Abstract
Gastric cancer (GC), one of the most common human cancers, is a heterogeneous disease with different phenotypes, prognoses, and responses to treatment. Understanding the pathogenesis of GC at the molecular level is important for prognosis prediction and determining treatments. Microsatellite instability (MSI), silencing of MLH1, MGMT, and CDKN2A genes by DNA hypermethylation, KRAS mutation, APC mutation, and ERBB2 amplification are frequently found in intestinal type GC. Inactivation of CDH1 and RARB by DNA hypermethylation, and amplification of FGFR and MET, are frequently detected in diffuse type GC. In addition, BST2 and PCDHB9 genes are overexpressed in intestinal type GC. Both genes are associated with GC progression. GC can be divided into gastric/intestinal mucin phenotypes according to mucin expression. MSI, alterations of TP73, CDH1 mutation, and DNA methylation of MLH are detected frequently in the gastric mucin phenotype. TP53 mutation, deletion of APC, and DNA methylation of MGMT are detected frequently in the intestinal mucin phenotype. FKTN is overexpressed in the intestinal mucin phenotype, and IQGAP3 is overexpressed in the gastric mucin phenotype. These genes are involved in GC progression. To characterize cancer stem cells, a useful method is spheroid colony formation. KIFC1 and KIF11 genes show more than twofold higher expression in spheroid-forming cells than that in parental cells. Both KIF genes are overexpressed in GC, and knockdown of these genes inhibits spheroid formation. Alterations of these molecules may be useful to understand gastric carcinogenesis. Specific inhibitors of these molecules may also be promising anticancer drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC), one of the most common human cancers, is a heterogeneous disease with different phenotypes, prognoses, and responses to treatment. Understanding GC pathogenesis at the molecular level is important for prognosis prediction and determining treatments. Various genetic and epigenetic alterations are associated with GC. Histologically, GC cases are classified into two major types: differentiated and undifferentiated types, as described by Nakamura et al. [1], or Lauren intestinal and diffuse types based on the glandular structure [2]. Intestinal and diffuse GC types show distinct clinical characteristics [3], and type-specific genetic and epigenetic alterations have been identified [4, 5]. Thus, the Lauren classification facilitates understanding the pathogenesis of GC. GC can also be classified into gastric or intestinal phenotypes according to mucin expression. Accumulating evidence has indicated that gastric/intestinal phenotypes of GC have distinct clinical characteristics and exhibit specific genetic and epigenetic changes [5]. Thus, mucin phenotype classification is also useful to understand GC pathogenesis. In addition to these histological classifications, studies employing next-generation sequencing (NGS) have proposed molecular classification of GC. Here, we focus on the molecular characteristics of GC according to histological and molecular classifications.
Lauren classification
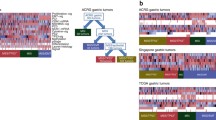
During the course of multistep carcinogenesis in the stomach, various genetic and epigenetic alterations accumulate [5]. Of these alterations, some are found in both intestinal and diffuse types of GC, while others are found in only one histological type. Microsatellite instability (MSI), silencing of MLH1, MGMT, and CDKN2A genes by CpG island hypermethylation, KRAS mutation, APC mutation, and ERBB2 amplification are frequently found in intestinal type GC. In contrast, inactivation of CDH1 and RARB genes by CpG island hypermethylation and amplification of FGFR and MET genes are frequently detected in diffuse type GC. Molecular alterations according to intestinal and diffuse types of GC are summarized in Fig. 1. These alterations facilitate understanding the pathogenesis of GC. In clinical practice, patients with intestinal type GC may be eligible for anti-HER2 therapy, and patients with diffuse type GC may be eligible for anti-MET therapy. Genes that encode transmembrane/secretory proteins and are expressed specifically in cancers can be ideal biomarkers for cancer diagnosis. If the gene product is involved in the neoplastic process, then the gene may be a therapeutic target. Thus, genes that encode transmembrane/secretory proteins are analyzed preferentially. Several transmembrane/secretory proteins are specifically expressed in GC.
BST2
BST2 encodes the BST-2 protein. BST-2, also known as HM1.24 or CD317, is a lipid raft-associated type II transmembrane glycoprotein that is overexpressed on multiple myeloma cells [6, 7]. A monoclonal antibody against BST-2 can induce antibody-dependent cellular cytotoxicity, suggesting that monoclonal antibodies against BST-2 may be an effective treatment for BST-2-positive malignancies. BST-2 overexpression has been reported in several human cancers including breast, lung, esophageal, and colorectal cancers, as well as GC [8,9,10]. BST-2 is overexpressed in 36% of GC tissue samples. BST-2 overexpression is preferentially found in intestinal type GC, and BST-2 expression is an independent prognostic classifier of GC patients [10]. Inhibition of BST2 by siRNA inhibits proliferation, induces apoptosis, and represses motility of GC cells, and these effects are mediated partly through NF-kB signaling [11]. Taken together, these findings suggest that BST-2 is involved in GC progression, and that BST-2 immunostaining is a clinically useful method to predict GC patient survival. Because expression of BST-2 is found in 35% of HER2-negative GC cases, BST-2 may be a useful therapeutic target for HER2-negative GC [10].
PCDHB9
Cadherins are a family of glycosylated transmembrane proteins that mediate cell–cell adhesion. The cadherin family is classified into classical cadherins, desmosomal cadherins, and protocadherins. Protocadherins encoded by PCDH genes are predominantly expressed in the nervous system and comprise the largest subfamily of the cadherin superfamily of cell adhesion molecules [12]. Protocadherin B9 is overexpressed in 36% of GC tissue samples. Overexpression of protocadherin B9 is frequently found in intestinal type GC and correlated with a poor prognosis of patients with intestinal type GC [13]. The function of protocadherin B9 has also been examined [13]. PCDHB9 knockdown or forced expression of PCDHB9 do not change growth or invasion activities of GC cell lines. In contrast, cell adhesion to fibronectin is reduced by PCDHB9 knockdown and enhanced by forced expression of PCDHB9. Expression of ITGA3, ITGA4, ITGA5, and ITGB1 is reduced by knockdown of PCDHB9 and enhanced by forced expression of PCDHB9. Both the number and size of spheres are decreased by PCDHB9 knockdown and increased by forced expression of PCDHB9. In the peritoneal dissemination mouse model, the weight of total disseminated nodules of the MKN-74 GC cell line is increased by forced expression of PCDHB9. In prostate cancer cells, expression of NF-kB p65 is induced by forced expression of PCDHB9 [14]. NF-kB p65 is a transcription factor that promotes GC cell invasion [15]. Therefore, in addition to ITGA genes, upregulation of NF-kB p65 is involved in the progression of protocadherin B9-positive GC. These studies indicate that protocadherin B9 plays an important role in the progression of intestinal type GC. Specific inhibitors of protocadherin B9 may also be promising anticancer drugs.
Genetic and epigenetic alterations of intestinal metaplasia (IM)
To understand gastric carcinogenesis, characterization of IM is important. IM is thought to be a premalignant condition of the gastric mucosa associated with an increased GC risk. IM shares various alterations similar to those seen in intestinal type GC [16]. Mutations of TP53, APC, or KRAS have been detected in IM, although at low frequencies [16]. A recent comprehensive analysis demonstrated that IMs exhibit low mutational burdens [17]. TP53 and ARID1A are two of the most frequently mutated tumor-suppressor genes in GC. However, only two cases of TP53 mutations (2%) and three cases of ARID1A mutations (3%) have been detected in 138 IM samples. Therefore, clonal TP53 and ARID1A mutations are likely infrequent in IM. In contrast, FBXW7 mutation is found in 4.7% of IM samples. Notably, the most frequent FBXW7 mutations are missense point mutations, causing a dominant negative activity. Therefore, monoallelic FBXW7 mutation is functionally active. Jiang et al. [18] have reported that the tumor incidence is obviously higher in Fbxw7+/− mice than in Fbxw7+/+ mice, and that IM and dysplasia are more severe in Fbxw7+/− mice than in Fbxw7+/+ mice. Taken together, FBXW7 plays a crucial role in gastric carcinogenesis derived from IM.
Several epigenetic alterations are observed in IM. Some IMs show DNA hypermethylation of MLH1, which reduces its expression [19]. Promoter hypermethylation of CDKN2A, RUNX3, MGMT, and DAPK is found in IM [20]. These alterations are also found in GC, suggesting that DNA hypermethylation is an early event in gastric carcinogenesis. Recent comprehensive analysis has also demonstrated that 78–99% of hypermethylated regions in IM are also hypermethylated in intestinal type GC [17]. However, IMs generally lack intragenic hypomethylation signatures. Therefore, aberrant DNA hypermethylation rather than genetic alteration is associated with IM. Aberrant DNA hypermethylation is an early event in IM, whereas global intragenic hypomethylation may be a late event in gastric carcinogenesis.
Mucin phenotype classification
Gastric cancer can be divided into gastric and intestinal phenotypes. The gastric and intestinal phenotypes of GC are analyzed by immunohistochemistry of MUC5AC and MUC6 as markers for the gastric phenotype, and MUC2 and CD10 as markers for the intestinal phenotype. Based on expression of these markers, GC cases are classified into four phenotypes: gastric (G type), intestinal (I type), gastric and intestinal mixed (GI type), and neither gastric nor intestinal (N type) [21]. It is important to note that the gastric phenotype is diminishes during GC progression. GC cases at early stages, independent of the histological type, mainly consist of the gastric phenotype, and a phenotypic shift from the gastric to intestinal phenotype is clearly observed with progression of the tumor stage [22]. Therefore, GC may develop from gastric foveolar cells, but not IM.
Several genetic and epigenetic alterations are frequently detected in gastric and intestinal phenotypes of GC. Molecular alterations according to gastric and intestinal phenotypes of GC are summarized in Fig. 1. MSI is detected more frequently in the gastric phenotype of GC [23]. Alterations of TP73, including loss of heterozygosity and abnormal expression, play an important role in the genesis of the gastric phenotype of GC [24]. CDH1 mutation is detected in differentiated type GC with the gastric phenotype [25]. TP53 mutation and allelic deletion of APC are detected more frequently in the intestinal phenotype of GC [26, 27]. Several epigenetic alterations have also been identified. DNA methylation of MLH1 frequently occurs in the gastric phenotype of GC, whereas MGMT is frequently methylated in the intestinal phenotype of GC [28].
Several transcription factors that induce the gastric/intestinal phenotypes have been identified. In the intestinal phenotype of GC, ectopic CDX2 expression has a crucial function [22]. In contrast, SOX2 may be an important transcription factor of the gastric phenotype of GC. SOX2 induces expression of MUC5AC and pepsinogen A, both of which are markers for the gastric phenotype [22]. Furthermore, SOX2 negatively regulates the CDX2 promoter by hampering the action of other transcription factors [29]. To characterize the intestinal phenotype of GC, identification of CDX2 target genes is essential. CDH17, REG4, DSC2, and ABCB1 are direct targets of CDX2, which are expressed in CDX2-positive GC cells [30,31,32,33]. Notably, in patients with the gastric phenotype of GC, 5-FU-based postoperative chemotherapy is beneficial. However, in patients with the intestinal phenotype of GC, 5-FU-based postoperative chemotherapy does not improve survival [34]. REG4 inhibits 5-FU-induced apoptosis and ABCB1 inhibits taxane-induced apoptosis [33, 35], suggesting that chemotherapy, including 5-FU-based or taxane-based chemotherapies, is not beneficial for patients with the intestinal phenotype of GC. For these patients, molecular-targeted therapies may be suitable. In addition to these genes, altered expression of several genes is observed in gastric/intestinal phenotypes of GC.
FKTN
FKTN, which encodes fukutin protein, is responsible for Fukuyama-type congenital muscular dystrophy [36]. Fukutin is presumably involved in the glycosylation of alpha-dystroglycan, which is involved in basement membrane formation. Overexpression of fukutin is observed in 43% of GC tissue samples and correlated with the advanced T grade and N grade [37]. Fukutin expression is frequently observed in the intestinal phenotype of GC. Inhibition of FKTN decreases GC cell proliferation. Therefore, fukutin may be a key regulator of the progression of GC with the intestinal phenotype.
IQGAP3
The IQGAP family includes three members, IQGAP1, IQGAP2, and IQGAP3 [38]. The name IQGAP is derived from the multiple functional domains these molecules that harbor four IQ motifs and a RasGAP-related domain (GRD) [39]. IQGAP1 regulates the cytoskeleton and cell migration, and plays a role in cancer progression [40, 41]. In contrast, IQGAP2 appears to act as a tumor-suppressor [42]. IQGAP3 enhances the proliferation of epithelial cells [43]. Therefore, IQGAP1 and IQGAP3 are thought to have oncogenic functions. Overexpression of IQGAP3 is detected in 21% of GC tissue samples and preferentially observed in the gastric phenotype GC [44]. IQGAP3 expression is an independent prognostic predictor for survival. The growth of GC cells is inhibited by knockdown of IQGAP3. Both the number and size of spheres formed by GC cells are significantly reduced by knockdown of IQGAP3. Furthermore, phosphorylation of Akt and Erk1/2 is inhibited by knockdown of IQGAP3. These results suggest that IQGAP3 plays an important role in GC progression. Because IQGAP3 protein is expressed on the cell membrane, IQGAP3 protein could be a therapeutic target for GC.
Cancer stem cells (CSCs)
In the past decade, cancer has been recognized as a stem cell disease [45]. To understand the pathogenesis of GC, identification and characterization of CSCs are essential. CSCs have been described in numerous solid tumors, and have been characterized by expression of specific cell surface markers including CD44, CD133, and aldehyde dehydrogenase 1 (ALDH1) [46].
To characterize CSCs, a useful methods is spheroid colony formation [47]. To form a spheroid colony, cells are cultured in serum-free medium on low attachment culture dishes. Spheroid colonies have characteristics of the CSC phenotype. Takahashi et al. identify GC-initiating cells using cell surface marker CD44 [48]. Among six GC cell lines, MKN-45, MKN-74, and NCI-N87 have a sizeable subpopulation of CD44(+) cells, and these cells show spheroid colony formation in serum-free medium in vitro, as well as a tumorigenic ability upon injection into the stomach and skin of severe combined immunodeficient mice in vivo. Gene expression profiles of spheroid colonies derived from these GC cell lines have also been analyzed [49]. KIF genes, including KIF11, KIF15, KIF2C, KIF20A, KIF20B, KIF22, KIF23, KIFC1, and KIF4A, are upregulated in spheroid-forming cells from both MKN-45 and MKN-74 cell lines. Kinesins are a family of molecular motors, which play important roles in intracellular transport or cell division [50]. Alterations of several kinds of kinesins have been reported in human cancers including GC. In human GC, overexpression of KIFC1, KIF11, KIF2A, and KIF26B has been reported [49, 51,52,53], indicating that kinesin proteins play important roles in GC pathogenesis. Inhibition of kinesin leads to cell cycle arrest at mitosis with the formation of characteristic monoaster spindles [54]. Ispinesib (SB-715992; Cytokinetics and GlaxoSmithKline) is the first potent, highly specific small molecule inhibitor of kinesin tested in clinical trials. Ispinesib achieved two complete and two partial responses among six evaluable tumors in the acute lymphoblastic leukemia xenografts panel [55]. Therefore, specific inhibitors of kinesins could be promising anticancer drugs.
KIFC1
KIFC1 (also known as HSET) is a C-type kinesin of the kinesin-14 family [56], which is assumed to be a minus end-directed motor protein [57]. Kinesins are a family of molecular motors and play important roles in intracellular transport or cell division [50]. Alteration of several kinds of kinesins has been reported in human cancers including GC. KIFC1 overexpression is found in 37% of GC tissue samples [49]. KIFC1-positive GC cases are frequently observed in the advanced stage and intestinal type of GC. Furthermore, expression of KIFC1 is detected in CD44- and ALDH1-positive GC cells. KIFC1 shows more than twofold higher expression in spheroid-forming cells than in parental cells of GC cell lines [49]. Both the number and size of spheres derived from GC cells are reduced by KIFC1 inhibition. In prostate cancer cells, inhibition of KIFC1 resensitizes docetaxel-resistant cell lines to docetaxel treatment [58]. Docetaxel alone has little effect on the viability of docetaxel-resistant cells. However, the combination of docetaxel and CW069 (KIFC1 inhibitor) reduces the viability of docetaxel-resistant cells, indicating that CW069 resensitizes docetaxel-resistant cell lines to docetaxel treatment. These results suggest that the combination of CW069 and docetaxel could be a potential strategy to overcome docetaxel resistance. Taken together, KIFC1 likely plays an important role in CSCs.
KIF11
KIF11 encodes KIF11 protein. KIF11 protein, also known as Eg5 protein or kinesin spindle protein, is a plus-end directed heterotetrameric motor protein capable of simultaneously moving along two microtubules [59]. KIF11 is overexpressed in human cancers including breast, lung, ovarian, bladder, and pancreatic cancers, as well as GC. KIF11 is overexpressed in 72% of GC tissue samples, and KIF11 overexpression is frequently observed in the intestinal phenotype of GC [51]. Both the number and size of spheres formed by GC cells are reduced by KIF11 inhibition. Levels of phosphorylated Erk1/2 are also reduced by KIF11 inhibition, indicating that KIF11 protein could be a therapeutic target for GC. Because KIF11 is not expressed in the adult peripheral nervous system, KIF11 inhibitors may not cause neuropathic side effects. KIF11 inhibitors have been developed as chemotherapeutic agents for the treatment of cancer [60]. Filanesib (ARRY-520) is a highly selective, targeted inhibitor of KIF11, which induces mitotic arrest and subsequent tumor cell death. In a phase 1 clinical study, ARRY-520 had acceptable tolerability and target-specific pharmacodynamic effects [60]. There is the possibility that ARRY-520 has an activity in GC patients.
Molecular classification of GC
By comprehensive molecular analysis using NGS, two kinds of molecular classification have been proposed for GC. The Cancer Genome Atlas (TCGA) Research Network has reported that GC can be classified into four distinct molecular subtypes: GCs positive for Epstein–Barr virus (EBV type); microsatellite-unstable GCs (MSI type); genomically stable GCs (GS type); GCs with chromosomal instability (CIN type) [61]. The Asian Cancer Research Group (ACRG) has divided GCs into four groups: microsatellite instability (MSI), microsatellite-stable and epithelial-to-mesenchymal transition (MSS/EMT), and microsatellite-stable and the presence of TP53 (MSS/TP53+) or no TP53 signature (MSS/TP53−) [62]. In general, the CIN type of TCGA classification and MSS/TP53 type of ACRG classification show the intestinal type of Lauran classification. The GS type of the TCGA classification and MSS/EMT type of the ACRG classification are associated with the diffuse type of Lauran classification. The TCGA classification is not correlated with prognosis. In contrast, MSS/EMT GCs are diagnosed mainly in advanced stages and correlate with a poor prognosis. The GS type of TCGA classification often shows mutations in genes responsible for cell adhesion, such as RHOA, CDH1, and CLDN18/ARHGAP. The MSS/TP53 type of the ACRG classification often shows mutations in genes including APC, ARID1A, KRAS, PIK3CA, and SMAD4. The MSS/TP53+ type of the ACRG classification is associated with EBV infection. Most EBV-positive GCs, such as the EBV type of the TCGA classification and MSS/TP53+ of the ACRG classification, are histologically GC with lymphoid stroma or lymphoepithelioma-like, which display PIK3CA and ARID1A mutations, genome-wide hypermethylation, and amplification of PD-L1, an important immune checkpoint regulator [63, 64]. Although further study should be performed, these classifications may provide personalized medicine in the near future.
Conclusions
Whole genome and exon sequencing in GC has been performed by NGS. The most frequently mutated gene is TP53 (32%) [65], and the second most frequently mutated gene is ARID1A (14%) [66]. The frequencies of other gene mutations are approximately 10% or below 10%. Thus, a driver gene mutation is a rare event in GC, and it is difficult to plan an effective treatment according to driver gene mutations. Therefore, alteration of gene expression is a critical event in gastric carcinogenesis. In the past few years, several targeted therapeutic agents against transmembrane proteins have been developed. For the treatment of advanced or metastatic GC, trastuzumab and ramucirumab have been approved [67, 68]. Unfortunately, the benefit from these agents is limited. Epidermal growth factor receptor (EGFR) overexpression occurs in 30–60% of GCs and is associated with a worse prognosis [69]. However, studies evaluating antibody inhibitors of EGFR have failed to demonstrate a survival advantage [70]. MET amplification is associated with a higher tumor stage, more aggressive phenotype, and significantly diminished survival [71]. However, monoclonal antibodies that target MET have limited activity as seen in phase III trials of rilotumumab and onartuzumab [72]. Further study is required to understand gastric carcinogenesis and treat GC.
References
Nakamura K, Sugano H, Takagi K (1968) Carcinoma of the stomach in incipient phase: its histogenesis and histological appearances. Gann 59:251–258
Lauren P (1965) The two histological main types of gastric carcinoma. Diffuse and so-called intestinal type carcinoma: an attempt at histological classification. Acta Pathol Microbiol Scand 64:31–49
Vauhkonen M, Vauhkonen H, Sipponen P (2006) Pathology and molecular biology of gastric cancer. Best Pract Res Clin Gastroenterol 20:651–674
Yasui W, Oue N, Ito R et al (2004) Search for new biomarkers of gastric cancer through serial analysis of gene expression and its clinical implications. Cancer Sci 95:385–392
Oue N, Sentani K, Sakamoto N et al (2015) Clinicopathologic and molecular characteristics of gastric cancer showing gastric and intestinal mucin phenotype. Cancer Sci 106:951–958
Kupzig S, Korolchuk V, Rollason R et al (2003) Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic 4:694–709
Goto T, Kennel SJ, Abe M et al (1994) A novel membrane antigen selectively expressed on terminally differentiated human B cells. Blood 84:1922–1930
Cai D, Cao J, Li Z et al (2009) Up-regulation of bone marrow stromal protein 2 (BST2) in breast cancer with bone metastasis. BMC Cancer 9:102
Wang W, Nishioka Y, Ozaki S et al (2009) HM1.24 (CD317) is a novel target against lung cancer for immunotherapy using anti-HM1.24 antibody. Cancer Immunol Immunother 58:967–976
Mukai S, Oue N, Oshima T et al (2017) Overexpression of transmembrane protein BST2 is associated with poor survival of patients with esophageal, gastric, or colorectal cancer. Ann Surg Oncol 24:594–602
Liu W, Cao Y, Guan Y et al (2018) BST2 promotes cell proliferation, migration and induces NF-kappaB activation in gastric cancer. Biotechnol Lett 40:1015–1027
Chen WV, Maniatis T (2013) Clustered protocadherins. Development 140:3297–3302
Mukai S, Oue N, Oshima T et al (2017) Overexpression of PCDHB9 promotes peritoneal metastasis and correlates with poor prognosis in patients with gastric cancer. J Pathol 243:100–110
Sekino Y, Oue N, Mukai S et al (2019) Protocadherin B9 promotes resistance to bicalutamide and is associated with the survival of prostate cancer patients. Prostate 79:234–242
Liu JY, Jiang L, He T et al (2019) NETO2 promotes invasion and metastasis of gastric cancer cells via activation of PI3K/Akt/NF-kappaB/Snail axis and predicts outcome of the patients. Cell Death Dis 10:162
Yasui W, Oue N, Kuniyasu H et al (2001) Molecular diagnosis of gastric cancer: present and future. Gastric Cancer 4:113–121
Huang KK, Ramnarayanan K, Zhu F et al (2018) Genomic and epigenomic profiling of high-risk intestinal metaplasia reveals molecular determinants of progression to gastric cancer. Cancer Cell 33(137–150):e5
Jiang Y, Qi X, Liu X et al (2017) Fbxw7 haploinsufficiency loses its protection against DNA damage and accelerates MNU-induced gastric carcinogenesis. Oncotarget 8:33444–33456
Oue N, Sentani K, Yokozaki H et al (2001) Promoter methylation status of the DNA repair genes hMLH1 and MGMT in gastric carcinoma and metaplastic mucosa. Pathobiology 69:143–149
Zou XP, Zhang B, Zhang XQ et al (2009) Promoter hypermethylation of multiple genes in early gastric adenocarcinoma and precancerous lesions. Hum Pathol 40:1534–1542
Oue N, Mitani Y, Aung PP et al (2005) Expression and localization of Reg IV in human neoplastic and non-neoplastic tissues: Reg IV expression is associated with intestinal and neuroendocrine differentiation in gastric adenocarcinoma. J Pathol 207:185–198
Tatematsu M, Tsukamoto T, Inada K (2003) Stem cells and gastric cancer: role of gastric and intestinal mixed intestinal metaplasia. Cancer Sci 94:135–141
Endoh Y, Tamura G, Ajioka Y et al (2000) Frequent hypermethylation of the hMLH1 gene promoter in differentiated-type tumors of the stomach with the gastric foveolar phenotype. Am J Pathol 157:717–722
Yokozaki H, Shitara Y, Fujimoto J et al (1999) Alterations of p73 preferentially occur in gastric adenocarcinomas with foveolar epithelial phenotype. Int J Cancer 83:192–196
Endoh Y, Tamura G, Watanabe H et al (1999) The common 18-base pair deletion at codons 418-423 of the E-cadherin gene in differentiated-type adenocarcinomas and intramucosal precancerous lesions of the stomach with the features of gastric foveolar epithelium. J Pathol 189:201–206
Endoh Y, Sakata K, Tamura G et al (2000) Cellular phenotypes of differentiated-type adenocarcinomas and precancerous lesions of the stomach are dependent on the genetic pathways. J Pathol 191:257–263
Wu LB, Kushima R, Borchard F et al (1998) Intramucosal carcinomas of the stomach: phenotypic expression and loss of heterozygosity at microsatellites linked to the APC gene. Pathol Res Pract 194:405–411
Motoshita J, Oue N, Nakayama H et al (2005) DNA methylation profiles of differentiated-type gastric carcinomas with distinct mucin phenotypes. Cancer Sci 96:474–479
Benahmed F, Gross I, Gaunt SJ, et al (2008) Multiple regulatory regions control the complex expression pattern of the mouse Cdx2 homeobox gene. Gastroenterology 135:1238–1247, 47e1–3
Hinoi T, Lucas PC, Kuick R et al (2002) CDX2 regulates liver intestine-cadherin expression in normal and malignant colon epithelium and intestinal metaplasia. Gastroenterology 123:1565–1577
Naito Y, Oue N, Hinoi T et al (2012) Reg IV is a direct target of intestinal transcriptional factor CDX2 in gastric cancer. PLoS ONE 7:e47545
Anami K, Oue N, Noguchi T et al (2010) Search for transmembrane protein in gastric cancer by the Escherichia coli ampicillin secretion trap: expression of DSC2 in gastric cancer with intestinal phenotype. J Pathol 221:275–284
Takakura Y, Hinoi T, Oue N et al (2010) CDX2 regulates multidrug resistance 1 gene expression in malignant intestinal epithelium. Cancer Res 70:6767–6778
Tajima Y, Shimoda T, Nakanishi Y et al (2003) Association of gastric and intestinal phenotypic marker expression of gastric carcinomas with tumor thymidylate synthase expression and response to postoperative chemotherapy with 5-fluorouracil. J Cancer Res Clin Oncol 129:683–690
Mitani Y, Oue N, Matsumura S et al (2007) Reg IV is a serum biomarker for gastric cancer patients and predicts response to 5-fluorouracil-based chemotherapy. Oncogene 26:4383–4393
Kobayashi K, Nakahori Y, Miyake M et al (1998) An ancient retrotransposal insertion causes Fukuyama-type congenital muscular dystrophy. Nature 394:388–392
Oo HZ, Sentani K, Mukai S et al (2016) Fukutin, identified by the Escherichia coli ampicillin secretion trap (CAST) method, participates in tumor progression in gastric cancer. Gastric Cancer 19:443–452
Yang Y, Zhao W, Xu QW et al (2014) IQGAP3 promotes EGFR-ERK signaling and the growth and metastasis of lung cancer cells. PLoS ONE 9:e97578
Johnson M, Sharma M, Henderson BR (2009) IQGAP1 regulation and roles in cancer. Cell Signal 21:1471–1478
Kim H, White CD, Sacks DB (2011) IQGAP1 in microbial pathogenesis: targeting the actin cytoskeleton. FEBS Lett 585:723–729
Mataraza JM, Briggs MW, Li Z et al (2003) IQGAP1 promotes cell motility and invasion. J Biol Chem 278:41237–41245
Schmidt VA, Chiariello CS, Capilla E et al (2008) Development of hepatocellular carcinoma in Iqgap2-deficient mice is IQGAP1 dependent. Mol Cell Biol 28:1489–1502
Nojima H, Adachi M, Matsui T et al (2008) IQGAP3 regulates cell proliferation through the Ras/ERK signalling cascade. Nat Cell Biol 10:971–978
Oue N, Yamamoto Y, Oshima T et al (2018) Overexpression of the transmembrane protein IQGAP3 is associated with poor survival of patients with gastric cancer. Pathobiology 85:192–200
Bessede E, Dubus P, Megraud F et al (2015) Helicobacter pylori infection and stem cells at the origin of gastric cancer. Oncogene 34:2547–2555
Wakamatsu Y, Sakamoto N, Oo HZ et al (2012) Expression of cancer stem cell markers ALDH1, CD44 and CD133 in primary tumor and lymph node metastasis of gastric cancer. Pathol Int 62:112–119
Takaishi S, Okumura T, Wang TC (2008) Gastric cancer stem cells. J Clin Oncol 26:2876–2882
Takaishi S, Okumura T, Tu S et al (2009) Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells 27:1006–1020
Oue N, Mukai S, Imai T et al (2016) Induction of KIFC1 expression in gastric cancer spheroids. Oncol Rep 36:349–355
Rath O, Kozielski F (2012) Kinesins and cancer. Nat Rev Cancer 12:527–539
Imai T, Oue N, Nishioka M et al (2017) Overexpression of KIF11 in gastric cancer with intestinal mucin phenotype. Pathobiology 84:16–24
Zhang S, Huang F, Wang Y et al (2016) KIF2A Overexpression and its association with clinicopathologic characteristics and poor prognoses in patients with gastric cancer. Dis Markers 2016:7484516
Zhang H, Ma RR, Wang XJ et al (2017) KIF26B, a novel oncogene, promotes proliferation and metastasis by activating the VEGF pathway in gastric cancer. Oncogene 36:5609–5619
Weil D, Garcon L, Harper M et al (2002) Targeting the kinesin Eg5 to monitor siRNA transfection in mammalian cells. Biotechniques 33:1244–1248
Mills CC, Kolb EA, Sampson VB (2017) Recent advances of cell-cycle inhibitor therapies for pediatric cancer. Cancer Res 77:6489–6498
Ando A, Kikuti YY, Kawata H et al (1994) Cloning of a new kinesin-related gene located at the centromeric end of the human MHC region. Immunogenetics 39:194–200
DeLuca JG, Newton CN, Himes RH et al (2001) Purification and characterization of native conventional kinesin, HSET, and CENP-E from mitotic hela cells. J Biol Chem 276:28014–28021
Sekino Y, Oue N, Koike Y et al (2019) KIFC1 inhibitor CW069 induces apoptosis and reverses resistance to docetaxel in prostate cancer. J Clin Med 8:225
Krzysiak TC, Grabe M, Gilbert SP (2008) Getting in sync with dimeric Eg5. Initiation and regulation of the processive run. J Biol Chem 283:2078–2087
LoRusso PM, Goncalves PH, Casetta L et al (2015) First-in-human phase 1 study of filanesib (ARRY-520), a kinesin spindle protein inhibitor, in patients with advanced solid tumors. Invest New Drugs 33:440–449
Network CGAR (2014) Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513:202–209
Cristescu R, Lee J, Nebozhyn M et al (2015) Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med 21:449–456
Saito R, Abe H, Kunita A et al (2017) Overexpression and gene amplification of PD-L1 in cancer cells and PD-L1(+) immune cells in Epstein-Barr virus-associated gastric cancer: the prognostic implications. Mod Pathol 30:427–439
Tan P, Yeoh KG (2015) Genetics and molecular pathogenesis of gastric adenocarcinoma. Gastroenterology 149:1153–1162
Forbes SA, Beare D, Gunasekaran P et al (2015) COSMIC: exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res 43:D805–D811
Wang K, Yuen ST, Xu J et al (2014) Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat Genet 46:573–582
Bang YJ, Van Cutsem E, Feyereislova A et al (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376:687–697
Wilke H, Muro K, Van Cutsem E et al (2014) Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 15:1224–1235
Wang KL, Wu TT, Choi IS et al (2007) Expression of epidermal growth factor receptor in esophageal and esophagogastric junction adenocarcinomas: association with poor outcome. Cancer 109:658–667
Lordick F, Kang YK, Chung HC et al (2013) Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol 14:490–499
Nakajima M, Sawada H, Yamada Y et al (1999) The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer 85:1894–1902
Jou E, Rajdev L (2016) Current and emerging therapies in unresectable and recurrent gastric cancer. World J Gastroenterol 22:4812–4823
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research (C) (18K07016) from the Japan Society for the Promotion of Science. This work was also supported by the Takeda Science Foundation. We thank Mitchell Arico from Edanz Group (http://www.edanzediting.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Oue, N., Sentani, K., Sakamoto, N. et al. Molecular carcinogenesis of gastric cancer: Lauren classification, mucin phenotype expression, and cancer stem cells. Int J Clin Oncol 24, 771–778 (2019). https://doi.org/10.1007/s10147-019-01443-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-019-01443-9