Abstract
Objectives
To investigate the functional roles of bone marrow stromal cell antigen 2 (BST2) in gastric cancer (GC) cells and its implications in the development of GC patients.
Results
BST2 was frequently overexpressed in GC tissues compared with the adjacent non-tumorous tissues, and high BST2 expression was correlated with tumor stage and lymphatic metastasis. Furthermore, in vitro experiments demonstrated that knockdown of BST2 by siRNA inhibited cell proliferation, induced apoptosis and repressed cell motility in GC cells. In addition, the pro-tumor function of BST2 in GC was mediated partly through the NF-κB signaling.
Conclusion
BST2 possesses the oncogenic potential in GC by regulating the proliferation, apoptosis, and migratory ability of GC cells, thereby BST2 could be a potential therapeutic target for the treatment of GC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is one of the most common malignancies with high morbidity and mortality. Each year around one million new cases of GC are diagnosed, and about 720,000 patients are estimated died from this disease worldwide (Bertuccio et al. 2009; Ferlay et al. 2015, 2013). Current treatment approaches including surgery, radiotherapy and chemotherapy can achieve certain effects. However, the prognosis in advanced GC remains extremely poor, which are primarily because of the high recurrence and metastasis rates (Cunningham et al. 2006; De Vita et al. 2014). Therefore, it is important to search for candidates that involve in GC development and value for treatment strategy.
Bone marrow stromal cell antigen 2 (BST2) is also known as CD317, tetherin, or HM 1.24 antigen (Kupzig et al. 2003; Mahauad-Fernandez and Okeoma 2016). It is constitutively detected in bone marrow stromal cells, dendritic cells, and terminally differentiated B cells, and plays critical roles in antiretroviral defense in the innate immune response (Neil et al. 2008). Recent studies have demonstrated that BST2 is also aberrantly expressed in many cancers, overexpression of BST2 is shown in breast cancer (Cai et al. 2009; Mahauad-Fernandez et al. 2014), bladder cancer (Shigematsu et al. 2017), oral cavity cancer (Fang et al. 2014), and multiple myeloma (Ozaki et al. 1997). In addition, anti-HM1.24 antibody therapy has been demonstrated effective in patients with multiple myeloma (Harada and Ozaki 2014), which suggested BST2 a strong potential for cancer therapy. Recently, Mukai et al. (Mukai et al. 2017) has demonstrated that BST2 is overexpressed in gastrointestinal cancer, and it may predict poor prognoses. However, its functional roles in GC require further investigation.
In this study, we first investigated the expression pattern of BST2 in clinical GC samples and analyzed its possible correlation with clinical parameters. Subsequently, the biological effects of BST2 on cell proliferation, apoptosis, and migratory ability were addressed by loss-of-function assay in vitro.
Materials and methods
Tissue specimens
A total of 95 paraffin-embedded GC samples were obtained from the Shengjing Hospital of China Medical University. None of the patients had received chemotherapy or radiotherapy before surgery. The clinical parameters of GC samples including age, histological type, tumor size, lymph nodes metastasis, TNM stage were summarized in Table 1, and the carcinomas were evaluated by using the American Joint Commmittee on Cancer (AJCC), 7th edition, staging system. Seven fresh GC specimens and paired adjacent non-cancerous tissues (located more than 5 cm away from cancer tissues) were immediately collected for BST2 protein analysis. The study was approved by the Ethical Committee of Shengjing Hospital of China Medical University.
Immunohistochemical analysis
Expression of BST2 in paraffin-embedded GC tissues was measured by immunohistochemical analysis. The paraffin-embedded sections (5-μm-thick) were processed with xylene, rehydrated and dehydrated by a gradient alcohol series, and heat-induced antigen retrieval. Thereafter, the sections were incubated with the primary antibody of BST2 (1:100, Abcam, Cambridge, UK) at 4 °C overnight, followed by incubation with peroxidase-conjugated streptavidin for 30 min. Images were analyzed by standard light microscope. BST2 expression was quantified according to the percentage of cells stained (0–100%) and the intensity of cell staining (3: strong; 2: moderate; 1: weak; or 0: no cell staining). The merged overall score was used for further analysis.
Cell culture and transfection
Human gastric cancer cell lines NCI-N87, MGC-803, SGC-7901, BGC-823, and a normal gastric cell line GES-1 were purchased from Chi Scientific Co. LTD (Shanghai, China). The cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) and kept at 37 °C in a humidified atmosphere of 5% CO2. For silencing BST2, short interfering RNAs (siRNA) targeting BST2 were synthesized by GenePharma (Shanghai, China), and then transfected into MGC-803 and SGC-7901 cells using lipofectamin 2000 reagent (Invitrogen, Carlsbad, CA, USA). The cells were subjected to subsequent experiments at 24 or 48 h post-transfection.
Real-time PCR
Total RNAs from cells were extracted using a simple Total RNA Kit (BioTeke, Beijing, China), then converted into cDNA using Super MMLV Reverse Transcriptase (BioTeke). Real-time PCR was performed with SYBR Green Master Mix (Solarbio, Beijing, China) in a quantitative fluorescence analyzer, and the specific primers used as follows: BST2, 5′- TTCTGGGGGTGCCCTTGATT -3′ (forward) and 5′- GGAGATGGGTGACATTGCGA -3′ (reverse). β-actin, 5′-CTTAGTTGCGTTACACCCTTTCTTG-3′ (forward) and 5′-CTGTCACCTTCACCGTTCCAGTTT-3′ (reverse).
Western blot
Western blot analysis was performed as described previously (Tang et al. 2016). Briefly, total proteins were extracted from cells using RIPA Lysis Buffer (Beyotime Institute of Biotechnology, Haimen, China). Equal amounts of proteins from each sample were separated by SDS-PAGE and transferred to PVDF membranes (Millipore, Bedford, MA, USA). The membranes were incubated with specific primary antibody overnight at 4 °C followed by incubation with corresponding secondary antibody for 45 min at room temperature. At last, the targeted blots were visualized using enhanced chemiluminescence reagent (Beyotime) and analyzed using the Gel-Pro Analyzer software. Primary antibodies included anti-BST2, and p65 (1:1000, Proteintech, Sangying, Wuhan, China), anti-cyclin A, and D1 (1:500, Boster, Wuhan, China), anti-Bcl-2, and Bax (1:1000, Sangon Biotech, Shanghai, China), anti-cleaved caspase-3 (1:1000, Cell signaling technology, USA), anti IκBα, and p-IκBα (1:1000 and 1:500, Bioss, Shanghai, China), anti-MMP-2, and MMP-9 (1:1000, Sangon Biotech, Shanghai, China).
MTT assay
The cell proliferation potential among different groups was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Briefly, cells at a density of 3 × 103 per well were seeded in 96-well plates, the cell growth was monitored at the indicated time point (24, 36, 48, and 72 h). Thereafter, MTT (5 mg/ml) was added to each well and incubated for additional 4 h. The optical density (OD) was measured at a wave-length of 570 nm under a microplate reader.
Flow cytometric analyses
For cell cycle analysis, cells were fixed with 70% ethanol, incubated in staining buffer containing propidium iodide (PI) and RNase A, and then analyzed by flow cytometry (BD Pharmingen, San Jose, CA, USA). For apoptosis analysis, collected cells were resuspended in binding buffer, followed by staining with annexin V-FITC and Propidium iodide (PI). Apoptotic cells were analyzed using the BD Accuri™ C6 flow cytometer (BD Pharmingen).
Wound-healing assay
Cell motility was assessed by a wound-healing assay. Briefly, cells were seeded in six-well plates and grown to 80% confluence. Then the cells were cultured in serum-free medium containing 1 μg/ml Mitomycin C (Sigma, St. Louis, MO, USA) for 1 h. A wound scratch was created on the cell monolayer with a 200 μl pipette tip. The cells were subsequently washed twice and cultured in complete medium. Wound gaps were observed for 48 h, and images were captured every 24 h at 100× magnification, then the migration rate was calculated accordingly.
Immunofluorescence
The cells were grown on glass coverslip until 80% confluent and then fixed with 4% formaldehyde and permeabilized with 0.1% Triton X-100. Thereafter, the cell slides were blocked with normal goat serum (Solarbio, Beijing, China) and incubated with a rabbit NF-кB p65 polyclonal antibody (1:50, Proteintech) at 4 °C overnight. Next, the cell slices were incubated with FITC-labeled goat anti-rabbit secondary antibody (1:500, Beyotime) at room temperature for 1 h, followed by counterstaining with DAPI. Images were analyzed under an OLYMPUS microscope with magnification 400×.
Statistical analyses
All data are expressed as the mean ± standard deviation (SD) and analyzed using GraphPad Prism 5.0 software (San Diego, CA, USA). A value of p < 0.05 was considered to be statistically significant. Correlation between BST2 expression and relevant clinical parameter was performed using the Chi square test or Fisher’s exact test. Between-group comparisons were analyzed using one-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test.
Results
Expression of BST2 was upregulated in human GC tissues
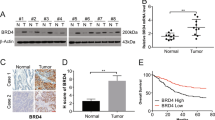
To determine the expression of BST2 in GC tissues, we first examined BST2 protein expression from seven paired GC tissues. Western blot results showed that BST2 was frequently overexpressed in GC tissues (5/7 cases) compared with the paired nontumorous tissue (Fig. 1a, p < 0.01). Next, we analyzed BST2 expression in 95 paired GC samples by immunohistochemical staining. As shown in Fig. 1b, weak or no staining of BST-2 was observed in nontumorous tissues, while BST2 expression was upregulated in GC tissues. Among 95 cases of GC samples, BST2 expression was high in 61 (64.2%) cases and low/negative in 34 (35.8%) cases. We further evaluated the association between BST2 expression and clinical-pathological parameters (Table 1). High BST2 expression was signifcantly associated with tumor-node-metastasis (TNM) stage (P = 0.0267) and lymph node metastasis (P = 0.005). Together, these results indicated BST2 is frequently overexpressed in GC tissues and was possibly associated with GC TNM stage and lymph node metastasis.
BST2 expression was increased in GC tissues. a Western blot analysis of BST2 expression in GC tissues (C) and matched adjacent nontumorous tissues (N).β-actin served as the internal control for densitometric analysis (n = 7, p < 0.05). b Representative immunohistochemical images of BST2 expression in paratumorous tissues and GC tissues. Magnification 400×, scale bar = 50 μm
Establishment of BST2-silenced GC cell lines
To investigate the biological functions of BST2 in GC cells, we first examined the expression levels of BST2 in four GC cell lines (NCI-N87, MGC-803, SGC-7901, and BGC-823) and one normal gastric cell line GES-1 by real-time PCR and Western blot (Fig. 2a). Among these cell lines, MGC-803 and SGC-7901 cells exhibited relatively high levels of BST2 than that in the normal GES-1 cells and other GC cells (NCI-N87 and BGC-823). Therefore, the MGC-803 and SGC-7901 cells were chosen for silencing of BST2 by siRNAs targeted BST2. The efficiency of transfection was confirmed by real time-PCR (Fig. 2b) and Western blot analysis (Fig. 2c). The mRNA and protein levels of BST2 significantly decreased in BST2-silenced MGC-803 and SGC-7901 cells (p < 0.01 vs. control cells), confirmed the construction of BST2-silenced GC cell lines.
Establishment of BST2-silenced GC cell lines and effect of BST2 silencing on cell proliferation. a The mRNA and protein levels of BST2 in different GC cell lines and one normal gastric cell line were detected by real-time PCR and Western blot. b MGC-803 and SGC-7901 cells were transfected with BST2 siRNA-1, -2, or control siRNA, the mRNA expression of BST2 in both cells was determined by real-time PCR. c The protein levels of BST2 in MGC-803 and SGC-7901 cells were determined by Western blot analysis, β-actin served as a loading control. d Cell proliferation was assessed by MTT assay at 24, 36, 48, and 72 h after seeding on 96-well plates. *p < 0.05 or **p < 0.01, compared with control siRNA-transfected cells
Silencing of BST2 inhibited cell proliferation and induced cell cycle arrest in GC cells
We next evaluated the effects of BST2 on cell proliferation by employing the cells acquired above. MTT results demonstrated that BST2 silencing significantly reduced the proliferation of MGC-803 and SGC-7901 cells compared with control cells (Fig. 2d, p < 0.01 or p < 0.05 vs. control cells). These results indicated that BST2 promotes the proliferation of GC cells.
It has been shown that the cell cycle is closely associated with the regulation of cell proliferation (Sherr 1996). We further examined whether BST2′s effect on cell proliferation was related with cell cycle. Representative images of cell cycle distribution in BST2-silenced MGC-803 and SGC-7901 cells were presented in Fig. 3a, b. Flow cytometry results showed that downregulation of BST2 significantly increased the cell numbers in G1 phase (p < 0.05 vs. control cells), and reduced the cell numbers in S phase (p < 0.01), implying G1/G0 cell cycle arrest by BST2 silencing. We further determined the protein levels of cell cycle regulators by Western blot. As excepted, knockdown of BST2 markedly downregulated the protein levels of cyclin A, and cyclin D1 in both MGC-803 and SGC-7901 cells (Fig. 3c, p < 0.01). Together, these results indicated that BST2-mediated proliferation of GC cells was associated with cell cycle regulation.
Silencing of BST2 induced cell cycle arrest in GC cells. a, b Parental, control, and BST2-silenced cells were stained with propidium iodide and then subjected to flow cytometry for cell cycle analysis. c Western blot analysis of cell cycle-related cyclinA, and D1 protein levels in different groups of MGC-803 and SGC-7901 cells. β-actin served as a loading control. *p < 0.05 or **p < 0.01, compared with control siRNA -transfected cells
Silencing of BST2 induced apoptosis in GC cells
We next examined the effect of BST2 silencing on cell apoptosis. Cell apoptosis status was analyzed with Annexin V/PI double staining. Flow cytometry data showed that the number of apoptotic cells significantly increased in BST2-silenced MGC-803 and SGC-7901 cells compared with control cells (Fig. 4a, b; p < 0.01). The levels of apoptotic-related factors were further detected by Western blot (Fig. 4c). Expression of anti-apoptotic Bcl-2 was markedly reduced in BST2-silenced MGC-803 and SGC-7901 cells compared with control cells. Meanwhile, the levels of pro-apoptotic factors Bax and cleaved caspase-3 were significantly increased in BST2-silenced cells. Together, these results indicated that downregulation of BST2 enhanced apoptosis in GC cells and BST2 could regulate apoptotic-related genes expression.
Silencing of BST2 promoted apoptosis in GC cells. a, b The cells were double stained with FITC-conjugated anti-Annexin V antibody and propidium iodide, and then subjected to flow cytometry for apoptosis analysis. Cells in the lower right and upper right quadrants were considered as apoptotic cells. c Western blot analysis of Bcl-2, Bax, cleaved caspase-3, and pro-caspase-3 in MGC-803 and SGC-7901 cells with or without BST2 silencing. β-actin served as a loading control. *p < 0.05 or **p < 0.01, compared with control siRNA-transfected cells
Downregulation of BST2 inhibited the migration of GC cells
Migration of cancer cells is a key step for cancer metastasis, thus we performed the wound-healing assay to evaluate the cell motility in MGC-803 and SGC-7901 cells. As shown in Fig. 5a, the migration distance of BST2-silenced cells was lower than that of control cells at 24 or 48 h (Fig. 5b; p < 0.01), indicated reduced cellular mobility by BST2 silencing. Moreover, we detected the expression of MMP-2 and MMP-9 in MGC-803 and SGC-7901 cells with or without BST2 silencing by Western blot. BST2 silencing markedly downregulated MMP-2 and MMP-9 expression (Fig. 5e, f; p < 0.01). Together, these results suggested that BST2 was a negative regulator of GC cells’ motility.
Downregulation of BST2 inhibited the migration of GC cells. a The migratory ability of MGC-803 and SGC-7901 cells was measured by wound-healing assay after BST2 silencing. The migration rate was determined accordingly. b Western blot analysis of MMP-2 and MMP-9 expression in MGC-803 and SGC-7901 cells with or without BST2 silencing. **P < 0.01, compared with control siRNA -transfected cells
Silencing of BST2 in GC cells inhibited the NF-κB signaling pathway
The NF-κB signaling pathway has been demonstrated to involve in numerous cellular processes, such as the proliferation, cell cycle and apoptosis (Biswas et al. 2004; Karin and Ben-Neriah 2000). Previous studies also reported that BST2 was an activator of NF-κB signaling (Galao et al. 2012; Kuang et al. 2017). Here we further examined the effect of BST2 on the NF-κB signaling in GC cells. Western blot results showed that the phosphorylation levels of IκBα, and nuclear p65 were significantly decreased in BST2-silenced cells compared to control cells (Fig. 6a; p < 0.05 or p < 0.01), suggested BST2 silencing inhibited the NF-κB signaling in GC cells. Consistently, immunoflorescence results also confirmed low levels of nuclear p65 appeared in BST2-silenced MGC-803 and SGC-7901cells (Fig. 6b), indicating decreased nuclear translocation of NF-κB p65 by BST2 silencing Together, these results suggested that BST2 silencing inhibited the NF-κB signaling pathway in GC cells.
Silencing of BST2 inhibited the NF-κB signaling pathway. a The protein levels of IκBα, p-IκBα, and nuclear p65 in MGC-803 and SGC-7901 cells were determined by Western blot analysis. Nuclear p65 was normalized to the nuclear marker protein Histone H3, and other proteins were normalized to β-actin. *p < 0.05 or **p < 0.01, compared with control siRNA -transfected cells. b Representative immunofluorescent images of cytoplasmic and nuclear p65 subunit of NF-κB localization, magnification 400× , scale bar = 50 μm
Discussion
Increasing evidence has demonstrated that BST2 is aberrantly expressed in multiple malignancies (Fang et al. 2014), and BST2 in the involvement of carcinogenesis has attracted much attentions (Mahauad-Fernandez et al. 2014; Wang et al. 2009). In accordance with previous studies, here we demonstrated that BST2 was overexpression in GC tissues compared with adjacent non-tumorous tissues, and high BST2 expression was correlated with tumor stage and lymphatic metastasis. Furthermore, in vitro experiments showed that knockdown of BST2 by siRNA interference inhibited cell proliferation, induced apoptosis and repressed cell motility of GC cells. In addition, the pro-tumor role of BST2 in GC cells was related to NF-κB activation. Together, these results suggested that BST2 may be a potential therapeutic target for the treatment of GC.
Uncontrolled cell growth and metastasis are key steps for tumor progression. Unlimited cell growth is closely associated with the imbalance between tumor cell proliferation and apoptosis. (Fan et al. 2010; Qi et al. 2012). The cell cycle is the fundamental process of cell proliferation, and it is regulated by cyclins and cyclin-dependent protein kinases (Hochegger et al. 2008; Marx 1994). Apoptosis is regulated by multiple apoptosis-related proteins, such as Bcl-2 and Bax (Cory and Adams 2002). Previous studies have shown that BST2 is essential for the regulation of cell proliferation and apoptosis in tumor cells. Forced BST2 expression in breast cancer cells increased the proliferation and the cell population at the S phase of cell cycles (Cai et al. 2009). Reduced BST2 expression inhibited the growth and invasive ability of renal cell carcinoma cells (Pham et al. 2017). In addition, knockdown of BST2 in nasopharyngeal cancer cells promoted cisplatin-induced apoptosis (Kuang et al. 2017), implying pro-tumor role of BST2. To evaluate the functional roles of BST2 in GC, we silenced BST2 expression in GC cells. Our results showed that silencing of BST2 inhibited cell proliferation, induced cell cycle arrest in G0/G1 phase, and promoted apoptosis in GC cells. Furthermore, knockdown of BST2 downregulated the expression levels of cell cycle related factors (cyclins) and apoptosis related factors (Bcl-2), which indicated BST2 through regulating these genes expression, thereby modulates GC cell proliferation and apoptosis. Moreover, we noticed that BST2 knockdown inhibited the motility of GC cells and suppressed the expression of MMP-2 and MMP-9. As previously reported, the expression level of MMPs were associated with the metastatic ability of cancer cells (Velinov et al. 2010). Abnormal expression of MMP-2 and MMP-9 often detected in solider tumor tissues and was associated with metastasis (Chu et al. 2011; Tang et al. 2016). Consistently, here we demonstrated BST2-mediated upregulation of MMP2 and MMP9 played an important role in GC cell metastasis.
NF-κB is a ubiquitous transcription factor that activated by a variety of stimuli and involved in numerous cell biological processes, including cell proliferation, cell cycle progression, and apoptosis (Biswas et al. 2004; Karin and Ben-Neriah 2000). The induction of NF-κB signaling requires activation of the IKK complex, followed by subsequent IκB degradation and NF-κB nuclear translocation (Kanarek and Ben-Neriah 2012; Vallabhapurapu and Karin 2009). NF-κB-driven gene products include cytokines/chemokines, pro and anti-apoptotic factors, and matrix metalloproteinases, and many of them contribute to carcinogenesis and cancer progression (Sokolova and Naumann 2017). Indeed, NF-κB is constitutively activated in human cancers (Maeda and Omata 2008; Sasaki et al. 2001), inhibition of this signaling may provide potential therapeutic targeting. Previous evidence has shown that BST2 acts BST-2 as an activator of NF-κB and exerts antiviral properties (Tokarev et al. 2013). Further, Kuang et al. revealed that BST2-mediated NF-κB activation was associated with cisplatin resistance in nasopharyngeal cancer cells (Kuang et al. 2017). These results indicated BST2-mediated NF-κB activation could play an important role in GC cells. As expected, our results demonstrated knockdown of BST2 markedly increased the IκBα levels and downregulated p-IκBα and nuclear p65 levels. Immunofluorescence further confirmed less nuclear translocation of p65 in BST2-silenced cells. Together, these results suggested that silencing of BST2 inhibits the activation of NF-κB, BST2-mediated NF-κB activation may play an important role in GC cells, and the contribution of NF-κB signaling to BST2-medicated proliferation and migration warrants further study. Considering that BST-2 acts as a critical regulator both in innate immune response and tumorigenesis, it could be a strong candidate for the treatment of GC.
In conclusion, our results demonstrate that BST2 is overexpressed in GC tissues and it correlates with tumor stage and lymphatic metastasis. Further in vitro studies indicate that BST2 silencing inhibits cell proliferation and migration, partly by regulating the NF-κB signaling and its downstream proteins. Thereby, BST2 could be a potential therapeutic for future GC therapies.
References
Bertuccio P et al (2009) Recent patterns in gastric cancer: a global overview. Int J Cancer 125:666–673. https://doi.org/10.1002/ijc.24290
Biswas DK, Shi Q, Baily S, Strickland I, Ghosh S, Pardee AB, Iglehart JD (2004) NF-kappa B activation in human breast cancer specimens and its role in cell proliferation and apoptosis. Proc Natl Acad Sci USA 101:10137–10142. https://doi.org/10.1073/pnas.0403621101
Cai D, Cao J, Li Z, Zheng X, Yao Y, Li W, Yuan Z (2009) Up-regulation of bone marrow stromal protein 2 (BST2) in breast cancer with bone metastasis. BMC cancer 9:102. https://doi.org/10.1186/1471-2407-9-102
Chu D, Zhang Z, Li Y, Zheng J, Dong G, Wang W, Ji G (2011) Matrix metalloproteinase-9 is associated with disease-free survival and overall survival in patients with gastric cancer. Int J Cancer 129:887–895. https://doi.org/10.1002/ijc.25734
Cory S, Adams JM (2002) The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2:647–656. https://doi.org/10.1038/nrc883
Cunningham D et al (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. New Engl J Med 355:11–20. https://doi.org/10.1056/NEJMoa055531
De Vita F et al (2014) Clinical management of advanced gastric cancer: the role of new molecular drugs. World J Gastroenterol 20:14537–14558. https://doi.org/10.3748/wjg.v20.i40.14537
Fan YZ, Zhao ZM, Fu JY, Chen CQ, Sun W (2010) Norcantharidin inhibits growth of human gallbladder carcinoma xenografted tumors in nude mice by inducing apoptosis and blocking the cell cycle in vivo. Hepatobiliary Pancreat Dis Int 9:414–422
Fang KH et al (2014) Overexpression of BST2 is associated with nodal metastasis and poorer prognosis in oral cavity cancer. The Laryngoscope 124:E354–360. https://doi.org/10.1002/lary.24700
Ferlay J et al (2013) Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 49:1374–1403. https://doi.org/10.1016/j.ejca.2012.12.027
Ferlay J et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–386. https://doi.org/10.1002/ijc.29210
Galao RP, Le Tortorec A, Pickering S, Kueck T, Neil SJ (2012) Innate sensing of HIV-1 assembly by Tetherin induces NFkappaB-dependent proinflammatory responses. Cell Host Microbe 12:633–644. https://doi.org/10.1016/j.chom.2012.10.007
Harada T, Ozaki S (2014) Targeted therapy for HM1.24 (CD317) on multiple myeloma cells. Biomed Res Int 2014:965384. https://doi.org/10.1155/2014/965384
Hochegger H, Takeda S, Hunt T (2008) Cyclin-dependent kinases and cell-cycle transitions: does one fit all? Nat Rev Mol Cell Biol 9:910–916. https://doi.org/10.1038/nrm2510
Kanarek N, Ben-Neriah Y (2012) Regulation of NF-kappaB by ubiquitination and degradation of the IkappaBs. Immunol Rev 246:77–94. https://doi.org/10.1111/j.1600-065X.2012.01098.x
Karin M, Ben-Neriah Y (2000) Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol 18:621–663. https://doi.org/10.1146/annurev.immunol.18.1.621
Kuang CM et al (2017) BST2 confers cisplatin resistance via NF-kappaB signaling in nasopharyngeal cancer. Cell Death Dis 8:e2874. https://doi.org/10.1038/cddis.2017.271
Kupzig S, Korolchuk V, Rollason R, Sugden A, Wilde A, Banting G (2003) Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic 4:694–709
Maeda S, Omata M (2008) Inflammation and cancer: role of nuclear factor-kappaB activation. Cancer Sci 99:836–842. https://doi.org/10.1111/j.1349-7006.2008.00763.x
Mahauad-Fernandez WD, Okeoma CM (2016) The role of BST-2/Tetherin in host protection and disease manifestation. Immunity, inflammation and disease 4:4–23. https://doi.org/10.1002/iid3.92
Mahauad-Fernandez WD, DeMali KA, Olivier AK, Okeoma CM (2014) Bone marrow stromal antigen 2 expressed in cancer cells promotes mammary tumor growth and metastasis. Breast Cancer Res 16:493. https://doi.org/10.1186/s13058-014-0493-8
Marx J (1994) How Cells Cycle Toward Cancer. Science 263:319–321
Mukai S et al (2017) Overexpression of transmembrane protein BST2 is associated with poor survival of patients with esophageal, gastric, or colorectal cancer. Ann Surg Oncol 24:594–602. https://doi.org/10.1245/s10434-016-5100-z
Neil SJ, Zang T, Bieniasz PD (2008) Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451:425–430. https://doi.org/10.1038/nature06553
Ozaki S, Kosaka M, Wakatsuki S, Abe M, Koishihara Y, Matsumoto T (1997) Immunotherapy of multiple myeloma with a monoclonal antibody directed against a plasma cell-specific antigen, HM1.24. Blood 90:3179–3186
Pham QT et al (2017) The expression of BTS-2 enhances cell growth and invasiveness in renal cell carcinoma. Anticancer Res 37:2853–2860. https://doi.org/10.21873/anticanres.11637
Qi S et al (2012) ZEB2 mediates multiple pathways regulating cell proliferation, migration, invasion, and apoptosis in glioma. PLoS ONE 7:e38842
Sasaki N et al (2001) Nuclear factor-kappaB p65 (RelA) transcription factor is constitutively activated in human gastric carcinoma tissue. Clin Cancer Res 7:4136–4142
Sherr CJ (1996) Cancer cell cycles Science 274:1672–1677
Shigematsu Y et al (2017) Overexpression of the transmembrane protein BST-2 induces Akt and Erk phosphorylation in bladder cancer. Oncol Lett 14:999–1004. https://doi.org/10.3892/ol.2017.6230
Sokolova O, Naumann M (2017) NF-kappaB Signaling in Gastric Cancer Toxins 9 https://doi.org/10.3390/toxins9040119
Tang Y, Lv P, Sun Z, Han L, Zhou W (2016) 14-3-3beta Promotes Migration and Invasion of Human Hepatocellular Carcinoma Cells by Modulating Expression of MMP2 and MMP9 through PI3 K/Akt/NF-kappaB Pathway. PLoS ONE 11:e0146070. https://doi.org/10.1371/journal.pone.0146070
Tokarev A, Suarez M, Kwan W, Fitzpatrick K, Singh R, Guatelli J (2013) Stimulation of NF-kappaB activity by the HIV restriction factor BST2. J Virol 87:2046–2057. https://doi.org/10.1128/JVI.02272-12
Vallabhapurapu S, Karin M (2009) Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol 27:693–733. https://doi.org/10.1146/annurev.immunol.021908.132641
Velinov N, Poptodorov G, Gabrovski N, Gabrovski S (2010) The role of matrixmetalloproteinases in the tumor growth and metastasis. Khirurgiia 1:44–49
Wang W et al (2009) HM1.24 (CD317) is a novel target against lung cancer for immunotherapy using anti-HM1.24 antibody. Cancer Immunol Immunother 58:967–976. https://doi.org/10.1007/s00262-008-0612-4
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Liu, W., Cao, Y., Guan, Y. et al. BST2 promotes cell proliferation, migration and induces NF-κB activation in gastric cancer. Biotechnol Lett 40, 1015–1027 (2018). https://doi.org/10.1007/s10529-018-2562-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-018-2562-z