Abstract
Background
Stoma closure has been associated with a high rate of surgical site infection (SSI) and the ideal stoma-site skin closure technique is still debated. The aim of this study was to compare the rate of SSI following primary skin closure (PC) versus a skin-approximating, subcuticular purse-string closure (APS).
Methods
All consecutive patients undergoing stoma closure between 2002 and 2007 by two surgeons at a single tertiary-care institution were retrospectively assessed. Patients who had a new stoma created at the same site or those without wound closure were excluded. The end point was SSI, determined according to current CDC guidelines, at the stoma closure site and/or the midline laparotomy incision.
Results
There were 61 patients in the PC group (surgeon A: 58 of 61) and 17 in the APS group (surgeon B: 16 of 17). The two groups were similar in baseline and intraoperative characteristics, except that patients in the PC group were more often diagnosed with benign disease (p = 0.0156) and more often had a stapled anastomosis (p = 0.002). The overall SSI rate was 14 of 78 (18%). All SSIs occurred in the PC group (14 of 61 vs. 0 of 17, p = 0.03).
Conclusions
Our study suggests that a skin-approximating closure with a subcuticular purse-string of the stoma site leads to less SSI than a primary closure. Randomized studies are needed to confirm our findings and assess additional end points such as healing time, cost, and patient satisfaction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of a diverting stoma has been shown to be a safe and effective adjunct in the treatment of many colorectal disorders (e.g., diverticular perforation, inflammatory bowel disease, colorectal cancer). Morbidity after stoma closure, however, is not negligible and the most common complication is postoperative superficial surgical site infection (SSI) [1–3]. The reported incidence of SSI following stoma closure is in the range of 2–41% [4, 5], a rate higher than expected for a clean-contaminated wound. To date, there is no consensus on the ideal closure technique of the stoma wound to minimize postoperative SSI and multiple techniques have been proposed: tight primary closure, loose primary closure, delayed primary closure, or no closure and healing by secondary intention. Primary closure of the wound leads to rapid healing but has been associated with higher postoperative SSI [4–7]. Benarjee [8] proposed a hybrid technique using a skin-approximating closure with a subcutaneous purse-string suture. This technique may lead to better cosmetic results and possibly less SSI [8, 9]. The aim of this study was to compare the incidence of SSI at the stoma site and/or at a concomitant laparotomy site after primary skin closure (PC) versus skin-approximating subcuticular purse-string closure (APS) of a stoma wound.
Materials and methods
We retrospectively reviewed the medical records of all consecutive patients who underwent surgery for stoma closure at the Division of Colon and Rectal Surgery at the University of Minnesota-Fairview Hospital, between January 2002 and December 2007. The study was approved by the University of Minnesota institutional review board. Ileostomy and colostomy takedown procedures were identified by procedure billing code. Two colorectal surgeons performed all procedures. We included only patients whose wound at the stoma site was closed either primarily (PC) or with an approximating subcuticular purse-string closure (APS) (Fig. 1). We reviewed both inpatient and outpatient medical records to retrieve data on potential risk factors for SSI (baseline characteristics, intraoperative variables, and postoperative variables) and recorded episodes of SSI. A SSI was defined by current Center for Disease Control (CDC) guidelines (Table 1) [10]. The endpoint of this study was SSI either at the stoma site and/or at the midline laparotomy wound.
Surgical technique
All procedures were performed with the patient under general anesthesia. Prophylactic antibiotics [second-generation cephalosporin in 68 (87%), other in 9 (12%), none in 1 (1%) patient in the APS group] were administered intravenously at a median of 5 min after induction of anesthesia (range = −70 to +40 min). Stoma takedown procedures included closure of diverting loop ileostomy and loop colostomy, or reversal of end ileostomy or end colostomy. A midline laparotomy was performed if the peristomal incision did not provide adequate exposure. Bowel continuity was re-established with either stapled or hand-sewn anastomosis at the discretion of the surgeon. The fascia at the stoma site and at the midline was closed with a long-term absorbable monofilament suture. Ventral hernias were repaired in five patients in the PC group and in one patient in the APS group, and parastomal hernias were repaired in one patient in each group. A biological mesh was used for one patient in each group (one patient received human acellular matrix, one patient had porcine acellular matrix implanted).
In the PC group, the peristomal incision was elliptical with a 2–3-mm margin from the mucocutaneous junction and was closed primarily with staples (n = 59) or suture (n = 2). A subcutaneous drain at the stoma site was left in place in 8/61 patients. The wound was then covered with at sterile dressing.
In the APS group, the peristomal incision was circular at the mucocutaneous junction. A subcuticular purse-string suture with a 2-0 resorbable monofilament was placed around the wound, then tightened and secured, leaving a skin defect size of approximately 1 cm. This opening was then packed with a wick dressing (Nu Gauze) and covered with a sterile dressing. Of note, extension of the stomal incision was required in one patient to aid in visualization during stoma takedown. For this case, the lateral margins of the incision were closed with staples and the central portion was then closed via the skin-approximating purse-string closure.
Statistical analysis
Categorical variables were compared using the χ2 test for multiple outcomes, the Fisher’s exact test for dichotomous outcomes involving small samples, Student’s t test for continuous variables with normal distribution, or the Mann–Whitney nonparametric test for non-normal distributions. All statistical analyses were performed using the GraphPad Instat software package (GraphPad Software Inc., San Diego, CA).
Results
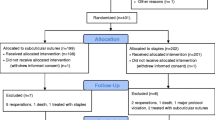
We identified 92 patients who underwent a stoma closure during the study period. Of those, we excluded 14 patients who did not have their stoma wound closed, either because a new stoma was created at the same site (n = 7) or because the wound was left open to heal by secondary intention (n = 7). Patients who had a new stoma created at a different site (n = 3) were included. In all, 78 patients were included in the analysis of whom 61 (78%) had the skin at the stoma site closed by primary closure (PC) and 17 (22%) had the stoma closed with an approximating purse-string suture (APS). Surgeon A preferably performed primary closures (58 of 61 patients in the PC group) while surgeon B preferably performed purse-string closure (16 of 17 patients in the APS group) (p < 0.001). Patient baseline characteristics and perioperative parameters in the two groups are shown in Tables 2 and 3, respectively.
The overall SSI rate was 18%. All SSI were encountered in the PC group (PC vs. APS, 14 of 61 vs. 0 of 17, p = 0.03). In eight patients, the SSI occurred at the stoma site only, in three patients at the midline laparotomy wound only, and in the remaining three patients at both locations. The SSI was treated by opening the wound at bedside or in the clinic in 11 cases and with antibiotics only in 3 cases.
At univariate analysis within the PC group, patients with SSI had significantly longer operations, higher preoperative blood loss, more frequent episodes of hypothermia, and more often chronic renal failure (Table 4). Because there was no SSI in the APS group, we were unable to construct a multivariate model to identify independent risk factors for SSI.
Discussion
It is still debated which closure technique of a stoma wound leads to the lowest SSI rate. Depending on the author and closure technique, SSI rates vary from 0 to 41% (Table 5) [4–23]. Although some case series have reported very low (0–3%) SSI rates after primary closure, most comparative studies—all retrospective—report better outcomes with closure by secondary healing or partial primary closure. The only randomized controlled study performed on stoma wound closure techniques compared primary closure to delayed primary closure and found that delayed primary closure was associated with higher SSI rates.
In our study, the SSI rate in the primary closure group (PC) was 18%, which is consistent with previous reports in the literature [12, 15, 16, 20]. No patient presented a SSI in the purse-string (APS) group. This is similar to the result of Sutton et al. [9] of a 0% SSI rate after purse-string closure in 52 patients. In a recent study, Milanchi et al. [23] compared 24 patients treated with a purse-string closure to 25 patients treated with primary closure. They also found no SSI in the APS group compared to 40% SSI in the PC group (p < 0.001). These results taken together suggest a very low risk of SSI with the APS technique.
Within the PC group, significant risk factors for SSI in univariate analysis were long operative time, increased blood loss, and chronic renal failure. Higher blood loss is associated with ostomy closures that required concurrent laparotomy and extensive adhesiolysis. Laparotomy was required in 4/14 (29%) of those with SSI and 12/47 (25%) of those with no SSI in the PC group. There was also a trend for higher SSI rates in patients with higher BMI and in those whose anastomosis was hand-sewn. These findings are in accordance with previous studies on risk factors for postoperative wound infection [1].
Our study has several limitations. The two groups were comparable for most preoperative, intraoperative, and postoperative characteristics but differed significantly in the distribution of primary diagnoses, surgeon, and type of bowel anastomosis. The clinical relevance of the difference in diagnosis distribution is unclear. Patients with stoma due to rectal cancer may be debilitated by adjuvant therapy, but patients with stomas for inflammatory disease are also frequently immunocompromised by their medical therapy. The surgeon was strongly correlated with a closure technique so unrecognized surgeon-related factors may have also influenced the outcome. In our study, there was a trend for more SSI in patients with hand-sewn anastomosis within the PC group, but in the APS group, where no SSI was encountered, there were significantly more hand-sewn anastomoses. In large studies, the type of anastomosis (hand-sewn vs. stapled) has not been found to influence significantly the risk for SSI [3, 4, 20]. Because of the retrospective nature of the study, its small size, and the fact that there were no events in one study arm, we were unable to control for confounding factors in a multivariate model. We recognize that there is the possibility of missed instances of SSI that may have been diagnosed by other care providers (e.g., primary care physicians); however, all subjects were seen by their surgeon in at least one postoperative visit within 30 days of surgery, where any postoperative complications were addressed and documented. We were also limited in our ability to evaluate other end points of interest such as time to wound healing, patient satisfaction (cosmetic outcome, difficulty of wound care), and cost analysis. These additional end points to SSI after stoma wound closure have been included in our ongoing prospective randomized trial comparing APC and PC.
Conclusion
Our experience suggests that the approximating subcuticular purse-string closure may lead to less postoperative SSI compared to primary closure. We are currently conducting a randomized trial to confirm our findings and further evaluate other end points of interest such as time to healing, cost-effectiveness, and overall patient satisfaction.
References
Konishi T, Watanabe T, Kishimoto J et al (2006) Elective colon and rectal surgery differ in risk factors for wound infection. Ann Surg 244(5):758–763
Kaidar-Person O, Person B, Wexner S (2005) Complications of temporary loop ileostomy. J Am Coll Surg 201(5):759–773
Riesener KP, Lehnen W, Hofer M et al (1997) Morbidity of ileostomy and colostomy closure: impact of surgical technique and perioperative treatment. World J Surg 21:103–108
Wong K, Remzi F, Gorgun E et al (2005) Loop ileostomy closure after restorative proctocolectomy: outcome in 1,504 patients. Dis Colon Rectum 48:243–250
Hackam D, Rotstein O (1995) Stoma closure and wound infection: an evaluation of risk factors. Can J Surg 38(2):144–148
van de Pavoordt H, Fazio V, Jagelman D et al (1987) The outcome of loop ileostomy closure in 293 cases. Int J Colorect Dis 2:214–217
Vermulst N, Vermeulen J, Haebroek E et al (2006) Primary closure of the skin after stoma closure. Dig Surg 23:255–258
Banerjee A (1997) Pursestring skin closure after stoma reversal. Dis Colon Rectum 40:993–994
Sutton E, Williams N, Marshall L et al (2002) A technique for wound closure that minimizes sepsis after stoma closure. ANZ J Surg 72:766–767
Mangram AJ, Horan TC, Pearson ML et al (1999) Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol 20(4):247–278
Amin S, Memon M, Armitage N et al (2001) Defunctioning loop ileostomy and staples side-to-side closure has low morbidity. Ann R Coll Surg Engl 83:246–249
Phang P, Hain J, Perez-Ramirez J et al (1999) Techniques and complications of ileostomy takedown. Am J Surg 177:463–466
Lewis P, Bartolo D (1990) Closure of loop ileostomy after restorative proctocolectomy. Ann R Coll Surg Engl 72:263–265
Feinberg S, McLeod R, Cohen A (1987) Complications of loop ileostomy. Am J Surg 153:102–107
Pittman D, Smith L (1985) Complications of colostomy closure. Dis Colon Rectum 28(11):836–843
Parks S, Hastings P (1985) Complications of colostomy closure. Am J Surg 149:672–675
Fasth S, Hulten L (1984) Loop-ileostomy: a superior diverting stoma in colorectal surgery. World J Surg 8:401–407
Williams LA, Sagar PM, Finan PJ et al (2007) The outcome of loop ileostomy closure: a prospective study. Colorectal Dis 10:460–464
Lahat G, Tulchinsky H, Goldman G et al (2005) Wound infection after ileostomy closure: a prospective randomized study comparing primary closure vs. delayed primary closure techniques. Tech Coloproctol 9:206–208
Garcia-Botello S, Garcia-Amengol J, Garcia-Granero E et al (2004) A prospective audit of the complications of loop ileostomy construction and takedown. Dig Surg 21:440–446
Khoo R, Cohen M, Chapman G et al (1994) Loop ileostomy for temporary fecal diversion. Am J Surg 167:519–522
Wexner S, Taranow D, Johansen O et al (1993) Loop ileostomy is a safe option for fecal diversion. Dis Colon Rectum 36:349–354
Milanchi S, Nasseri Y, Kidner T et al (2009) Wound infection after ileostomy closure can be eliminated by circumferential subcuticular wound approximation. Dis Colon Rectum 52(3):469–474
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marquez, T.T., Christoforidis, D., Abraham, A. et al. Wound Infection Following Stoma Takedown: Primary Skin Closure versus Subcuticular Purse-string Suture. World J Surg 34, 2877–2882 (2010). https://doi.org/10.1007/s00268-010-0753-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-010-0753-4