Abstract
Background
To evaluate the expression of programmed cell death-ligand 1 (PD-L1) and CD8 in high-grade endometrial carcinomas and relate it to several clinicopathological parameters.
Methods
One hundred and one (101) patients with high-grade endometrial carcinomas who were completely surgically staged were included in this study. PD-L1 and CD8 + expression was evaluated by immunohistochemistry.
Results
In our cohort, 47 women (46.5%) had endometrioid carcinomas and 54 patients (53.5%) were diagnosed with non-endometrioid cancers. In endometrioid carcinomas, there was a significantly higher rate of positivity for PD-L1 expression (p = 0.042) and of intraepithelial CD8 + cell counts (p = 0.004) as opposed to non-endometrioid cancers. There were no significant relationships with any of the other clinicopathological features under study. Univariate and multivariate analysis revealed that only high intraepithelial CD8 + counts (p = 0.01) was associated with longer progression-free survival. Tumors positive for PD-L1 and high intraepithelial CD8 expression were mainly of endometrioid histology, whilst PD-L1-positive/CD8 low and PD-L1-negative/CD8 low tumors were mostly non-endometrioid carcinomas (p = 0.01). PD-L1 negative/CD8 high tumors had the longest progression-free survival (p = 0.032).
Conclusions
In grade 3 endometrial carcinomas, both of endometrioid and non-endometrioid type, high intraepithelial CD8 + counts represent an independent favorable prognostic factor and when related to PD-L1-negative tumors, a longer progression-free survival can be predicted. Immunotherapy could probably be considered for PD-L1-positive/CD8 + high tumors, which were mostly of endometrioid histology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endometrial cancer is the most common gynecological malignancy in western countries. It is estimated that around 11,000 deaths will be attributed to this disease in 2018 in the United States [1]. Due to early clinical signs, such as postmenopausal or prolonged menstrual hemorrhage, endometrial cancer is often diagnosed in early stage and in that case is treated with curable intent. On the other hand, the prognosis of patients with recurrent or metastatic disease remains dismal despite combined treatment modalities [2].
Elucidation of the molecular aspects of endometrial carcinogenesis has led to the establishment of two clinicopathological disease types that emerge through different pathogenetic mechanisms and are consequently characterized by distinct molecular profiles. Type I endometrial carcinoma, which is the most frequent and mostly of endometrioid histology, has a relatively indolent biological behavior. Type II endometrial cancer, on the other hand, comprises mainly serous or clear-cell sub-types and has a more aggressive clinical course [3]. This dualistic model proposed by Bokhman in 1983 [3] has served greatly in the understanding of endometrial cancer carcinogenesis and in the establishment of treatment protocols [4]. However, some questions arose regarding the existence of some carcinomas with ambiguous or overlapping pathological features that could not be classified in any of the existing groups or the different clinical course of cancers that phenomenally belonged to the same category [5]. Recently, some positive steps towards informative classification and risk stratification of endometrial carcinomas have emerged. Specifically, in 2013, the Cancer Genome Atlas (TCGA) research project presented a new genomic classification of endometrial cancer, dividing the disease in 4 subgroups, using a combination of whole genome sequencing, exome sequencing, microsatellite instability assays and copy number analysis. Between other findings, two of the above sub-types, the polymerase epsilon (POLE) ultra-mutated and the microsatellite instability-hyper-mutated (MSI-H), are characterized by a high mutational load providing the rationale for a potential activity of immunotherapy [5, 6].
It is accepted that the immune system has a key role in carcinogenesis, specifically in the arrest of tumor development. Increased concentration of tumor-infiltrating lymphocytes is associated with a better prognosis in a wide variety of cancers, including endometrial carcinoma [7,8,9]. Intra-tumoral cytotoxic (CD8 +) lymphocytes have been described in up to 95% of endometrial tumors [10, 11]. T-lymphocytes can attack tumor cells via recognition of antigens associated with the tumor and are presented by antigen-presenting cells. However, several different ways have been discovered through which tumor cells can escape the immune response [12]. One primary mechanism occurs within the tumor microenvironment, through the programmed cell death-1 (PD-1) receptor/programmed death-ligand 1 (PD-L1) pathway which represents an adaptive immune-resistance mechanism that is exerted by tumor cells in response to endogenous immune anti-tumor activity [13]. It has been reported that PD-L1 is expressed in 20.0–83.0% of endometrial carcinomas [14,15,16,17]. In general, endometrial carcinomas with tumor PD-L1 expression tend to have increased infiltration by CD8 + T-lymphocytes [18, 19] and these cancers usually belong to the POLE-ultra-mutated and MSI-H molecular subgroup [5, 14]. Despite findings in other malignancies, in endometrial cancer, the association between PD-L1 expression and clinicopathological features as well as its prognostic significance remains under debate. High-grade histology and lymphovascular space invasion have been correlated with positive PD-L1 expression [20, 21], however, this is not a universal finding [22, 23]. In addition, although in several reports PD-L1 expression is recognized as a worse prognostic indicator [24], this relationship is not widely accepted, and, in fact, the exact opposite effect has also been described [25]. The vague criteria defining positivity of PD-L1 expression in endometrial tumors and the variations in commonly used assays may contribute to the diversity of these reports. Conduction of more studies under broadly accepted and standardized PD-L1 evaluation protocols would likely shed light on these unclear topics.
The PD-1/PD-L1 axis has already been an established target in the treatment approach of several malignancies such as melanoma and non-small cell lung cancer [26]. Initial studies have shown that this strategy with immune checkpoint inhibitors could also be effective in certain molecular subgroups of endometrial cancer, which present with an increased neoantigen load such as POLE ultra-mutated and MSI-H cancers and, thus, produce a more robust immune response along with PD-1 and PD-L1 overexpression [27]. The recent Food and Drug Administration approval of an immune checkpoint inhibitor, the programmed cell death 1 antibody (Pembrolizumab, Keytruda), for the treatment of patients with metastatic mismatch repair deficient cancers (dMMR) regardless of tumor location, might be the initial step for the use of immunotherapy in metastatic endometrial cancers [28].
Despite the fact that both POLE and MSI-H tumors are usually of endometrioid type, it is known that any histologic type can fall into any of the Cancer Genome Atlas subgroups [5]. Patients with high-grade aggressive endometrial tumors experience limited benefits from standard treatment and alternate approaches are essential [29]. It has been suggested that the expression of PD-L1 and CD8 are useful indicators of tumor response to immunotherapy [30, 31]. However, current literature series evaluating these markers in endometrial cancer include limited number of grade 3 carcinomas. This is, to our knowledge, the largest cohort with combined consideration of PD-L1 and CD8 expression in high-grade endometrial carcinomas of all histologic types, correlating their expression with various clinicopathological characteristics and the course of the disease.
Materials and methods
Out of 159 patients with high-grade endometrial carcinoma, diagnosed and treated between 2001 and 2017, 101 were completely surgically staged and constituted the final population of this study. None of these women had received any pre-operative chemotherapy. Routinely, a total hysterectomy, bilateral salpingo-oophorectomy and pelvic ± para-aortic lymphadenectomy was performed. Omentectomy was restricted to selected cases. However, in extensive disease more radical surgery was implemented. Post-operatively, 27.7% of the patients received at least 4 cycles of platinum-based chemotherapy and pelvic radiation, 24.6% were treated with chemotherapy alone, 16.9% with chemotherapy and brachytherapy, 10.8% with brachytherapy alone, while 7.7% of the patients received no adjuvant therapy.
The hematoxylin–eosin and all immunohistochemically stained slides were reviewed by two pathologists specialized in gynecological pathology (PY, EG) who were unaware of the histologic diagnosis. In cases of disagreement the slides were re-evaluated by a third pathologist (AN). Tumors with ambiguous or overlapping morphological and immunohistochemical features that could not be characterized as of endometrioid or serous histology were considered as “unclassifiable”.
Immunohistochemistry
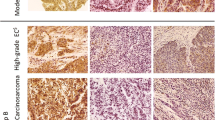
From each representative tissue block, two 4-μm tissue sections were cut and were used for the immunohistochemical analysis. For PD-L1 staining, the DAKO certified kit clone 22C3 (dilution 1:40 overnight) was used. For each case, the percentage of positively stained tumor cells over the whole section was evaluated. PD-L1 expression was considered positive when there was ≥ 1% membranous staining of any intensity in tumor cells; conversely, expression < 1% was considered negative [32]. Representative images of the PD-L1-stained samples are shown in Fig. 1a, b.
Representative photomicrographs of the immunohistochemical analysis. a PD-L1 membranous positivity. b Absence of PD-L1 expression, with positive internal control (immune cells). c High intraepithelial CD8 expression. d Low intraepithelial CD8 expression. e High stromal CD8 expression. f Low stromal CD8 expression
For CD8 lymphocytes, a mouse monoclonal CD8 antibody clone C8/1448 (DAKO, dilution 1:70) was used. The number of intraepithelial as well as stromal CD8 + cells was manually counted in five random high-power fields X40 (HPF). The total median count for intraepithelial and stromal cells was 17.6 and 66.8, respectively. Cases with a level of expression at or above the median were considered to have high expression, Fig. 1c–f [33].
For CD8, histologic sections from tonsils were used as positive controls. For PD-L1, placental chorionic villi were used as positive controls and immune cells as positive internal controls. Histologic sections of the tumor without application of the antibodies were used as negative controls.
Statistical analysis
All statistical analyses were performed using SPSS version 23.0. Pearson χ2 or Fisher’s exact test were used to explore statistically significant relationships between categorical variables, whereas staining results or other continuous variables were examined with independent samples t test. Kaplan–Meier method was used for univariate survival analysis (log rank test). Variables significant on univariate analysis (p < 0.05) such as stage on both progression-free and overall survival and intra-tumoral CD8 + infiltration on progression-free survival were entered in the multivariate model. In addition, clinically relevant variables (i.e., PD-L1 expression, histologic type) were also entered in the multivariate model regardless of univariate analysis results. Multivariate survival analyses were performed using Cox proportional hazards regression analysis. Any p value < 0.05 was considered of statistical significance.
Results
Patients
One hundred and one patients with completely staged disease were included in the analysis. Follow-up period ranged from 12 to 178 months. All clinical information was retrieved from the patients’ files. The clinicopathological features related to the study group are presented in Table 1.
PD-L1 expression: association with CD8 infiltration and clinicopathological features
The positive rate of PD-L1 expression was significantly higher in endometrioid than in non-endometrioid carcinomas (55.3% vs 35.2%, p = 0.042). PD-L1 was not associated with any of the clinicopathologic features examined, even when tested separately within the endometrioid and the non-endometrioid group of tumors (data not shown). Carcinomas positive for PD-L1 were significantly related to high counts of both intraepithelial (p = 0.003) and stromal (p = 0.005) CD8 + lymphocytes as compared to PD-L1-negative carcinomas. The association between PD-L1 expression and the clinicopathologic factors under evaluation is shown in Table 2.
Intraepithelial and stromal CD8 expression: association with clinicopathologic features
There was a significantly higher rate of intraepithelial CD8 + lymphocytes in the group of endometrioid as opposed to non-endometrioid carcinomas (p = 0.004). The relationship between intraepithelial CD8 + counts and the clinicopathological characteristics is presented in Table 3. No statistically significant association was found between “high” and “low” stromal CD8 + counts and the histologic type (p = 0.661) nor any of the other clinicopathological parameters under evaluation (Table 3). Furthermore, higher stromal CD8 + cell counts were statistically significantly associated with increased intraepithelial CD8 + cell numbers (p = 0.005).
Survival analysis
In univariate analysis, there was no significant difference neither in progression-free survival nor in overall survival between PD-L1-positive and PD-L1-negative tumors (p = 0.578 and p = 0.243, respectively). There were no statistically significant differences in progression-free or overall survival between patients with high stromal CD8 + cell infiltration and patients with low stromal CD8 + cells (p = 0.849 and 0.651, respectively). Patients with high intraepithelial CD8 + cell counts had a longer progression-free survival compared to those with low counts (p = 0.01), whereas no significant differences were observed for overall survival (p = 0.091), Fig. 2a–f. The relationship between high intraepithelial CD8 expression and improved progression-free survival remained significant in multivariate Cox regression analysis (hazard ratio = 0.039; 95% confidence intervals 0.039–0.295; p = 0.039) (Table 4). High stage was significantly associated with worse progression-free and overall survival in both univariate (p = 0.012 and p = 0.007, respectively) and multivariate analysis (hazard ratio 2.516, 95% CI 1.06–5.97, p = 0.036 and hazard ratio 4.16, 95% CI 1.34–12.92, p = 0.014, respectively).
Survival curves. On univariate analysis (Kaplan–Meier method), high intraepithelial CD8 expression (panel c) and the combined negative PDL1 expression/high intraepithelial CD8 expression (panel g) were significantly associated with longer PFS and there was no association with OS (panels d and h). PD-L1 and stromal CD8 expression had no significant impact neither on PFS nor on OS (panels a, b, e, f). Log rank test for p values. PD-L1 programmed cell death-ligand 1, PFS progression-free survival, OS overall survival, iCD8 intraepithelial CD8 + cells, high iCD8 ≥ 17.6 CD8 + cells/high-power field, low iCD8 < 17.6 CD8 + cells/high-power field
Grouped PD-L1 and intraepithelial CD8 + cell analysis
To evaluate the significance in disease progression of the coupled PD-L1/intraepithelial CD8 expression, four groups of tumors were isolated as suggested by Teng et al. [34]. Specifically, group 1 tumors were PD-L1 positive/CD8 high, group 2 PD-L1 negative/CD8 low, group 3 PD-L1 positive/CD8 low and group 4 PD-L1 negative/CD8 high. 28 (27.7%), 33 (32.7%), 17 (16.8%) and 23 (22.8%) patients belonged to groups 1, 2, 3 and 4, respectively. These groups were found to be significantly associated with the histology (p = 0.01). Group 1 tumors were mostly of endometrioid type (71.4%), while group 2 and group 3 tumors were mainly non-endometrioid carcinomas (64.7% and 69.7%, respectively). In group 4, there were nearly equal percentages of endometrioid and non-endometrioid carcinomas (47.8% and 52.2%, respectively). Statistical analysis on the relationship between each different group and the clinicopathologic features under evaluation yielded no statistically significant results, neither within the endometrioid nor within the non-endometrioid carcinomas (data not shown). In multivariate Cox regression analysis, group 3 tumors had significantly worse progression-free survival compared to group 4 tumors (hazard ratio 10.399; 95% confidence intervals 1.176–91.974; p = 0.035) (Supplemental Table 1).
Among the four groups, there were significant differences in progression-free survival (p = 0.032). Group 4 patients had the longest progression-free survival, whereas group 3 patients the shortest. No statistically significant differences were found regarding overall survival (p = 0.336), Fig. 2g–h.
Discussion
Grade 3 endometrial carcinomas represent a heterogeneous group of tumors that comprises both endometrioid and non-endometrioid histologic types and is characterized by a high risk for pelvic recurrence and distant metastases. For advanced stage or recurrent disease that has not responded to the initial treatment, there is evidence favoring the use of different drugs targeting angiogenesis or altered signaling pathways, such as mammalian target of rapamycin (mTOR) inhibitors [35]. Growing knowledge on cancer treatment revealed that both cytotoxic agents and targeted therapies modulate immune responses, thereafter, treatment strategies combining immunotherapy might improve clinical outcomes [36]. Recently, the PD-1/PD-L1 interaction has been suggested as a possible target for immune intervention in cancer [26]. Nevertheless, knowledge on the therapeutic potential of this pathway in endometrial carcinoma is limited. Some data derive from retrospective analysis of scattered cases [37, 38]. However, the phase II KEYNOTE-028 trial, a hallmark study for immunotherapy in endometrial cancer, has demonstrated a favorable safety profile and a long-lasting anti-tumor effect of pembrolizumab in advanced endometrial cancer [32].
Regarding PD-L1-positive immunohistochemical expression, it has been recognized as a parameter indicating higher response rates in PD-1/PD-L1 blockade treatment [39]. In addition, positive PD-L1 expression was found to be related to favorable prognosis in several solid tumors, such as breast cancer, while being a negative prognostic indicator in others [40, 41]. As for endometrial cancer, the results were controversial while favoring no correlation between PD-L1 expression and outcome [20, 21, 24]. In the present series, we also failed to show any significant relationship between PD-L1 expression and neither progression-free survival nor overall survival.
In our study, the rate of PD-L1-positive tumors was statistically higher in carcinomas of endometrioid than of non-endometrioid histology. This finding is consistent with the results of previous studies indicating that PD-L1 expression is associated with tumors characterized by an increased neoantigen load, which are primarily of endometrioid histology [5]. However, in another study, the above finding was confirmed only for PD-L1 positivity in immune cells and not in tumor cells [42]. Apart from tumor histology, in our group of tumors, PD-L1 expression was not associated with any of the clinicopathological parameters under evaluation which is in agreement with results already published [21].
Our results on CD8 expression revealed a significantly higher rate of intraepithelial CD8 + lymphocytes in the group of endometrioid when compared to non-endometrioid carcinomas, except for those belonging to the “unclassifiable” category. Indeed, studies on polymerase epsilon ultra-mutated carcinomas, which are mainly of endometrioid type, demonstrated a massive infiltration of the tumors by CD8 + lymphocytes [43]. Moreover, there is growing evidence that the estimation of CD8 + cells in the pathologic examination of a tumor can offer important information in terms of prognosis, treatment options and response to therapy [44]. Our finding that stromal CD8 + cells are increased in tumors with high intraepithelial CD8 + cells have been reported previously [45]. Hendry et al. [46] proposed a uniform protocol for the evaluation of tumor-infiltrating lymphocytes. It was stated that intraepithelial and stromal lymphocytes should be reported individually, since depending on the type of cancer, each compartment might have different prognostic relevance [44]. For example, both intraepithelial and stromal CD8 + cells were found to be associated with worst prognosis in gastric adenocarcinoma [47], while only stromal CD8 + cells were considered as independent positive prognostic indicators in non-small cell lung cancer [48]. Concerning endometrial cancer, there is initial evidence that only intraepithelial immune cells have a positive prognostic value [49, 9, 7], a finding which is questioned by others [45].
In the present study, both intraepithelial and stromal CD8 + cells were evaluated. Only high intraepithelial CD8 counts were found to be an independent prognostic factor associated with longer progression-free survival, whilst this association was not statistically significant for stromal immune cells. In a study conducted by Jung et al. [50], on endometrial carcinomas of all grades, high CD8 counts were associated with < 50% of myometrial invasion and with the absence of lymph node metastases. In our cohort, neither intraepithelial nor stromal CD8 high counts were correlated to any of the clinical and pathological factors examined. Most probably, this finding might be related to the fact that our results were based solely on high-grade tumors which are usually of higher stage at initial diagnosis.
Of specific importance is our analysis on the prognostic relevance of tumor PD-L1 expression in combination with intraepithelial CD8 + tumor-infiltrating lymphocytes score. It was found that PD-L1-positive/CD8 low (group 3) tumors had the shortest progression-free survival, indicating that the expression of PD-L1 in tumor cells may function as a mechanism of adaptive immune resistance and contribute to CD8 + cells dysfunction and tumor escape [51]. The longest progression-free survival was observed in group 4 tumors which were both of endometrioid and non-endometrioid histology and characterized by a PD-L1-negative/CD8 high phenotype. It seems that in the above group, other suppressors than PD-L1 contribute in the enhancement of immune tolerance [27]. Finally, the group of tumors characterized by a PD-L1-positive/CD8 high phenotype seems to be of importance in our study. This group is mostly represented by endometrioid tumors exhibiting an adaptive immune resistance and likely to respond to immune checkpoint inhibitors [13]. However, the validity of using PD-L1 protein expression as a hallmark for the design of a treatment protocol is questioned. Indeed, several reports indicate that anti-PD-1 antibodies are effective even in patients without PD-L1 expression [25]. Those controversies underline the need for establishing universal criteria for the evaluation of PD-L1 immunohistochemical expression and the use of specific platforms related to each different reagent. The significance of combined PD-L1/CD8 immunohistochemical analysis in endometrial carcinomas has also been mentioned in the past [24]. Nevertheless, contrary to our study, the above authors evaluated both PD-L1 and CD8 combined expression in immune cells of different tumor sites, while in our study, the expression of PD-L1 was evaluated in tumor cells. Despite the aforementioned difference, our results were similar, indicating that evaluating PD-L1 expression in immune cells of the center of the tumor or in tumor cells and combining it with CD8 expression has the same prognostic significance. Specifically, in both studies, PD-L1−/CD8 + tumors were associated with a better progression-free survival as opposed to PD-L1+/CD8 tumors, suggesting that the combined assessment of these markers could be used as a prognostic indicator.
Our study has some limitations. It is a retrospective study and several factors that could probably interfere with the course of the disease are not estimated. Moreover, for some of our cases, the follow-up period was short, rendering information on progression-free and overall survival of limited value.
In conclusion, our study on PD-L1 and CD8 immunohistochemical evaluation in high-grade endometrial carcinomas revealed that endometrioid tumors had a higher rate of PD-L1 and of intraepithelial CD8 + tumor-infiltrating lymphocytes expression as opposed to non-endometrioid tumors. However, in univariate and multivariate analyses, only a high intraepithelial CD8 + count was an independent prognostic factor correlating with better outcomes. A longer progression-free survival was also observed in tumors characterized by the coupled PD-L1-negative/CD8 high phenotype, which were both of endometrioid and of non-endometrioid type. Immunotherapy should probably be considered for metastatic PD-L1-positive/CD8 high tumors, which are characterized by an increased neoantigen load and were mostly of endometrioid histology.
References
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68(1):7–30. https://doi.org/10.3322/caac.21442
Miller KD, Siegel RL, Lin CC et al (2016) Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 66(4):271–289. https://doi.org/10.3322/caac.21349
Bokhman JV (1983) Two pathogenetic types of endometrial carcinoma. Gynecol Oncol 15(1):10–17
Colombo N, Creutzberg C, Amant F et al (2016) ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Ann Oncol 27(1):16–41. https://doi.org/10.1093/annonc/mdv484
Suarez AA, Felix AS, Cohn DE (2017) Bokhman redux: endometrial cancer "types" in the 21st century. Gynecol Oncol 144(2):243–249. https://doi.org/10.1016/j.ygyno.2016.12.010
Piulats JM, Guerra E, Gil-Martin M et al (2017) Molecular approaches for classifying endometrial carcinoma. Gynecol Oncol 145(1):200–207. https://doi.org/10.1016/j.ygyno.2016.12.015
Kondratiev S, Sabo E, Yakirevich E et al (2004) Intratumoral CD8+ T lymphocytes as a prognostic factor of survival in endometrial carcinoma. Clin Cancer Res 10(13):4450–4456. https://doi.org/10.1158/1078-0432.CCR-0732-3
de Jong RA, Boerma A, Boezen HM et al (2012) Loss of HLA class I and mismatch repair protein expression in sporadic endometrioid endometrial carcinomas. Int J Cancer 131(8):1828–1836. https://doi.org/10.1002/ijc.27449
Suemori T, Susumu N, Iwata T et al (2015) Intratumoral CD8+ lymphocyte infiltration as a prognostic factor and its relationship with cyclooxygenase 2 expression and microsatellite instability in endometrial cancer. Int J Gynecol Cancer 25(7):1165–1172. https://doi.org/10.1097/IGC.0000000000000482
de Jong RA, Leffers N, Boezen HM et al (2009) Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol Oncol 114(1):105–110. https://doi.org/10.1016/j.ygyno.2009.03.022
Iurchenko NP, Glushchenko NM, Buchynska LG (2014) Comprehensive analysis of intratumoral lymphocytes and FOXP3 expression in tumor cells of endometrial cancer. Exp Oncol 36(4):262–266
Ladanyi A, Somlai B, Gilde K et al (2004) T-cell activation marker expression on tumor-infiltrating lymphocytes as prognostic factor in cutaneous malignant melanoma. Clin Cancer Res 10(2):521–530
Gadducci A, Guerrieri ME (2017) Immune checkpoint inhibitors in gynecological cancers: update of literature and perspectives of clinical research. Anticancer Res 37(11):5955–5965. https://doi.org/10.21873/anticanres.12042
Howitt BE, Shukla SA, Sholl LM et al (2015) Association of polymerase e-mutated and microsatellite-instable endometrial cancers with neoantigen load, number of tumor-infiltrating lymphocytes, and expression of PD-1 and PD-L1. JAMA Oncol 1(9):1319–1323. https://doi.org/10.1001/jamaoncol.2015.2151
Vanderstraeten A, Luyten C, Verbist G et al (2014) Mapping the immunosuppressive environment in uterine tumors: implications for immunotherapy. Cancer Immunol Immunother 63(6):545–557. https://doi.org/10.1007/s00262-014-1537-8
Jones NL, Xiu J, Chatterjee-Paer S et al (2017) Distinct molecular landscapes between endometrioid and nonendometrioid uterine carcinomas. Int J Cancer 140(6):1396–1404. https://doi.org/10.1002/ijc.30537
Liu J, Liu Y, Wang W et al (2015) Expression of immune checkpoint molecules in endometrial carcinoma. Exp Ther Med 10(5):1947–1952. https://doi.org/10.3892/etm.2015.2714
Asaka S, Yen TT, Wang TL et al (2019) T cell-inflamed phenotype and increased Foxp3 expression in infiltrating T-cells of mismatch-repair deficient endometrial cancers. Mod Pathol 32(4):576–584. https://doi.org/10.1038/s41379-018-0172-x
Crumley S, Kurnit K, Hudgens C et al (2019) Identification of a subset of microsatellite-stable endometrial carcinoma with high PD-L1 and CD8+ lymphocytes. Mod Pathol 32(3):396–404. https://doi.org/10.1038/s41379-018-0148-x
Li Z, Joehlin-Price AS, Rhoades J et al (2018) Programmed death ligand 1 expression among 700 consecutive endometrial cancers: strong association with mismatch repair protein deficiency. Int J Gynecol Cancer 28(1):59–68. https://doi.org/10.1097/IGC.0000000000001120
Bregar A, Deshpande A, Grange C et al (2017) Characterization of immune regulatory molecules B7-H4 and PD-L1 in low and high grade endometrial tumors. Gynecol Oncol 145(3):446–452. https://doi.org/10.1016/j.ygyno.2017.03.006
Sungu N, Yildirim M, Desdicioglu R et al (2018) Expression of immunomodulatory molecules PD-1, PD-L1, and PD-L2, and their relationship with clinicopathologic characteristics in endometrial cancer. Int J Gynecol Pathol. https://doi.org/10.1097/PGP.0000000000000543
Tawadros AIF, Khalafalla MMM (2018) Expression of programmed death-ligand 1 and hypoxia-inducible factor-1alpha proteins in endometrial carcinoma. J Cancer Res Ther 14(Supplement):S1063–S1069. https://doi.org/10.4103/0973-1482.202891
Kim J, Kim S, Lee HS et al (2018) Prognostic implication of programmed cell death 1 protein and its ligand expressions in endometrial cancer. Gynecol Oncol 149(2):381–387. https://doi.org/10.1016/j.ygyno.2018.02.013
Yamashita H, Nakayama K, Ishikawa M et al (2018) Microsatellite instability is a biomarker for immune checkpoint inhibitors in endometrial cancer. Oncotarget 9(5):5652–5664. https://doi.org/10.18632/oncotarget.23790
Thallinger C, Fureder T, Preusser M et al (2018) Review of cancer treatment with immune checkpoint inhibitors: current concepts, expectations, limitations and pitfalls. Wien Klin Wochenschr 130(3–4):85–91. https://doi.org/10.1007/s00508-017-1285-9
Mittica G, Ghisoni E, Giannone G et al (2017) Checkpoint inhibitors in endometrial cancer: preclinical rationale and clinical activity. Oncotarget 8(52):90532–90544. https://doi.org/10.18632/oncotarget.20042
US Food and Drug Administration (2017) FDA approves first cancer treatment for any solid tumor with a specific genetic feature. US Food and Drug Administration, Silver Spring
De Felice F, Marchetti C, Tombolini V et al (2019) Immune check-point in endometrial cancer. Int J Clin Oncol. https://doi.org/10.1007/s10147-019-01437-7
Tumeh PC, Harview CL, Yearley JH et al (2014) PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515(7528):568–571. https://doi.org/10.1038/nature13954
Eggink FA, Van Gool IC, Leary A et al (2017) Immunological profiling of molecularly classified high-risk endometrial cancers identifies POLE-mutant and microsatellite unstable carcinomas as candidates for checkpoint inhibition. Oncoimmunology 6(2):e1264565. https://doi.org/10.1080/2162402X.2016.1264565
Ott PA, Bang YJ, Berton-Rigaud D et al (2017) Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1-positive endometrial cancer: results from the KEYNOTE-028 study. J Clin Oncol 35(22):2535–2541. https://doi.org/10.1200/JCO.2017.72.5952
Kraft S, Fernandez-Figueras MT, Richarz NA et al (2017) PDL1 expression in desmoplastic melanoma is associated with tumor aggressiveness and progression. J Am Acad Dermatol 77(3):534–542. https://doi.org/10.1016/j.jaad.2017.05.007
Teng MW, Ngiow SF, Ribas A et al (2015) Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res 75(11):2139–2145. https://doi.org/10.1158/0008-5472.CAN-15-0255
Husseinzadeh N, Husseinzadeh HD (2014) mTOR inhibitors and their clinical application in cervical, endometrial and ovarian cancers: a critical review. Gynecol Oncol 133(2):375–381. https://doi.org/10.1016/j.ygyno.2014.02.017
Vanneman M, Dranoff G (2012) Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer 12(4):237–251. https://doi.org/10.1038/nrc3237
Santin AD, Bellone S, Buza N et al (2016) Regression of chemotherapy-resistant polymerase epsilon (POLE) ultra-mutated and MSH6 hyper-mutated endometrial tumors with nivolumab. Clin Cancer Res 22(23):5682–5687. https://doi.org/10.1158/1078-0432.CCR-16-1031
Mehnert JM, Panda A, Zhong H et al (2016) Immune activation and response to pembrolizumab in POLE-mutant endometrial cancer. J Clin Investig 126(6):2334–2340. https://doi.org/10.1172/JCI84940
Garon EB, Rizvi NA, Hui R et al (2015) Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372(21):2018–2028. https://doi.org/10.1056/NEJMoa1501824
Sabatier R, Finetti P, Mamessier E et al (2015) Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget 6(7):5449–5464. https://doi.org/10.18632/oncotarget.3216
Tamura T, Ohira M, Tanaka H et al (2015) Programmed death-1 ligand-1 (PDL1) expression is associated with the prognosis of patients with stage II/III gastric cancer. Anticancer Res 35(10):5369–5376
Mo Z, Liu J, Zhang Q et al (2016) Expression of PD-1, PD-L1 and PD-L2 is associated with differentiation status and histological type of endometrial cancer. Oncol Lett 12(2):944–950. https://doi.org/10.3892/ol.2016.4744
Bellone S, Bignotti E, Lonardi S et al (2017) Polymerase epsilon (POLE) ultra-mutation in uterine tumors correlates with T lymphocyte infiltration and increased resistance to platinum-based chemotherapy in vitro. Gynecol Oncol 144(1):146–152. https://doi.org/10.1016/j.ygyno.2016.11.023
Fridman WH, Pages F, Sautes-Fridman C et al (2012) The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 12(4):298–306. https://doi.org/10.1038/nrc3245
Cermakova P, Melichar B, Tomsova M et al (2014) Prognostic significance of CD3+ tumor-infiltrating lymphocytes in patients with endometrial carcinoma. Anticancer Res 34(10):5555–5561
Hendry S, Salgado R, Gevaert T et al (2017) Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the international immuno-oncology biomarkers working group: part 1: assessing the host immune response, TILs in invasive breast carcinoma and ductal carcinoma in situ, metastatic tumor deposits and areas for further research. Adv Anat Pathol 24(5):235–251. https://doi.org/10.1097/PAP.0000000000000162
Thompson ED, Zahurak M, Murphy A et al (2017) Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut 66(5):794–801. https://doi.org/10.1136/gutjnl-2015-310839
Al-Shibli KI, Donnem T, Al-Saad S et al (2008) Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res 14(16):5220–5227. https://doi.org/10.1158/1078-0432.CCR-08-0133
Hendry S, Salgado R, Gevaert T et al (2017) Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the international immuno-oncology biomarkers working group: part 2: TILs in melanoma, gastrointestinal tract carcinomas, non-small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors. Adv Anat Pathol 24(6):311–335. https://doi.org/10.1097/PAP.0000000000000161
Jung IK, Kim SS, Suh DS et al (2014) Tumor-infiltration of T-lymphocytes is inversely correlated with clinicopathologic factors in endometrial adenocarcinoma. Obstet Gynecol Sci 57(4):266–273. https://doi.org/10.5468/ogs.2014.57.4.266
Wang Q, Lou W, Di W et al (2017) Prognostic value of tumor PD-L1 expression combined with CD8(+) tumor infiltrating lymphocytes in high grade serous ovarian cancer. Int Immunopharmacol 52:7–14. https://doi.org/10.1016/j.intimp.2017.08.017
Acknowledgements
This research work was supported by the Onassis Foundation—Scholarship ID: G ZO 001-1/2018-2019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Onassis Foundation did not influence on the decision to submit this manuscript or on its content. We declare that we have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Vagios, S., Yiannou, P., Giannikaki, E. et al. The impact of programmed cell death-ligand 1 (PD-L1) and CD8 expression in grade 3 endometrial carcinomas. Int J Clin Oncol 24, 1419–1428 (2019). https://doi.org/10.1007/s10147-019-01484-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-019-01484-0