Abstract
Purpose
To determine whether patients can avoid systematic prostate biopsy (PBx) if their Prostate Imaging Reporting and Data System version 2 (PI-RADs v2) score is ≤ 3 and how we clinicians make decisions that can maximize benefit.
Materials and methods
We reviewed our prospectively maintained database of consecutive men who received transrectal ultrasound-guided 24-core biopsy as well as pre-biopsy multi-parametric magnetic resonance imaging (mp-MRI). Of the 1276 men who were performed PBx in our institution from 2012 to July 2018, 491 patients conformed to the criteria. Negative predictive value (NPV) of negative mp-MRI (defined as PI-RADs < 3) combined prostate-specific antigen density (PSAD) were calculated. Models based on PI-RADs v2 were developed to predict the absence of clinically significant prostate cancer (CSPCa) and prostate cancer (PCa). Nomograms as well as receiver operating curves (ROC) were established to estimate the discrimination. Calibration curves were used to assess the concordance between predictive value and true risk. Decision curves were made to measure the overall net benefit.
Results
Prostate cancer and CSPCa detection rates were 21.6%, 7.3% and 36.7%, 23.4% in PIRADs v2 < 3 cohort and PIRADs v2 = 3 cohort, respectively. Men with biopsy-proved CSPCa had higher prostate-specific antigen (PSA), lower prostate volume (PV) and higher PSAD (all p < 0.05 in the two cohorts) than patients with clinically insignificant prostate cancer (CIPCa) or negative results. NPV of negative mp-MRI for detection of PCa was much higher when the PSAD was less than 0.15 (p < 0.001) and 0.2 for CSPCa (p = 0.007). According to multivariate analysis, we developed the model comprising Age, PSAD and PI-RADs v2 to predict the absence of CSPCa and PCa. The area under the curve (AUC) of the model for non-CSPCa was 0.75 (95% CI 0.68–0.80, PSAD cutoff 0.20), better than 0.71 (95% CI 0.65–0.80, PSAD cutoff 0.15). As for model for non-PCa, the AUC was 0.76 (95% CI 0.70–0.80, PSAD cutoff 0.15), higher than 0.71(95% CI 0.67–0.78, PSAD cutoff 0.20). Internally validated calibration curves showed that the model might overestimated the risk of the absence of CSPCa when the threshold was between 53 and 72%, and if the threshold was between 72 and 87%, it might underestimate the risk. As for the absence of PCa, the model might overestimate the risk between 52 and 76%. Decision curves showed that a better clinical net benefit was met when the threshold was 55% for non-PCa and 70% for non-CSPCa.
Conclusions
NPV of negative mp-MRI for detection of CSPCa and PCa was improved with decreasing PSAD. The nomograms based on PI-RADs v2, age and PSAD showed internally validated high discrimination and calibration for the absence of PCa and CSPCa. When the predictive value was greater than 70% for the absence of CSPCa and 55% for the absence of PCa, we could avoid unnecessary PBx to maximize net benefit.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Although prostate cancer (PCa) screening based on elevated prostate-specific antigen (PSA) reduces the incidence of advanced disease, trade-offs include overdiagnosis and resultant overtreatment. It has been a dominant concern how clinicians can differentiate the indolent tumor from clinically significant prostate cancer (CSPCa) [1].
Multi-parametric magnetic resonance imaging (mp-MRI) is recommended to be performed before prostate biopsy (PBx) due to its priority in visualization of prostate, high soft-tissue contrast, high resolution, and simultaneous image functional parameters [2]. Prostate Imaging Reporting and Data System version 1 (PI-RADs v1) was developed by European Society of Urogenital Radiology (ESUR) to set standardized reporting and propose criteria for interpreting data of mp-MRI in 2012 and modified in December 2014, which was known as PI-RADs v2 [3]. It defined a five-point assessment scale indicating the likelihood that mpMRI findings correlate with the presence of CSPCa at a particular anatomic location. And previous studies have validated the diagnostic performance of positive mp-MRI in detection of CSPCa [4, 5]. However, little clinical studies concern the negative predictive value (NPV) of negative mp-MRI (defined as PI-RADs v2 < 3) and whether it could predict the absence of CSPCa or PCa.
In our study, we aimed to investigate how to make clinical decisions when the PI-RADs v2 was ≤ 3. We also explored whether the men with negative mpMRI could safely avoid PBx. Besides, seeing that there have been several studies clarifying the diagnostic performance of PIRADs v2 in detection of CSPCa [6, 7], we developed a model based on PI-RADs v2 to help predict the absence of CSPCa and PCa and maximize the clinical net benefit.
Materials and methods
Data source and study population
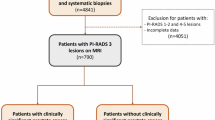
From our prospectively maintained institutional review board-approved PBx database, we identified 1276 consecutive men who underwent PBx at our institution (2012 to July 2018). Men who were biopsy naïve and had PIRADs v2 ≤ 3 as well as PSA < 25 ng/ml met the criteria. The exclusive criteria were as follows: (1) patients whose mp-MRI could not be assessed by PIRADs v2; (2) men who received surgical treatment for benign prostatic hypertrophy. After that, 491 eligible men composed the cohort, including 273 patients with negative mp-MRI (defined as PIRADs v2 < 3) and 218 patients whose PIRADs v2 = 3.
MRI protocol
As recommended [3], the prostate mpMRI was performed at a 3-Tesla (T) system. The acquisition protocol consisted of T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI) with apparent diffusion coefficient map (ADC), and dynamic contrast-enhanced (DCE) sequences and calculated b value of 1000 or above. Each sequence used a five-point assessment scale (except for DCE) which graded the level of suspicion for the presence of PCa from 1 to 5. A urologic clinician who was experienced with PI-RADs v2 and blinded to histopathology as well as clinical data performed scoring newly replacing the scores on the image report to eliminate interobserver heterogenicity.
Biopsy and histopathological analysis
Transrectal ultrasound-guided systematic prostate biopsy of 24 needle cores was performed in our institution, which conformed to the concept of saturation biopsy. Compared to magnetic resonance imaging-targeted biopsy, it showed no difference in overall PCa detection but a higher rate of clinically insignificant prostate cancer (CIPCa) and lower rate of CSPCa [8, 9]. There were 20 needles in the peripheral zone from apex and mid to posterior base in case of missing the CSPCa in the anterior part of the prostate [10] and four needles in the transitional zone. A uropathologist with more than 20 years in urological pathology revised the histopathology results and assigned Gleason scores (GS). According to Epstein criteria [11], the CSPCa was defined as GS ≥ 7, or > 3 biopsy cores positive, or at least one biopsy core with > 50% involvement. CIPCa was defined as GS < 7 without Gleason pattern 4 or 5, less than 3 core samples, and no core sample > 50% involved. We did not use the updated Epstein criteria [12] to define the CSPCa or CIPCa without the combination of PSAD.
Statistical analysis
The demographic data of all the patients involved were shown in three subgroups: CSPCa, CIPCa and Benign. To present the data better, we separate them into PI-RADs v2 < 3 cohort and PI-RADs v2 = 3 cohort. NPV with 95% confidence interval (CI) was calculated for predictive accuracy of negative mp-MRI combined with PSAD in contrast to histological findings. Analysis of variance and chi-square test were used to determine significant differences in baseline characteristics. Univariate and multivariate logistic regression was applied to explore the relationship between variables and results (absence of PCa and absence of CSPCa). Multivariate logistic regression model was constructed using statistically significant variables on multivariate analysis. Nomograms as well as receiver operating curves (ROC) were established to assess the discrimination. Calibration curves were performed to assess the extent of over- or underestimation of the models. Decision curves and clinical impact curves were made to determine the clinical net benefit derived from the use of the model. p < 0.05 was considered to indicate a statistical significance. All analyses were performed with SPSS software (Version 25.0. IBM), and R version 3.5.1.
Results
From 1276 consecutive men undergoing PBx, there were 966 patients whose mp-MRI could be graded by PI-RADs v2. We excluded those who had been performed biopsy previously (n = 59), whose PI-RADs v2 score was > 3 (n = 399) and who had been given transurethral resection of prostate (n = 17). Of the all 491 men involved, 273 patients had negative mp-MRI and 218 patients had the PI-RADs v2 score category 3. Tables 1 and 2 summarize the demographics. Detection rate of PCa and CSPCa in the PI-RADs v2 < 3 cohort was 21.6% (59/273) and 7.3% (20/273), and 37.2% (81/218) and 23.4% (51/218) in the PI-RADs v2 = 3 cohort. The rate of diagnosis of CIPCa was 14.3% (39/273) and 13.3 (29/218), respectively. A statistical significance was met in the three subgroups (CSPCa, CIPCa and benign) when it came to PSA, PV (prostate volume), PSAD, positive cores per patient and cancer core percent (all p < 0.05). CSPCa patients had higher PSA and PSAD, more positive cores per patient and greater cancer core percent, but lower PV compared to CIPCa or benign men. NPV of mp-MRI for detection of CSPCa and PCa was improved with decreasing PSAD.
The NPV was much higher when the cutoff of PSAD was 0.15 for PCa (p < 0.001) and 0.20 for CSPCa (p = 0.007, Table 3).
Of 140 patients diagnosed with PCa on PBx, 38 underwent radical prostatectomy (RP). 14 patients were diagnosed as CIPCa according to biopsy outcomes. And 7 of them were confirmed as CSPCa by radical prostatectomy specimens, due to upgrading of GS. Two CSPCa people had the GS < 7 after radical prostatectomy, but they remained clinically significant because of high tumor volume. Biopsy and RP histology were concordant in 82% of the cases (Table 4).
Univariate analysis revealed that age, PSA, PV, PSAD and PI-RADs v2 were the predictors of the absence of CSPCa and PCa. Multivariate analysis confirmed that age, PSAD and PI-RADs v2 were independent predictors of the absence of CSPCa as well as the absence of PCa (all p < 0.05,). PV was associated with predicting non-PCa (p < 0.001) but not non-CSPCa (p = 0.84, Table 5). ROC analysis showed that the model based on age, PSAD and PI-RADs v2 had a larger area under the curve (AUC) in comparison to other parameters. And the AUC of the model in predicting non-PCa was larger when the threshold of PSAD was 0.15 (0.76, 95% CI 0.70–0.80, Fig. 1). However, a larger AUC was met if the cutoff of PSAD was 0.20 when predicting non-CSPCa (0.75, 95% CI 0.68–0.80, Fig. 1).
ROC curves of the two models. a Model for predicting the absence of CSPCa. b Model for predicting the absence of PCa. Model 1 takes PSAD = 0.15 as cutoff, Model 2 takes PSAD = 0.20 as cutoff. As shown in the figures, Model 2 has a higher AUC than Model 1 when predicting non-CSPCa. However, Model 1 was better when predicting non-PCa compared to Model 2
Nomograms predicting the absence of CSPCa and PCa were developed based on the three parameters (Fig. 2). The internally validated c-index of the two nomograms were 0.77 and 0.75. Besides AUC and c-index, calibration is an important indicator of diagnostic performance that assesses the agreement between the predicted risk and actual detection rate for each prediction model [13]. Non-CSPCa and non-PCa estimates were well correlated with observed outcome (Fig. 3). Internally validated calibration curves showed that the nomogram might overestimate the risk of the absence of CSPCa when the predicted probability was between 53 and 72%, and if the predicted probability was between 72 and 87%, it might underestimate the risk. As for the absence of PCa, the model might overestimate the risk between 52 and 76% and accord with the actual probability msore suitably at the range above 76% (Fig. 3).
a Nomogram predicting the absence of CSPCa. (B) Nomogram predicting the absence of PCa. PSAD values were grouped by 1-PSAD < 0.15, 2–0.15 ≤ PSAD < 0.20, 3–0.30 > PSAD ≥ 0.2, 4-PSAD ≥ 0.30. PI-RADs (−) means PI-RADs v2 < 3 and PI-RADs (+) means PI-RADs v2 = 3. As shown, a man aged 80 with PSAD < 0.15 and PI-RADs v2 < 3 has the probability of 87% for non-CSPCa and 60% for non-PCa. PSAD prostate-specific antigen density
Calibration curves of the nomograms. a Calibration curve of the nomogram for the absence of CSPCa. b Calibration curve of the nomogram for the absence of PCa. As shown by the curves, the nomogram for predicting non-CSPCa might overestimate the risk at the probability range 53–72% and underestimate the risk at the range 72–87%. And the nomogram predicting non-PCa might overestimate the risk between 52 and 76%
The net benefit in the decision curve (DCA) was higher when the threshold was 55% for non-PCa and 70% for non-CSPCa (Fig. 4). And we could avoid unnecessary biopsy and maximize the clinical benefit when the predicted probability was higher than that threshold. The clinical impact curves were drawn to better show the number of estimated high risk and actual risk if we applied the model to predict non-CSPCa and PCa in one thousand men (Fig. 5).
Decision curves of the nomograms. a Clinical net benefit of predicting the absence of CSPCa was met when the threshold was greater than 70%. b Clinical net benefit of predicting the absence of PCa was met when the threshold was greater than 55%. All for “treat all” means that all the patients received no biopsy. And none for “treat none” means that all patients received PBx
Clinical impact curves based on decision curves. a Clinical impact curve of the nomogram applied to predict the absence of CSPCa in one thousand men. b Clinical impact curve of the nomogram applied to predicting the absence of PCa in one thousand men. Pale lines represent the 95% confidence interval
Discussion
Multivariable models have been developed and validated for application in the primary screening setting of PCa or CSPCa [14, 15]. And several studies confirmed the use of mp-MRI before PBx and the value of PI-RADs v2 in detection of CSPCa [6, 16, 17]. They focused on the diagnostic performance of mp-MRI. However, in this single institution study, we mainly concentrated on predicting the absence of CSPCa as well as PCa and maximizing the clinical net benefit by avoiding unnecessary PBx. From 1276 consecutive patients in our database, we identified 491 men including 273 patients with PI-RADs v2 < 3 and 218 patients with PI-RADs v2 = 3. The NPV of CSPCa and PCa in negative mp-MRI cohort was 93% and 78%. And the NPV was improved with decreasing PSAD, which meant that a negative mp-MRI result combining a low PSAD implied a high probability of non-CSPCa or non-PCa. The nomograms predicting the absence of CSPCa and the absence of PCa were shown to have high discrimination and good calibration. Decision curves indicated that the over-diagnosis of PCa as well as CSPCa could be avoided when the threshold probability was above 55% for non-PCa and 70% for non-CSPCa.
The definitions of CIPCa and CSPCa in this study were based on pathological assessment of biopsy specimens and were in accordance with Epstein criteria other than justified by GS < 7 or not. Compared to other similar studies [18, 19] that defined the CSPCa as GS≥ 7, we might upgrade the men with GS < 7 to CSPCa due to at least one biopsy core with > 50% involvement. And we brought biopsy-naïve patients into consideration, differing from them which contained biopsy-naïve people, previous negative biopsy people and active surveillance patients. Therefore, the NPV of negative mp-MRI for CSPCa in current study was higher than theirs (93% vs 83% and 87%) [18, 19]. Systematic extended-sextant 24-core saturation biopsies were performed in our zinstitution and no MRI-targeted or fusion biopsies were given for the cases with PI-RADS score ≤ 3. And this might lead to more detection of CIPCa [20, 21].
How to manage men with negative MRI enjoyed a wide range of concerns among clinicians. As mentioned previously, several studies [18, 19, 22] have tried to verify whether negative mp-MRI can conduct non-essential PBx or not. But the results were not all the same. Washino et al. [23] concluded that patients with a PI-RADS v2 score of ≤ 3 and PSA density of < 0.15 ng/mL/mL might avoid unnecessary biopsies. However, Oishi et al. [18] drew a conclusion that men with negative mpMRI, PSAD < 0.15 ng/mL/mL and prior negative biopsy might safely avoid re-biopsy. Conversely, PBx should be considered in biopsy-naïve men, regardless of negative mpMRI, particularly those with PSAD > 0.15 ng/mL/mL. In our research, the NPV was much higher when the cutoff of PSAD was 0.15 for PCa and 0.20 for CSPCa, similar to their conclusions that PSAD < 0.15 was a safe range for non-CSPCa. However, the false-negative rate for a negative mp-MRI in our institution should not be neglected. If we chose to not perform PBx when the PI-RADs v2 was < 3, we would have missed 22% (59/273) PCa patients and 7% (20/273) CSPCa patients.
To validate whether we could give up PBx when the PI-RADS v2 was ≤ 3, we developed models comprising PI-RADs v2, age and PSAD. The ROC curves showed a larger AUC predicting the absence of CSPCa when the cutoff of PSAD was 0.2 and a larger AUC predicting the absence of PCa when the cutoff of PSAD was 0.15. The findings conformed to the NPV calculated by negative mp-MRI in combination with PSAD.
Nomograms were also established to predict the probability of non-CSPCa and non-PCa directly. To our best knowledge, there was little literature reporting the structure of nomograms in predicting the absence of CSPCa. Distinct facets of our nomogram deserve attention. First, it was developed to estimate the possibility of non-tumor based on PI-RADs v2 and PSAD. Distler et al. [24] also generated a nomogram comprising PI-RADs v2 and PSAD. However, it was used to predict the risk of CSPCa. In their analyses, patients with PSAD values were stratified to 3 PSAD groups (PSAD-G), including group 1—less than 0.07, group 2—0.07 to 0.15 and group 3—greater than 0.15 ng/ml/ml. The authors concluded that combining PSAD-G with mp-MRI information can help identify patients at very low risk for harboring significant and high-grade PCa. The distinctions were that we stratified PSAD to groups less than 0.15, 0.15 to 0.20, 0.20 to 0.30 and greater than 0.30. And we did not include the patients with previous negative biopsy. Second, we could predict not only the probability of non-CSPCa, but also the possibility of non-PCa. Combing the two consequences, the risk of the men suffering from CIPCa could be assessed. As shown in Fig. 2, the men (80 years old, PI-RADs v2 = 3, PSAD < 0.15) had 87% probability of non-CSPCa and 60% of benign. The chance he suffered from CIPCa was just a little more than 20%. But if the likelihood of non-PCa was less than 40%, the probability of CIPCa might be more than 50%, which meant the patient had to undergo active surveillance or active follow-up even if he avoided PBx. Third, we contained only PI-RADs v2 ≤ 3 in the nomograms, because it was highly likely to be PCa or CSPCa if the PI-RADs v2 score was greater than 3. We should still perform PBx in view of its high sensitivity and positive predictive value if PI-RADs v2 was > 3, though it did have benign or CIPCa in the cohort PI-RADs v2 > 3 on the basis of other studies [16, 22,23,24].
From a clinical standpoint, our nomograms improve our ability to identify patients at a high probability of non-CSPCa or non-PCa, and its implementation in the clinical practice would reduce the overdiagnosis and resultant overtreatment. In consideration of its high discrimination, the extent of over- or underestimation of the histologically confirmed versus nomogram-predicted probability was graphically explored using a calibration plot (internally validated). The calibration plot of predicted probabilities against observed non-tumor rates indicated excellent concordance and showed that the nomogram might overestimate the risk of non-CSPCa (predicted probability 53–72%) as well as non-PCa (52–76%). That warned us if the calculated probability was just little greater than 50%, the actual risk of non-tumor might be under 50% due to the overestimation. And whether to perform PBx or not needs further assessment. As seen in Fig. 3, the deviation from actual probability was a little greater in predicting the absence of PCa than the absence of CSPCa.
The use of an arbitrary cutoff to select patients who could avoid biopsy implies the acceptance of the possibility of overdiagnosis in a certain proportion of individuals below that cutoff. And decision curves were made to solve the concern. The DCA demonstrated that the novel nomogram improved clinical risk prediction against threshold probabilities of 55% for non-PCa and 70% for non-CSPCa. That implied we could safely avoid performing PBx and maximize net benefit if the predictive value was greater than the threshold.
As Gandaglia et al. [25] stated, the problem is not what to do with indolent prostate cancer—the problem is how to avoid finding these cancers. Can negative mp-MRI give us the reassurance we need to avoid PBx? Panebianco et al. [26] assess the outcomes of men with negative MRI and clinical follow-up after 7 years of activity at a reference center. After 48 months of follow-up, any-grade PCa diagnosis-free survival probability was 84% in group A (naïve patients, n = 659) and 96% in group B (previous negative biopsy, n = 596). They recommended that systematic biopsy cannot be routinely omitted after negative MRI, especially in patients with strong clinical suspicion of PCa (rising PSAD), given the possibility of missing clinically significant, MRI-silent tumors. What is noteworthy, there were still 59 (22%) PCa patients and 20 (7%) CSPCa men in our PI-RADs v2 < 3 cohort. Based on our study, we suggest that we clinicians combine PI-RADs v2, PSAD as well as age to decide whether to perform PBx and distinctly find the tumor which is histologically different from the PCa detected by mp-MRI [27].
There are still some problems that need to be further investigated. First, the concordance between biopsy-proven pathological outcomes and RP specimens needs to be assessed. According to our current study, biopsy and radical prostatectomy histology were concordant in 82% of the patients with PI-RADs v2 ≤ 3. Second, PI-RADs v2 = 3 has no obvious indication for PCa or non-PCa, which is called MRI equivocal. The decisions we make for MRI-equivocal patients should be further studied. Last, the histological differences and even prognostic differences between mp-MRI detected and mp-MRI missed prostate cancers need to be further discussed.
This study has several limitations. First, it was based on the data acquired in a single institution and the consequences need external validation. Besides, the NPV was calculated according to biopsy specimens but not radical prostatectomy specimens. As Le et al. reported [28], the overall mp-MRI sensitivity for tumor detection which was confirmed by whole-mount histopathology was only 47% (132/283). This might result in a higher NPV in our research as well as a higher diagnostic performance of the nomograms for non-CSPCa and non-PCa. Finally, only 38 patients out of PI-RADs ≤ 3 received RP, and 7 were upgraded to CSPCa after RP, which means that 18%(7/38) of all the RP-operated and 50%(7/14) of the biopsy-proven CIPCa were not in accordance with the biopsy pathology. However, in the current study, we did not concentrate on the differences.
Conclusions
NPV of negative mp-MRI for detection of CSPCa and PCa was improved with decreasing PSAD. The nomograms based on PI-RADs v2, age and PSAD showed internally validated high discrimination and calibration for the absence of PCa and the absence of CSPCa. When the predictive value was greater than 70% for non-CSPCa and 55% for non-PCa, we could avoid unnecessary PBx to maximize net benefit. The concordance between biopsy-proven pathological outcomes and RP specimens needs to be further investigated.
References
Loeb S, Bjurlin MA, Nicholson J et al (2014) Overdiagnosis and overtreatment of prostate cancer. Eur Urol 65(6):1046–1055
Meng X, Rosenkrantz AB, Mendhiratta N et al (2016) Relationship between prebiopsy multiparametric magnetic resonance imaging (MRI), biopsy indication, and MRI-ultrasound fusion–targeted prostate biopsy outcomes. Eur Urol 69(3):512–517
Barentsz JO, Weinreb JC, Verma S et al (2016) Synopsis of the PI-RADS v2 guidelines for multiparametric prostate magnetic resonance imaging and recommendations for use. Eur Urol 69(1):41–49
Porpiglia F, Manfredi M, Mele F et al (2017) Diagnostic pathway with multiparametric magnetic resonance imaging versus standard pathway: results from a randomized prospective study in biopsy-naïve patients with suspected prostate cancer. Eur Urol 72(2):282–288
Ahmed HU, El-Shater Bosaily A, Brown LC et al (2017) Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 389(10071):815–822
Zhang Y, Zeng N, Zhu YC et al. (2018) Development and internal validation of PI-RADs v2-based model for clinically significant prostate cancer. World J Surg Oncol 16(1):102
Woo S, Suh CH, Kim SY et al. (2017) Diagnostic performance of prostate imaging reporting and data system version 2 for detection of prostate cancer: a systematic review and diagnostic meta-analysis. Eur Urol 72(2):177–188
Schoots IG, Roobol MJ, Nieboer D et al. (2015) Magnetic resonance imaging–targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol 68(3):438–450
Radtke JP, Kuru TH, Boxler S et al (2015) Comparative analysis of transperineal template saturation prostate biopsy versus magnetic resonance imaging targeted biopsy with magnetic resonance imaging-ultrasound fusion guidance. J Urol 193(1):87–94
Schouten MG, van der Leest M, Pokorny M et al (2017) Why and where do we miss significant prostate cancer with multi-parametric magnetic resonance imaging followed by magnetic resonance-guided and transrectal ultrasound-guided biopsy in biopsy-naïve men? Eur Urol 71(6):896–903
Ploussard G, Epstein JI, Montironi R et al (2011) The contemporary concept of significant versus insignificant prostate cancer. Eur Urol 60(2):291–303
Jeldres C, Suardi N, Walz J et al (2008) Validation of the contemporary Epstein criteria for insignificant prostate cancer in European men. Eur Urol 54(6):1306–1313
Chen R, Xie L, Xue W et al (2016) Development and external multicenter validation of Chinese Prostate Cancer Consortium prostate cancer risk calculator for initial prostate biopsy. Urol Oncol 34(9):416 e411–417 e411
Hansen J, Auprich M, Ahyai SA et al (2013) Initial prostate biopsy: development and internal validation of a biopsy-specific nomogram based on the prostate cancer antigen 3 assay. Eur Urol 63(2):201–209
Roobol MJ, van Vugt HA, Loeb S et al (2012) Prediction of prostate cancer risk: the role of prostate volume and digital rectal examination in the ERSPC risk calculators. Eur Urol 61(3):577–583
Hofbauer SL, Maxeiner A, Kittner B et al (2018) Validation of prostate imaging reporting and data system version 2 for the detection of prostate cancer. J Urol 200:767–773
Fütterer JJ, Briganti A, De Visschere P et al (2015) Can clinically significant prostate cancer be detected with multiparametric magnetic resonance imaging? A systematic review of the literature. Eur Urol 68(6):1045–1053
Oishi M, Shin T, Ohe C et al (2018) Which patients with negative magnetic resonance imaging can safely avoid biopsy for prostate cancer? J Urol 201:268–277
Wang RS, Kim EH, Vetter JM et al (2017) Determination of the role of negative magnetic resonance imaging of the prostate in clinical practice: is biopsy still necessary? Urology 102:190–197
Kasivisvanathan V, Rannikko AS, Borghi M et al (2018) MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 378(19):1767–1777
Kasivisvanathan V, Dufour R, Moore CM et al (2013) Transperineal magnetic resonance image targeted prostate biopsy versus transperineal template prostate biopsy in the detection of clinically significant prostate cancer. J Urol 189(3):860–866
Perlis N, Al-Kasab T, Ahmad A et al (2018) Defining a cohort that may not require repeat prostate biopsy based on PCA3 score and magnetic resonance imaging: the dual negative effect. J Urol 199(5):1182–1187
Washino S, Okochi T, Saito K et al (2017) Combination of prostate imaging reporting and data system (PI-RADS) score and prostate-specific antigen (PSA) density predicts biopsy outcome in prostate biopsy naive patients. BJU Int 119(2):225–233
Distler FA, Radtke JP, Bonekamp D et al (2017) The value of PSA density in combination with PI-RADS for the accuracy of prostate cancer prediction. J Urol 198(3):575–582
Gandaglia G, Briganti A, Fossati N et al (2016) The problem is not what to do with indolent and harmless prostate cancer-the problem is how to avoid finding these cancers. Eur Urol 70(4):547–548
Panebianco V, Barchetti G, Simone G et al (2018) Negative multiparametric magnetic resonance imaging for prostate cancer: what's next? Eur Urol 74(1):48–54
Rosenkrantz AB, Mendrinos S, Babb JS et al (2012) Prostate cancer foci detected on multiparametric magnetic resonance imaging are histologically distinct from those not detected. J Urol 187(6):2032–2038
Le JD, Tan N, Shkolyar E et al (2015) Multifocality and prostate cancer detection by multiparametric magnetic resonance imaging: correlation with whole-mount histopathology. Eur Urol 67(3):569–576
Funding
No funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors; and it receives ethics approval from Capital Medical University affiliated Beijing Friendship Hospital Ethics Committee.
Informed consent
No informed consent was obtained because we applied for exception to informed consent from our ethics committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Zhang, Y., Zeng, N., Zhang, F. et al. How to make clinical decisions to avoid unnecessary prostate screening in biopsy-naïve men with PI-RADs v2 score ≤ 3?. Int J Clin Oncol 25, 175–186 (2020). https://doi.org/10.1007/s10147-019-01524-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-019-01524-9