including prospective randomized trials. The evaluation of the metrics of clinical significance mafde it possible to reach methodologically and clinically sound conclusions. Moreover, the subgroup analysis of all RCTs as well as RCTs including patients with loop ileostomy only strengthened the core finding deriving from the analysis of observational studies.
The main limitation of this meta-analysis was that majority of the eligible studies involved a small sample size. Seven of 20 included studies involved patients with colostomy. Despite the experimental design, all RCTs were subject to high risk for performance and detection bias. There was heterogeneity in the definition of study interventions, such as midline laparotomy, technique of anastomosing, technique of closure of the stoma site layers.
Clinical and scientific implications
The evidence provided in this meta-analysis is sufficient to favor PSC over LC as a skin closure technique following loop ileostomy reversal to reduce incisional SSI rates. However, the limitations of the existing evidence should be taken into account. The optimal type of suture for PSC and the diameter of the central skin defect are yet to be determined. The rate of incisional SSI should be considered the only outcome associated with skin closure technique.
Further studies on this subject should use a standardized technique and focus on the SSI rate. Moreover, the timing of the patient-reported outcomes should also be standardized (e.g., 30 days postoperatively), and they should be based on previously validated questionnaires avoiding self-established 4- or 5-point Likert scales. Future larger scale experimental design studies including patients with loop ileostomy only (undergoing reversal with biologic mesh) with the null hypothesis built using the findings of this meta-analysis would add to the existing evidence. Based on inequality design given an α-error of 0.05 and statistical power of 80%, the required sample size is at least 72 patients (36 patients in each arm). Such sample size was achieved in one RCT only with the statistical power of 94% at post-hoc power analysis [24]. A separate observational or experimental study including patients with colostomy only is needed. A pooled analysis of patient-level data from the included RCTs might help to determine whether incisional hernia after ostomy reversal is correlated to SSI. Moreover, such pooled analysis may make it possible to find any correlations between SSI and the details of the intervention, such as aperture size or use of packing.
Conclusions
This meta-analysis found that PSC was associated with significantly decreased rates of SSI in patients undergoing loop ileostomy reversal. Further studies are required to identify the impact of PSC on colostomy reversal as well as the role of biologic mesh in the development of adverse events.
References
Bax TW, McNevin MS (2007) The value of diverting loop ileostomy on the high-risk colon and rectal anastomosis. Am J Surg 193(5):585–587
Nagle DA (2013) Toward better understanding of readmissions for physiologic complications of ileostomy. Dis Colon Rectum 56(8):933–934
Kaidar-Person O, Person B, Wexner DS (2005) Complications of construction and closure of temporary loop ileostomy. J Am Coll Surg 201(5):759–773
Herrle F, Sandra-Petrescu F, Weiss C, Post S, Runkel N, Kienle P (2016) Quality of life and timing of stoma closure in patients with rectal cancer undergoing low anterior resection with diverting stoma: a multicenter longitudinal observational study. Dis Colon Rectum 59(4):281–290
Sutton CD, Williams N, Marshall LJ, Lloyd G, Thomas WM (2002) A technique for wound closure that minimizes sepsis after stoma closure. ANZ J Surg 72(10):766–767
Perez RO, Habr-Gama A, Seid VE et al (2006) Loop ileostomy morbity: timing of closure matters. Dis Colon Rectum 49(10):1539–1545
Hackam DJ, Rotsein OD (1995) Stoma closure and wound infection: an evaluation of risk factors. Can J Surg 38(2):144–148
Pan HD, Wang L, Peng YF et al (2015) Subcutaneous vacuum drains reduce surgical site infection after primary closure of defunctioning ileostomy. Int J Colorectal Dis 20(7):977–982
Uchino M, Hirose K, Toshihiro B, Chohno T, Takesue Y, Ikeuchi H (2016) Randomized controlled trial of prophylactic negative-pressure wound therapy at ostomy closure for the prevention of delayed wound healing and surgical site infection in patients with ulcerative colitis. Dig Surg 33(6):449–454
Banerjee A (1997) Pursestring skin closure after stoma reversal. Dis Colon Rectum 40(8):993–994
Li LT, Hicks SC, Davila JA, Kao LS, Berger RL, Arita NA, Lian MK (2014) Circular closure is associated with the lowest rate of surgical site infection following stoma reversal: a systematic review and multiple treatment meta-analysis. Colorectal Dis 16(6):406–416
McCartan DP, Burke JP, Walsh SR, Coffey JC (2013) Purse-string approximation is superior to primary skin closure following stoma reversal: a systematic review and meta-analysis. Tech Coloproctol 17(4):345–351
Higgins JP, Green S (2011) Cochrane handbook for systematic reviews of interventions, vol 4. Wiley, London
Moher D, Liberati A, Tetzlaff J et al (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8:336–341
Stroup DF, Berlin JA, Morton SC et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283(15):2008–2012
Horan TC, Gaynes RP, Martone WJ et al (1992) CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol 13:606–608
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13
Higgins JP, Thompson SG, Deeks JJ et al (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Cook RJ, Sackett DL (1995) The number needed to treat: a clinically useful measure of treatment effect. BMJ 310(6977):452–454
Altman DG (1998) Confidence intervals for the number needed to treat. BMJ 317(7168):1309–1312
Sureshkumar S, Jubel K, Ali MS et al (2018) Comparing surgical site infection and scar cosmesis between conventional linear skin closure versus purse-string skin closure in stoma reversal—a randomized controlled trial. Cureus 10(2):e2181
Alvandipour M, Gharedaghi B, Khodabakhsh H, Karami MY (2016) Purse-string versus linear conventional skin wound closure of an ileostomy: a randomized clinical trial. Ann Coloproctol 32(4):144–149
Camacho-Mauries D, Rodriguez-Díaz JL, Salgado-Nesme N, González QH, Vergara-Fernández O (2013) Randomized clinical trial of intestinal ostomy takedown comparing pursestring wound closure vs conventional closure to eliminate the risk of wound infection. Dis Colon Rectum 56(2):205–211
Dusch N, Goranova D, Herrle F, Niedergethmann M, Kienle P (2013) Randomized controlled trial: comparison of two surgical techniques for closing the wound following ileostomy closure: purse string vs direct suture. Colorectal Dis 15(8):1033–1040
Habbe N, Hannes S, Liese J, Woeste G, Bechstein WO, Strey C (2014) The use of purse-string skin closure in loop ileostomy reversals leads to lower wound infection rates—a single high-volume centre experience. Int J Colorectal Dis 29(6):709–714
Juratli MA, Nour-Eldin NA, Ackermann H et al (2018) Purse-string closure technique reduces the incidence of incisional hernias following the reversal of temporary ileostomy. Int J Colorectal Dis 33:973–977
Klink CD, Wunschmann M, Binnebosel M et al (2013) Influence of skin closure technique on surgical site infection after loop ileostomy reversal: retrospective cohort study. Int J Surg 11(10):1123–1125
Lee JR, Kim YW, Sung JJ et al (2011) Conventional linear versus purse-string skin closure after loop ileostomy reversal: comparison of wound infection rates and operative outcomes. J Korean Soc Coloproctol 27(2):58–63
Lee JT, Marquez TT, Clerc D et al (2014) Pursestring closure of the stoma site leads to fewer wound infections: results from a multicenter randomized controlled trial. Dis Colon Rectum 57(11):1282–1289
Li LT, Brahmbhatt R, Hicks SC, Davila JA, Berger DH, Liang MK (2014) Prevalence of surgical site infection at the stoma site following four skin closure techniques: a retrospective cohort study. Dig Surg 31(2):73–78
Marquez TT, Christoforidis D, Abraham A, Madoff RD, Rothenberger DA (2010) Wound infection following stoma takedown: primary skin closure versus subcuticular purse-string suture. World J Surg 34(12):2877–2882
Milanchi S, Nasseri Y, Kidner T, Fleshner P (2009) Wound infection after ileostomy closure can be eliminated by circumferential subcuticular wound approximation. Dis Colon Rectum 52(3):469–474
Mirbagheri N, Dark J, Skinner S (2013) Factors predicting stomal wound closure infection rates. Tech Coloproctol 17(2):215–220
O’Leary DP, Carter M, Wijewardene D et al (2017) The effect of purse-string approximation versus linear approximation of ileostomy reversal wounds on morbidity rates and patient satisfaction: the ‘STOMA’ trial. Tech Coloproctol 21(11):863–868
Reid K, Pockney P, Pollitt T, Draganic B, Smith SR (2010) Randomized clinical trial of short-term outcomes following purse-string versus conventional closure of ileostomy wounds. Br J Surg 97(10):1511–1517
Suh YJ, Park JW, Kim YS, Park SC, Oh JH (2014) A beneficial effect of purse-string skin closure after ileostomy takedown: a retrospective cohort study. Int J Surg 12(6):615–620
Wada Y, Miyoshi N, Ohue M et al (2015) Comparison of surgical techniques for stoma closure: a retrospective study of purse-string skin closure versus conventional skin closure following ileostomy and colostomy reversal. Mol Clin Oncol 3(3):619–622
Yamamoto M, Tanaka K, Masubuchi S et al (2018) Risk factors for surgical site infection after stoma closure comparison between pursestring wound closure and conventional linear wound closure: propensity score matching analysis. Am J Surg 215(1):58–61
Yoon SI, Bae SM, Namgung H, Park DG (2015) Clinical trial on the incidence of wound infection and patient satisfaction after stoma closure: comparison of two skin closure techniques. Ann Coloproctol 31(1):29–33
Younis J, Chowdhury Y, Scott HJ (2011) Purse-string closure versus conventional primary linear closure of ileostomy wounds. Colorectal Dis 13(Supp5):87
Zhou P, Hrabe J, Byrn J (2016) A retrospective, single-institution review of loop ileostomy reversal outcomes. Ostomy Wound Manag 62(8):22–33
Hseih MC, Kuo LT, Chi CC, Huang WS, Chin CC (2015) Pursestring closure versus conventional primary closure following stoma reversal to reduce surgical site infection rate: a meta-analysis of randomized controlled trials. Dis Colon Rectum 61(2):808–815
Funding
None.
Author information
Authors and Affiliations
Contributions
MG, RB: Intellectual concept and design of the work. MG, HL, AC, AD, NZ, and RB: Acquisition, analysis, or interpretation of the data. MG, HL, AC, AD, NZ, and RB: Drafting the manuscript. MG, HL, AC, AD, NZ, and RB: Revising and final approval of the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gachabayov, M., Lee, H., Chudner, A. et al. Purse-string vs. linear skin closure at loop ileostomy reversal: a systematic review and meta-analysis. Tech Coloproctol 23, 207–220 (2019). https://doi.org/10.1007/s10151-019-01952-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10151-019-01952-9