Abstract
Background
No previous reports have characterized national bone sarcoma profiles overall. We examined the nationwide statistics for bone sarcoma in Japan using data from the National Cancer Registry (NCR), a population-based cancer registry.
Methods
We identified 3,755 patients with bone sarcomas entered in the NCR during 2016–2019 using International Classification of Diseases-Oncology, Third Edition codes for cancer topography and morphology. We extracted data on patient demographics, tumor details (reason for diagnosis, tumor location, histology, extent of disease), hospital volume/type, treatment, and prognosis for each patient.
Results
Bone sarcoma showed a slight male preponderance. The age distribution peaked at ages 10–20 and 60–80; approximately 44% of patients were aged over 60 years. Chordoma, chondrosarcoma, and malignant fibrous histiocytoma of bone peaked in the elderly, and Ewing’s sarcoma peaked in children. Osteosarcoma had two peaks in Japan as well as in Western countries. The most frequent tumor locations were the limb (45%) and the pelvis (21%). Extent of disease was categorized as: “localized” (39%), “regional” (27%), and “distant” (11%). We found significant associations between overall survival and age, tumor location, facility type, hospital volume, histologic subtype, reason for diagnosis, and extent of disease. The latter had the poorest survival.
Conclusions
This is the first study to outline the epidemiology, clinical features, treatment, prognosis, and significant factors affecting prognosis of bone sarcoma in Japan using the NCR. Documenting our data regarding elderly patients’ outcomes is essential so other countries showing similar population-aging trends can learn from our experiences.
Level of evidence
Prognostic studies, Level III.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone sarcomas are heterogeneous malignant neoplasms originating from mesenchymal cells. There are a number of histological types, and the tumors can arise at any anatomic site, creating considerable variety of histologic and primary site combinations. In addition to the heterogeneity of bone sarcoma, its rarity inevitably makes diagnosis and treatment difficult.
A few previous reports have focused on the epidemiology of osteosarcoma or Ewing’s sarcoma using the Surveillance, Epidemiology, and End Results (SEER) database in the United States [1,2,3,4,5], and on the epidemiology of bone sarcoma using a nationwide, organ-specific cancer registry for bone and soft-tissue tumors in Japan [6, 7]. However, to our knowledge, no attempt has yet been made to characterize the population-based overall profiles of bone sarcoma at the national level. There has also been little information available regarding the prognostic factors for bone sarcoma based on a nationwide cohort.[1, 2, 6, 7].
Therefore, in the present study, we attempted to clarify the nationwide statistics for bone sarcoma in Japan by analyzing data from the National Cancer Registry (NCR), which is a nationwide, population-based cancer registry that was launched in 2016. This study is the first to use the NCR to study bone sarcoma since it became available for the purpose of clinical research in 2019.
Patients and methods
Data source
The NCR was developed as a reliable cancer-surveillance system on the legal basis of the Cancer Registry Promotion Act of 2013, for the purpose of promoting cancer control in Japan. The NCR was transitioned from prefectural, population-based cancer registries and the data of cancer patients diagnosed since January 2016 has been collected.
The details of enrollment of the NCR are stipulated in the manual (https://ganjoho.jp/med_pro/cancer_control/can_reg/national/hospital/pdf/ncr_manual_2022.pdf). Briefly, the timing of patient enrollment is basically at the time of diagnosis and gathered data must be submitted by the end of next year (ex. The data on patient diagnosed on January 10, 2020 must be submitted by December 31, 2021). All hospitals in Japan are obliged to submit basic data to prefectures when they diagnose new patients with cancer. Therefore, the NCR is a population-based cancer registry which corresponds to the SEER database in the United States, but has unique advantage in terms of registry completeness. The unique advantage of the NCR is that it represents almost all newly diagnosed cancer cases in Japan. One of the main advantages of the NCR is that it can avoid double enrollment of the patient because the national government leads the registry. On the other hand, former population-based cancer registry (Regional Cancer Registry) in Japan was led by each prefecture, and it had remained possible that one patient is registered twice (double registration) in case the patient was diagnosed at the hospital in one prefecture then transitioned to another hospital in a different prefecture for treatment.
The data collected include prefecture of the patient’s address; cancer type and stage; treatment; circumstance of cancer detection, diagnosis, and treatment; survival; etc. For the patient follow-up, the ministry provides death certificate data to the national cancer registry office once a year and the office matches them to the registered cases every year. The collected data were thoroughly filtered by the government and have been available to researchers since 2019. This study conducted investigative research based on the Cancer Registry Act. According to the procedure stipulated by the law, the protocol was reviewed by the Data Utilization Committee of the National Cancer Registration Office. As per the research ethics guidelines in Japan, our study was exempted from an ethics review by our institutional review board.
Data extraction
Patients eligible for study inclusion were diagnosed with bone sarcoma between 2016 and 2019, as defined by the cancer topography codes (C400–C403, C408–C414, C418–C419) and morphology codes (8000–8934, 8940–9138, 9141–9582) of the International Classification of Diseases-Oncology, Third Edition (ICD-O-3) [8]. The final cohort was composed of 3,755 patients. The data in this study were independently created and processed in accordance with relevant data-sharing laws.
The final classifications for bone sarcomas included osteosarcoma, chondrosarcoma, chordoma, Ewing’s sarcoma, malignant fibrous histiocytoma (MFH) of bone, and other unspecified bone sarcomas (Table 1, Appendix 1). Although the term “MFH of bone” is no longer used in the latest World Health Organization (WHO) classification and replaced by undifferentiated pleomorphic sarcoma (UPS), we used MFH of bone in accordance with the ICD-O-3 as a synonym for UPS of bone. Tumor location was categorized according to topography codes into “chest wall,” “craniofacial bone,” “limb,” “pelvis,” and “spine.” The extent of disease was categorized into “localized” (confined to the organ of origin and not spread to other parts of the body), “regional” (the spread of cancer from its original site to nearby areas, such as regional lymph nodes and adjacent organs, but not to distant sites), and “distant” (spread to organs or tissues that are farther away). Type of facility was categorized based on Japanese Orthopedic Association (JOA) certification. The number of patients with bone and soft-tissue sarcoma for each hospital was assessed, and the hospital volumes were determined using the hospital’s unique identifier and categorized by patient tertiles into “low” (≤ 49 cases/4 years), “medium” (50–158 cases/4 years), or “high” volume (≥ 159 cases/4 years). This is because facilities treating bone sarcoma usually treat also soft-tissue sarcoma.
Statistical analyses
An age-adjusted rate is a measure that controls for the effects of age differences on the rate of health events. In this study, we used direct age adjustment for calculating incidence where the sum of the products of age-specific rates observed in a population, multiplied by the proportion of each age group in a standard population, was the age-adjusted incidence. The 1985 model population of Japan was used for this purpose. The incidences were described per 100,000 population. To compare the incidences among different prefectures, standardized incidence ratios (SIR) were calculated where the SIRs were obtained by dividing the observed number of cases of bone sarcoma by the expected number of cases estimated using the entire Japanese population as a reference.
We performed univariate comparisons of proportions using the chi-squared test. Overall survival (OAS) was defined as the period from the date of diagnosis to the date on which the patient was last contacted, or died. The OAS was estimated using the Kaplan–Meier method, and comparisons were assessed using the log-rank test. Univariate and multivariate analyses were conducted using the Cox proportional hazards model.
Differences were considered to be statistically significant at P < 0.05. All statistical analyses were conducted using IBM SPSS version 19.0 (IBM SPSS, Armonk, NY, USA).
Results
Clinical characteristics of the patients overall
For the period of 2016–2019, we identified the records of 3,755 patients who were diagnosed with bone sarcomas. The characteristics of the study population according to subtypes are summarized in Table 1. Approximately 900–950 new cases were diagnosed as bone sarcoma per year, with the age-adjusted incidence of bone sarcoma being 0.68/100,000/year, which meets the criteria for a rare cancer (< 6/100,000/year) [9]. The SIRs of bone sarcoma among different prefectures ranged from 0.72 (Okinawa Prefecture) to 1.38 (Yamagata Prefecture) (Fig. 1).
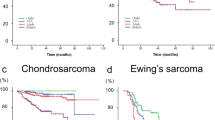
There was a slight male preponderance in the diagnoses. The age distribution had two peaks overall: one in the 10–20 age range, and the other in the 60–80 age range (Table 1, Fig. 2). Among major histologic subtypes, chordoma, chondrosarcoma, and MFH of bone had a peak in the elderly, and Ewing’s sarcoma had a peak in children; whereas osteosarcoma had two peaks: one in the 10–19 age range and one in the 60–79 age range.
The most common sarcoma location was the limb (N = 1,705; 45%), followed by the pelvis (N = 787; 21%) and craniofacial bones (N = 508; 14%). According to the histologic subtypes, osteosarcoma had a preponderance in the limb (68%) while specific subtypes tended to affect the axial skeleton (chest wall, pelvis, and spine): chordoma (65%), Ewing’s sarcoma (54%), and chondrosarcoma (45%).
Only a small proportion of patients (2.7%) were diagnosed with bone sarcoma by cancer screening or routine health check-up. As expected, patients with osteosarcoma or Ewing’s sarcoma were rarely diagnosed by cancer screening or routine health check-up. However, patients with chondrosarcoma (5.4%) or chordoma (3.7%) were more frequently found by cancer screening or routine health check-up, reflecting the indolent nature of the disease and its prevalence in the elderly population.
Distant metastasis was present at the time of diagnosis in 423 cases (11.3%), overall. These rates were extremely high in Ewing’s sarcoma (25%) and MFH of bone (24%), while few cases had distant metastasis in chordoma (2.4%) and chondrosarcoma (6.5%).
Approximately half of the patients were diagnosed with bone sarcoma in JOA-certified hospitals, and this trend was more evident for osteosarcoma (63%) and Ewing’s sarcoma (63%).
Treatment
Overall, 1,984 patients (65%) underwent surgical treatment. The proportion of patients who underwent surgery was relatively low for chordoma (21%) and Ewing’s sarcoma (59%). This was probably due to the widely recognized efficacy of carbon-ion radiotherapy for chordoma, the radiosensitivity of Ewing’s sarcoma, and their tendency to arise in the torso. This explanation is supported by the evidence that the proportion of patients who underwent radiotherapy was high for chordoma (57%) and Ewing’s sarcoma (37%), compared with other subtypes of bone sarcoma.
As for chemotherapy, 1,133 patients (39%) underwent this treatment. This included the majority of patients with osteosarcoma (73%) and Ewing’s sarcoma (98%), whereas few patients with chordoma and chondrosarcoma underwent chemotherapy.
Survival and prognostic factors
The cumulative 3-year OAS was 67% (follow-up period, mean 579 [standard deviation 419] days) (Fig. 3). The unadjusted associations of various factors with the OAS rate determined using Kaplan–Meier plots are shown in Fig. 4A–K.
Table 2 shows the unadjusted and adjusted hazard ratios obtained from the Cox proportional hazards model for OAS. Female patients had significantly better OAS than male patients (hazard ratio [HR], 0.82; 95% confidence interval [CI], 0.70–0.97; P = 0.023). Patients aged 40–59 years (HR: 1.84; 95% CI 1.22–2.78; P = 0.004) or ≥ 60 years (HR: 4.38; 95% CI 3.01–6.36; P < 0.001) had significantly worse OAS than those aged ≤ 14 years. Tumor location in the pelvis (HR: 1.54; 95% CI 1.24–1.92; P < 0.001) or spine (HR: 1.53; 95% CI 1.17–1.99; P = 0.002) was associated with significantly worse OAS than tumor location in the limb. In terms of histologic subtype, chondrosarcoma (HR: 3.22; 95% CI 1.98–5.23; P < 0.001), osteosarcoma (HR: 6.29; 95% CI 3.91–10.11; P < 0.001), Ewing’s sarcoma (HR: 4.68; 95% CI 2.50–8.78; P < 0.001), and MFH of bone (HR: 6.14; 95% CI 3.48–10.83; P < 0.001) were associated with significantly worse OAS than was the case for chordoma. The extent of disease was the strongest predictor for OAS when compared with localized disease: regional (HR: 1.56; 95% CI 1.24–1.95; P < 0.001) and distant (HR: 5.72; 95% CI 4.54–7.19; P < 0.001). Patients who were diagnosed with bone sarcoma by cancer screening or routine health check-up had significantly better outcomes (HR: 0.21; 95% CI 0.07–0.65; P = 0.007). Patients diagnosed at low-volume centers had significantly worse OAS than those diagnosed at high-volume centers (HR: 1.30; 95% CI 1.04–1.61; P = 0.019).
Discussion
In the present study, we analyzed 3,755 patients with bone sarcoma who were registered in the NCR between 2016 and 2019, focusing on descriptive epidemiology and treatment statistics. In addition, we obtained an overview of the prognosis and identified several significant factors affecting OAS for patients with bone sarcoma. To our knowledge, the current study is the first to have characterized the profiles of bone sarcoma on a national basis in Japan, using population-based data.
Epidemiology data from population-based cancer registries is valuable in the development of health policy and for improving the quality of cancer control strategies, and population-based cancer registries have been developed mainly in Western countries. Although the latest SEER data on bone sarcoma incidence is not published in the academic literature, the age-adjusted incidence of bone sarcoma based on 2017–2021 cases is reported as 1.0/100,000 in the “Cancer Stat Facts: Bone and Joint Cancer” in the SEER website [10]. Although population-based cancer registries are less developed in Asian countries, recent article [11] reported the age-adjusted incidence of bone sarcoma in Thailand as 0.51/100,000/year. Because comparing epidemiology data of different countries could help contextualize the findings within a global framework and may reveal unique trends or common challenges in sarcoma management, we are planning a comparative analysis of bone sarcoma using registries from different countries in the future study.
The proportion of the elderly population is growing rapidly worldwide, and this trend is particularly evident in Japan, where the elderly (those aged 65 years or older) accounted for approximately 30% of the population in 2022—the highest such percentage in the world [12]. Consequently, it is expected that the number of elderly patients diagnosed with bone sarcoma will increase. Our data demonstrate a bimodal age distribution of bone sarcoma patients, with one peak in the 10–20 age range and another in the 60–80 age range. Also, the proportion of elderly patients aged 65 years and over was approximately 37%. We also demonstrated that bone sarcoma in the elderly had a worse prognosis than in younger patients after adjustment for patient background characteristics. As this trend is expected to continue in the next few decades, it is necessary to establish a treatment strategy of elderly patients with bone sarcoma, who usually have decreased performance status and several comorbidities. Furthermore, it is anticipated that documenting our experiences using the NCR will be helpful to other countries also experiencing population aging.
Previously, it was believed that osteosarcoma in Japan had a single age peak because of the low rate of Paget’s disease of bone among the Japanese population—whereas this disease shows two age peaks in Caucasians [13]. Ogura et al. [6], reported there was no second age peak in the elderly for sarcoma based on 3,256 cases that had been entered in the Bone and Soft Tissue Tumor (BSTT) Registry in Japan between 1972 and 2003. The age distribution became bimodal among 1,152 cases of osteosarcoma diagnosed in Japan during 2006–2012. However, this registry is not population based, and the cases were mainly orthopedic cases because the JOA is in charge of this registry; therefore, the actual incidence and age distribution have been unclear. In the present study, we first verified that osteosarcoma in Japan, as well as Western countries, had bimodal age peaks in a population-based approach. This may have been attributable to the increased proportion of the elderly population or a change in awareness regarding the diagnosis of osteosarcoma in the elderly.
One of the shortcomings of the organ-specific cancer registries organized by academic societies, such as the BSTT Registry, is the underreporting and bias of the cases treated by physicians of different specialties. This background makes it difficult for the BSTT Registry to capture the actual number of cases with bone sarcoma which were not treated by orthopedic oncologists, including those originating from the spine and craniofacial bones. In fact, in the report of national statistics of bone sarcoma based on the BSTT Registry, bone sarcoma in the spine and craniofacial bones accounted for 4% and 1% of bone sarcoma, respectively [6], whereas they accounted for 9.5% and 13.5% in the NCR, respectively. The present study revealed the actual incidence of bone sarcoma, especially those that were not treated by orthopedic oncologists and covered by the BSTT Registry.
Several important limitations of the present study should be acknowledged. First, database studies usually have incomplete or inaccurate data that can bias the results. There may be an under- or overestimation of the data due to this incomplete reporting. Second, we were unable to control for several potentially important clinical parameters that may have affected the OAS, including tumor size, the severity of preoperative comorbidities, response to chemotherapy, surgery type, and surgical margin status because the NCR does not collect such information. Similarly, it should be noted that some other important data specifically relevant to the tumor, including extent of the disease, were absent in 22% of the patients. Third, we have to admit that the follow-up time currently possible in the NCR is not currently long enough because it started in January 2016. Fourth, the different SIRs of bone sarcoma among different prefectures can be due to the magnitude of a single case resulting from the nature of rare incidence, therefore, should be interpreted carefully. Despite these limitations, we believe that the present study is valuable because it is the first to have characterized the profiles of bone sarcoma, including the demographic features, treatment data, survival, and prognostic factors, on a national basis in a population-based manner in Japan.
In addition, it reflects the current statistics and treatment outcomes that can be expected with modern treatment strategies as the cases included in this study were treated within a short period during 2016–2019, when it can be assumed that treatment strategies would have been relatively uniform. As this would exclude any historical changes in statistics or treatment outcomes, the present data reflect not only the national trend in Japan but also provide the best indication of what can be expected from modern multidisciplinary treatment in a worldwide context. Although the present study is the first to have used the NCR since it became available for the purposes of clinical research in 2019, we anticipate that further informative data for bone sarcoma will emerge, thus improving both the level of medical care offered by clinicians and outcomes for patients through the sharing of such data and the promotion of clinical research using this database.
In conclusion, this study has presented an overview of the epidemiology, clinical features, treatment, and prognosis of patients with bone sarcoma in Japan based on the large-scale cohort of the NCR. The current study is the first to have characterized the profiles of bone sarcoma on a national basis in a population-based manner in Japan and should provide a valuable starting point for further understanding of bone sarcoma and future clinical studies aimed at determining therapeutic strategies for management of bone sarcoma patients.
Abbreviations
- BSTT:
-
Bone and Soft Tissue Tumor Registry in Japan
- CI:
-
Confidence interval
- HR:
-
Hazard ratio
- ICD-O-3:
-
International Classification of Diseases-Oncology, Third Edition
- JOA:
-
Japanese Orthopedic Association
- MFH:
-
Malignant fibrous histiocytoma (of bone)
- NCR:
-
National Cancer Registry
- OAS:
-
Overall survival
- SEER:
-
Surveillance, Epidemiology, and End Results
- SIR:
-
Standardized incidence ratios
References
Duchman KR, Gao Y, Miller BJ (2015) Prognostic factors for survival in patients with Ewing’s sarcoma using the surveillance, epidemiology, and end results (SEER) program database. Cancer Epidemiol 39:189–195
Duchman KR, Gao Y, Miller BJ (2015) Prognostic factors for survival in patients with high-grade osteosarcoma using the Surveillance, Epidemiology, and End Results (SEER) Program database. Cancer Epidemiol 39:593–599
Duong LM, Richardson LC (2013) Descriptive epidemiology of malignant primary osteosarcoma using population-based registries, United States, 1999–2008. J Registry Manag 40:59–64
Mirabello L, Troisi RJ, Savage SA (2009) Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer 115:1531–1543
Schrager J, Patzer RE, Mink PJ et al (2011) Survival outcomes of pediatric osteosarcoma and Ewing’s sarcoma: a comparison of surgery type within the SEER database, 1988–2007. J Registry Manag 38:153–161
Ogura K, Higashi T, Kawai A (2017) Statistics of bone sarcoma in Japan: Report from the Bone and Soft Tissue Tumor Registry in Japan. J Orthop Sci 22:133–143
Ogura K, Higashi T, Kawai A (2017) Statistics of soft-tissue sarcoma in Japan: Report from the Bone and Soft Tissue Tumor Registry in Japan. J Orthop Sci 22:755–764
Cates JMM (2018) The AJCC 8th Edition Staging System for Soft Tissue Sarcoma of the Extremities or Trunk: A Cohort Study of the SEER Database. J Natl Compr Canc Netw 16:144–152
Stiller CA, Trama A, Serraino D et al (2013) Descriptive epidemiology of sarcomas in Europe: report from the RARECARE project. Eur J Cancer 49:684–695
National Cancer Institute (2017) Cancer Stat Facts: Bone and Joint Cancer. National Cancer Institute, Bethesda, MA
Klangjorhor J, Pongnikorn D, Phanphaisarn A et al (2022) An analysis of the incidence and survival rates of bone sarcoma patients in thailand: reports from population-based cancer registries 2001–2015. Cancer Epidemiol 76:102056
Statistics Bureau of Japan. https://www.stat.go.jp/data/jinsui/2022np/index.html
Ishikawa Y, Tsukuma H, Miller RW (1996) Low rates of Paget’s disease of bone and osteosarcoma in elderly Japanese. Lancet 347:1559
Funding
This study was funded by the National Cancer Center Research and Development Funds (31-A-14, 2022-A-15).
Author information
Authors and Affiliations
Contributions
KO, TH, and AK conceived of and designed the study. KO, CM, TS, SI, YT, SM, EK, AA, CO, YK acquired and analyzed data. All authors read, critically revised, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Ethics statement
Approval of the research protocol by an Institutional Reviewer Board: According to the procedure stipulated by the law, the protocol was reviewed by the Data Utilization Committee of the National Cancer Registration Office. As per the research ethics guidelines in Japan, our study was exempted from an ethics review by our institutional review board.
Informed consent
N/A.
Registry and the registration No. of the study/trial
N/A.
Animal studies
N/A.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
About this article
Cite this article
Ogura, K., Morizane, C., Satake, T. et al. Statistics of bone sarcoma in Japan: report from the population-based cancer registry in Japan. Int J Clin Oncol 29, 1209–1219 (2024). https://doi.org/10.1007/s10147-024-02566-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-024-02566-4