Abstract
Background
The optimal access for thermal ablation of the liver has not been evaluated in the literature for the laparoscopic versus percutaneous techniques. The aim of this manuscript was to determine the optimal ablation technique and patient selection for hepatic malignancies by comparing the efficacy and recurrence-free survival of laparoscopic and percutaneous thermal ablation.
Methods
A detailed literature search was made in PubMed, Web of Science, Google scholar, and EMBASE for related research publications. The data were extracted and assessed by two reviewers independently. Analysis of pooled data was performed, and Odds Ratio (OR) or Hazard Ratio (HR) with corresponding confidence intervals (CIs) was calculated and summarized respectively.
Results
A total of 10 articles were included with 1916 ablation patients. Laparoscopic ablation success (Median 100%) was found to be higher than percutaneous ablation success (median 89.4%) (p = ns). There was a higher percentage of both local and non-local hepatic recurrence in the patients treated with percutaneous ablation versus laparoscopic ablation. Meta-analysis indicated no difference in the adjusted hazard rate of recurrence by procedure type (p = 0.94). Laparoscopic ablation had a higher percentage of complications compared to percutaneous ablation (median lap 14.5% vs. perc 3.3%).
Conclusions
While laparoscopic and percutaneous ablation are both effective interventions for hepatic malignancies, laparoscopic ablation was found to have improved ablation success and less local and non-local hepatic recurrence compared to percutaneous ablation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hepatic malignancies are one of the most common and fatal cancers worldwide. It is the second leading cause of cancer in men and the sixth leading cause of cancer in women [1]. Hepatocellular carcinoma (HCC) is the most common primary liver malignancy, and it is the third leading cause of cancer deaths worldwide [2]. Other types of liver tumors include intrahepatic cholangiocarcinoma, gallbladder cancer, secondary liver malignancies (Colorectal and Neuroendocrine) as well as benign liver tumors [3]. HCC, metastases, and benign lesions have varying treatment modalities including surgical resection, thermal ablation, cryoablation, embolization, and liver transplantation [4]. When resection is not indicated, thermal ablation is an effective alternative treatment strategy that can be used as an adjunct in cancer therapy overall [5].
Two types of thermal ablation are currently being used as treatment modalities for hepatic tumors—microwave ablation (MWA) and radiofrequency ablation (RFA). The goal of both modalities is the induction of cellular damage or death [6]. It has been found that that cell death in the ablation zone (AZ) is indistinguishable between MWA and RFA [7]. However, there are some major differences between these two ablation modalities. RFA is limited by the level of temperature that can be reached in the AZ and by the variation in size of the AZ [6]. In contrast, due to the mechanism of action of MWA, heat can be continuously generated in much larger volumes of tissue; clinically, this results in fewer applications of energy and enhanced ease of obtaining ablation margins [8]. MWA is a relatively newer ablation modality, compared to RFA. Studies that examined MWA, RFA, or both modalities are included in this systematic review.
Laparoscopic and percutaneous approaches are currently being used for both MWA and RFA of hepatic tumors. Importantly, laparoscopic ablation has been found to be advantageous for preventing damage to nearby organs as well as for ablating tumors that are relatively inaccessible percutaneously [9]. Laparoscopic ablation is also beneficial for HCC tumors located on the surface, multiple tumors, or tumors that are undetectable by computerized tomography (CT) or magnetic resonance imaging (MRI) [9]. Perceptions remain that laparoscopic ablation is more invasive and carries more risk than does percutaneous ablation, however both require general anesthesia commonly and the length of stay has been equivalent [9].
It is vital that physicians opt for the most efficacious and safe ablative technique to enhance patient survival and prevent morbidity, recurrence, and death. Thus far, very few studies have compared laparoscopic ablation and percutaneous ablation of hepatic tumors with regards to optimal patient selection for each technique, efficacy and recurrence-free survival. The aim of this study was to compare these two ablative techniques for efficacy and recurrence-free survival.
Methods
Literature search
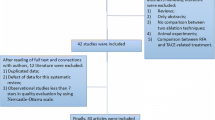
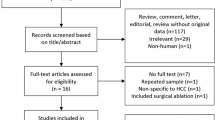
A systematic review of the literature was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines using PubMed, Web of Science, Google scholar, and EMBASE. The initial search was conducted using the terms “liver”, “ablation”, “laparoscopy”, “percutaneous”, and “oncology” in all fields. The initial search yielded 124 results (Fig. 1). An additional 5 articles were hand-selected and added. From the combined 129 articles, 119 articles were excluded after being screened for the following criteria: English only, human subjects only, ablation of liver tumors, and comparison of percutaneous and laparoscopic ablation. Duplicates, meta-analyses, and systematic reviews were also removed, yielding 10 total articles. The remaining 10 articles were examined in their entirety and searched for quality reporting data related to the key inclusion criteria, which is outlined in the section below. After this review, the 10 articles remained, and the efficacy data and recurrence-free survival outcomes based upon ablation modality type were extracted (Fig. 1).
Inclusion and exclusion criteria
Inclusion was limited to English articles and included observational and comparative cohort studies. Original articles that focused on ablation of liver tumors with the direct or indirect comparison of laparoscopic ablation versus percutaneous ablation were identified and included. Their reference lists were further examined to identify additional studies not captured by the primary literature search. Exclusion criteria included non-English studies, reviews, letters, abstracts, studies in animals, and laboratory studies. Additionally, any articles that examined resection or hepatectomy, organs that were not the liver, microwave ablation versus radiofrequency ablation, or only one ablative technique were excluded.
Data extraction and quality assessment
One author independently screened the titles and abstracts of all articles identified in the primary search strategy. Based on the inclusion and exclusion criteria, the author assessed the full text of 10 articles, and then subsequently performed the data extraction (JM). Enduring conflicts and questions were resolved following review by a second author (RM). Extracted perioperative and operative variables included tumor histology, tumor size and range, ablation modality, differences between percutaneous and laparoscopic groups, postoperative morbidity and mortality, and recurrence-free survival. The primary endpoint was recurrence-free survival. Secondary endpoints included ablation success, local recurrence and postoperative morbidity and mortality. Studies were given a “strength score” based on experimental design and number of participants. Randomized controlled trials (RCT) were assigned a value of + 4.0 points towards their strength score total. Non-randomized trials received a value of + 3.0 points. Observational studies with controls were given a value of + 2.0 points, and observational studies without controls were scored with + 1.0 points. Studies with more than 100 participants were given (+ 0.3) points, between 50 and 100 participants were given (+ 0.2) points, and less than 50 participants received (+ 0.1) points toward the total strength score.
Definitions
This systematic review follows the definitions of ablation success, local recurrence, and non-local hepatic recurrence as proposed by North et al. [4]. Ablation success was defined as “complete eradication of the tumor using high-quality cross-section contrast-enhanced imaging (CT or MRI) within 4 weeks of ablation, specifically disappearance of any intratumoral contrast enhancement as described in modified Response Evaluation Criteria in Solid Tumors (RECIST) criteria.” Local recurrence after confirmation of ablation success was defined as “evidence for viable tumor at or within 1.0 cm of a prior ablation site for which ablation success was documented—confirmed by multi-slice, multi-phase dynamic imaging.” Further, North et al. defined nonlocal hepatic recurrence as “evidence for viable intrahepatic tumor more than 1.0 cm from any prior ablation site at any time interval after ablation.” The authors of this current review defined post-ablation mortality as death within 30–90 days post-ablation that occurred due to the ablation.
Statistical methodology and risk of bias assessment
Meta-analysis was performed to obtain combined estimates across manuscripts for the following four outcomes: ablation success, local recurrence, surgical complication, and adjusted hazard of recurrence. The first three outcomes are binary, and counts/percentages were extracted from the manuscripts. Similarly, the adjusted hazard ratios (aHR) for recurrence were extracted from manuscripts that included such information. Groeschl et al. [13] did not report an aHR directly comparing laparoscopic and percutaneous procedures but including aHR comparing each relative to open surgery. An aHR for the contrast of interest was estimated from the difference of the log(aHR)s compared to open; a conservative standard error estimate was taken by assuming additive variances of the log(aHR)s. Sensitivity analyses were performed by excluding potential non-representative manuscripts and investigating any differences in the results. To account for known differences in the design and data collection across studies (difference in definition of ablation success, follow-up time for local recurrence, the choice of confounders in aHRs, etc.), a random effects model is used throughout to account for this known source of study heterogeneity; analysis using the alternative fixed effect model (results not shown) did not lead to any substantive differences in results. Statistical analysis was performed using R statistical software, version 4.1.2.
Results
Ten articles met the inclusion criteria and were included in the analysis [4, 9,10,11,12,13,14,15,16,17] (Fig. 1). These studies were largely observational and comparative cohort studies. A total of 2268 patients with liver tumors were subjects for interventions in these studies. Among them, 498 patients underwent laparoscopic ablation, while 1418 patients underwent percutaneous ablation (Table 1). Patient selection and technique are described in detail for each study. Patients were predominantly male (69%, 1861 of 2706) in the included studies. Some patients received neoadjuvant therapy before undergoing ablation, however, only 5 studies reported these data [9, 10, 13, 17, 18]. The decision to perform laparoscopic or percutaneous ablation was at the physician’s discretion based on tumor location and accessibility, tumor size, intraoperative assessment, patient comorbidities, past therapy, and patient preference. Generally, patients with tumors in more challenging locations were candidates for laparoscopic ablation, while patients that had tumors in more accessible locations were candidates for percutaneous ablation.
Tumor characteristics and ablation modalities
Most of the hepatic tumors included in the studies were classified as HCC (2460 of 3106 total tumors). The remainder of the tumors were classified as either a metastasis or some other tumor type (570 and 76, respectively) (Table 2). Tumor size and range were reported in various modes in each article, with 5 articles reporting the mean or average [9, 11, 12, 16, 18], 4 articles reporting the median [10, 13, 14, 17], and 1 article not specifying [15]. In one of the articles that reported the tumor size as a mean, the range was not reported. [9]. Seven of the 10 articles reported the tumor size for both the laparoscopic group and the percutaneous group (Table 2). Ablation modality varied between studies, with 6 examining RFA, 3 examining MWA, and 1 examining both modalities (Table 2).
Ablation success and recurrence
Ablation success was defined in 8 of 10 studies; however, these definitions varied. Two studies did not report a definition for ablation success (Table 2) [9, 11]. The percentage of laparoscopic ablation success versus percutaneous ablation success was higher in the 4 of 5 studies that reported these data (Table 2). On meta-analysis, the odds of a successful ablation were no different for laparoscopic vs percutaneous procedure (OR 1.12, 95% CI 0.60–2.09, p = 0.72) (Supplement Figure). Wong et al. was the only study that reported a higher percutaneous ablation success percentage compared to the laparoscopic ablation success percentage (84.6% vs. 82.5%, respectively) (Table 2); however, this 82.5% success rate corresponds to a combined cohort of 22 laparoscopic and 75 open surgeries, which we treated as the laparoscopic cohort. Repeating the meta-analysis excluding this manuscript (Wong et al.) provides some evidence in favor of higher success for laparoscopic technique (OR 3.12, 0.79–12.34, p = 0.11) (Fig. 2a).
Local recurrence was reported in 6 of the 10 studies and non-local hepatic recurrence was reported in 2 studies (Table 3). For each of these studies, it was found that there was a higher percentage of both local recurrence and non-local hepatic recurrence in the patients treated with percutaneous ablation versus laparoscopic ablation (Table 3). The reported percentages of the local recurrence for the laparoscopic groups were 2.8–57.9%, whereas the reported comparative percentages of the local recurrence for the percutaneous groups were 11–70.1%, respectively. Meta-analysis (Supplemental Figure) finds a lower odds of local recurrence using a laparoscopic procedure (OR 0.50, 0.28–0.92, p = 0.02); re-analysis excluding the Wong et al. [14] again supports a lower rate of recurrence for laparoscopic groups (OR = 0.36, 0.17–0.74, p < 0.01) (Fig. 2b). The authors of the sixth study that reported the local recurrence percentages only reported data for the percutaneous group (14.1%) and stated that this percentage is higher than the laparoscopic groups’ percentage [13]. As for non-local hepatic recurrence, two papers reported 36.6% [9] and 41.2% [14] for the laparoscopic groups and 49.7% and 42.6% for the percutaneous groups, respectively. Only the Zhang et al. paper reported recurrence-free survival percentages that compared the laparoscopic group with the percutaneous group. They found higher recurrence-free survival percentages at the 1-, and 3-year follow-up time periods for the laparoscopic group compared to the percutaneous group. At the 5-year follow-up period, the percutaneous group was found to have a higher percentage of recurrence-free survival (Table 3).
Postoperative complications and mortality
Postoperative complications comparing laparoscopic ablation and percutaneous ablation were reported in six studies. Five of the six studies reported a higher percentage of complications with laparoscopic ablation compared to percutaneous ablation (Table 3), and meta-analysis indicates higher odds of complications for laparoscopic procedures (OR 3.08, 1.52–6.25, p < 0.01, (Supplemental Figure) analysis without Wong et al. (2012): OR 2.19, 1.10–4.35, p = 0.03) (Fig. 2c). Postoperative mortality occurred in 3 of the 7 articles that reported it (Table 3). Ding et al. noted that two deaths occurred within 30 days of the MWA. Groeschl et al. stated that seven patients died within 30 days of the MWA. Wood et al. noted that of the patients that died, one death was directly due to the percutaneous ablation that was performed.
As most manuscripts included in this analysis are non-randomized cohort studies, the above difference may be impacted by treatment selection bias. To that end, we consider a meta-analysis for recurrence-free survival based on the three manuscripts including RFS adjusted hazard ratios (Fig. 3) as these may provide a fairer comparison that accounts for differences in treatment groups. Based on this analysis, no significant difference was found in the hazard associated with laparoscopic versus percutaneous surgery (meta-analysis adjusted HR 1.03, 95% CI 0.53–1.99, p = 0.94). Excluding the Groeschel et al. [13] manuscript that required approximating the standard error did not meaningfully change the results (adjusted HR 0.85, 0.20–3.51, p = 0.82).
Heterogeneity and risk of bias
Overall, the articles included in this review were non-randomized observational cohort studies; therefore, they received a relatively poor strength score (median 1.25 out of a possible 4.3, range 1.1–1.3). The lack of randomization, controls, and large patient cohorts were major contributors to the studies’ low strength scores.
Discussion
The present systematic review summarizes the available data on the efficacy and outcomes of laparoscopic ablation versus percutaneous ablation of various hepatic tumor types. To date, there have been no systematic reviews published that compare these two modalities of treatment for hepatic tumors. This review examines 1916 patients, of whom, approximately 26% underwent laparoscopic ablation and 74% underwent percutaneous ablation. At baseline analysis, there were some differences noted between the two groups in 5 of the 10 studies. It was reported in three studies that there was a higher number of patients with multinodular disease in the laparoscopic group versus the percutaneous group (Table 4) [9, 16, 18]. All other differences listed in Table 4 were only found in one study each. Many of these group differences were found to be statistically significant and may have contributed to the outcome of the studied ablation treatment. The reported median and mean sizes of the laparoscopic versus percutaneously treated tumors were comparable.
According to the data presented in the included studies, there was found to be a higher percentage of ablation success in laparoscopic groups compared to percutaneous groups in 4 of 10 studies [9, 10, 16, 18], with only one study reporting a higher percentage of ablation success in the percutaneous group [14]. It should be noted that in this study, the percutaneous group was being compared to a surgical cohort group that included both the laparoscopic and open approach. It is difficult to distinguish if the combining of both laparoscopic and open surgical approaches had any role in decreasing the ablation success rate.
Of note, the definition of ablation success varied widely across studies. Out of the 8 studies that reported a definition for ablation success, technique efficacy, complete ablation, or incomplete ablation, 5 mentioned post-ablation imaging (CT or MRI), 3 mentioned a post-ablation 1-month timeframe, 5 mentioned disappearance or absence of enhancement, and 4 mentioned complete destruction, necrosis, or eradication of the tumor (Table 2). North et al. states that the completeness of the initial ablation is the most important factor for ablation success and improved progression-free survival [4].
The local and non-local hepatic recurrence percentages were found to be lower in the laparoscopic group compared to the percutaneous group in every study that reported these data. Regardless of the ablation modality (MWA or RFA), laparoscopic ablation seems to lead to better recurrence outcomes for patients when compared to percutaneous ablation. Unfortunately, only one study reported comparison percentages of recurrence-free survival for the laparoscopic group versus the percutaneous group (Table 3) [11]. It is interesting to note that the laparoscopic recurrence-free survival percentages are higher for both 1- and 3-year survival; however, the 5-year laparoscopic recurrence-free survival percentage is lower when compared to the percutaneous percentage. This might suggest that percutaneous ablation may have some longer-term effects in warding off recurrence, whereas laparoscopic ablation may lead to more robust and immediate protection due to initial accessibility and visualization of the lesion. More research must be done comparing the recurrence and recurrence-free survival of these two ablation techniques to accurately and equivocally determine if there is a significant difference in patient outcome.
While laparoscopic ablation is reported to have a higher ablation success rate and fewer recurrences than percutaneous ablation, the included studies also reported a higher complication rate for the laparoscopic modality. Of the three studies reporting complications in a comparative manner, two reported a higher complication rate in the laparoscopically treated patients versus the percutaneously treated patients [9, 18]. While laparoscopic ablation for hepatic tumors has shown great promise, it may come with greater risk for complications.
Limitations of the present review include the limited sample size of patients who underwent laparoscopic ablation (n = 498) compared to percutaneous ablation (n = 1418), baseline differences between the two groups in 5 of the 10 studies, as well as different indications for ablation from center to center. Also, the heterogeneity of tumor histologies in this review may decrease the generalizability of the findings. The quality of reporting in these studies continues to be problematic to as clearly define ablation quality and optimal liver segment tumor location. Other critical limitations are based on the inherent fact that optimal liver segment locations and prior abdominal surgery play a significant role in choosing a laparoscopic or percutaneous approach. Continued reviews and discussion are critical for referring physicians to understand these metrics.
Ultimately, this systematic review portrays the need for randomized controlled trials and controlled observational studies that compare efficacy and outcomes of laparoscopic ablation and percutaneous ablation of hepatic tumors. Based on the data presented and in our opinion and current clinical exposure we believe that a laparoscopic technique should be considered for larger lesions (3–5 cm), (for ease of overlapping access), multi-focal lesions (for improved staging, i.e. identifying other lesions), segment 7 and 8 lesions, and lesions close to extra-hepatic structures. Furthermore, there must be a standardization of definitions for ablation success, local recurrence, and non-hepatic local recurrence to accurately compare and repeat studies done on these ablation modalities.
Conclusions
In conclusion, the cumulative data from this systematic review suggests that laparoscopic ablation has a higher ablation success rate and a lower recurrence rate when compared to percutaneous ablation, and similar recurrence-free survival outcomes. Further studies are warranted to examine longer-term recurrence data as well as the complication rates for both ablation techniques.
Data availability
The datasets supporting the conclusions of this article are included within the article.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- MWA:
-
Microwave ablation
- RFA:
-
Radiofrequency ablation
- AZ:
-
Ablation zone
- CT:
-
Computerized tomography
- MRI:
-
Magnetic resonance imaging
- CI:
-
Confidence interval
- HR:
-
Hazard ratio
- OR:
-
Odds ratio
- RCTs:
-
Randomized controlled trials)
References
Global Cancer Facts & Figures 4th Edition (Ebook) (2018). Atlanta, USA: American Cancer Society
Galle PR (2016) Extended abstract: management of liver cancer. Dig Dis 34(4):438–9 (PubMed PMID: 27170399. Epub 2016/05/14)
AC S. What Is Liver Cancer? 2019; Available from: https://www.cancer.org/cancer/liver-cancer/about/what-is-liver-cancer.html. Accessed June 2021
North DA, Groeschl RT, Sindram D et al (2014) Microwave ablation for hepatic malignancies: a call for standard reporting and outcomes. Am J Surg 208(2):284–94 (PubMed PMID: 24970652. Epub 2014/06/28)
Frezza EE (2004) Therapeutic management algorithm in cirrhotic and noncirrhotic patients in primary or secondary liver masses. Dig Dis Sci 49(5):866–71 (PubMed PMID: 15259511. Epub 2004/07/21)
Hinshaw JL, Lubner MG, Ziemlewicz TJ et al (2014) Percutaneous tumor ablation tools: microwave, radiofrequency, or cryoablation–what should you use and why? Radiographics 34(5):1344–62 (PubMed PMID: 25208284. PMCID: PMC4319523. Epub 2014/09/11)
Wright AS, Sampson LA, Warner TF et al (2005) Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology 236(1):132–9 (PubMed PMID: 15987969. Epub 2005/07/01)
Andreano A, Brace CL (2013) A comparison of direct heating during radiofrequency and microwave ablation in ex vivo liver. Cardiovasc Intervent Radiol 36(2):505–11 (PubMed PMID: 22572764. Pubmed Central PMCID: PMC3437379. Epub 2012/05/11)
Eun HS, Lee BS, Kwon IS et al (2017) Advantages of laparoscopic radiofrequency ablation over percutaneous radiofrequency ablation in hepatocellular carcinoma. Dig Dis Sci 62(9):2586–600 (PubMed PMID: 28744835. Epub 2017/07/27)
De Cobelli F, Marra P, Ratti F et al (2017) Microwave ablation of liver malignancies: comparison of effects and early outcomes of percutaneous and intraoperative approaches with different liver conditions : new advances in interventional oncology: state of the art. Med Oncol 34(4):49 (PubMed PMID: 28220346. Epub 2017/02/22)
Zhang W, Jiang L, Yan L et al (2016) Radiofrequency ablation for HCC patients with multifocal tumours meeting the Milan criteria: a single-centre experience. Dig Liver Dis 48(12):1485–91 (PubMed PMID: 27495779. Epub 2016/08/09)
Ding J, Jing X, Wang Y et al (2016) Thermal ablation for hepatocellular carcinoma: a large-scale analysis of long-term outcome and prognostic factors. Clin Radiol 71(12):1270–6 (PubMed PMID: 27510559. Epub 2016/08/12)
Groeschl RT, Pilgrim CH, Hanna EM et al (2014) Microwave ablation for hepatic malignancies: a multiinstitutional analysis. Ann Surg 259(6):1195–200 (PubMed PMID: 24096760. Epub 2013/10/08)
Wong J, Lee KF, Yu SC et al (2013) Percutaneous radiofrequency ablation versus surgical radiofrequency ablation for malignant liver tumours: the long-term results. HPB (Oxford) 15(8):595–601 (PubMed PMID: 23458320. Pubmed Central PMCID: PMC3731580. Epub 2013/03/06)
Hirooka M, Kisaka Y, Uehara T et al (2009) Efficacy of laparoscopic radiofrequency ablation for hepatocellular carcinoma compared to percutaneous radiofrequency ablation with artificial ascites. Dig Endosc 21(2):82–6 (PubMed PMID: 19691779. Epub 2009/08/21)
Yokoyama T, Egami K, Miyamoto M et al (2003) Percutaneous and laparoscopic approaches of radiofrequency ablation treatment for liver cancer. J Hepatobiliary Pancreat Surg 10(6):425–7 (PubMed PMID: 14714162. Epub 2004/01/10)
Wood TF, Rose DM, Chung M et al (2000) Radiofrequency ablation of 231 unresectable hepatic tumors: indications, limitations, and complications. Ann Surg Oncol 7(8):593–600 (PubMed PMID: 11005558. Epub 2000/09/27)
Della Corte A, Ratti F, Monfardini L et al (2020) Comparison between percutaneous and laparoscopic microwave ablation of hepatocellular carcinoma. Int J Hyperthermia 37(1):542–8 (PubMed PMID: 32469252. Epub 2020/05/30)
Funding
The authors have no financial support to declare.
Author information
Authors and Affiliations
Contributions
RM designed the research process. JM searched the database for corresponding articles. JM extracted useful information from the articles above. JG performed the meta-analysis. JM drafted the systematic review. All authors had read and approved the manuscript and ensured that this was the case.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study did not require ethical approval since it was a review of published articles and did not directly involve the use of human or animal subjects.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10147_2023_2304_MOESM1_ESM.tiff
Supplementary file1 Figure 1 Forest plot with meta-analysis with all articles included of (a) ablation success (b) local recurrence, and (c) complications (TIFF 6328 KB)
About this article
Cite this article
Musick, J.R., Gaskins, J.T. & Martin, R.C.G. A meta-analysis and systematic review of the comparison of laparoscopic ablation to percutaneous ablation for hepatic malignancies. Int J Clin Oncol 28, 565–575 (2023). https://doi.org/10.1007/s10147-023-02304-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-023-02304-2