Abstract
Purpose
Although recurrence after hepatectomy for colorectal liver metastases (CRLM) is common, the optimal treatment strategy remains unclear. The aims of this study were to clarify the impact of repeat surgery and identify the predictive factors for repeat surgery.
Methods
Among the 170 patients who underwent potentially curative surgery for CRLM, 113 developed recurrence. The predictive factors for the performance of repeat surgery were identified and a predictive model was constructed.
Results
The patterns of recurrence were as follows; single site [n = 100 (liver, n = 61; lung, n = 22; other, n = 17)], multiple site (n = 13). Repeat surgery was performed in 54 patients (47.8%) including re-hepatectomy (n = 25), radiofrequency ablation (n = 12), and resection of the extrahepatic recurrent disease (n = 17), and their overall survival (OS) was significantly better than that of those who could not (5-year OS 60.7 vs 19.5%, P < 0.0001). A multivariate analysis revealed that a primary N-negative status [relative risk (RR) 2.93, P = 0.017], indocyanine retention rate at 15 min ≤ 10% before hepatectomy (RR 2.49, P = 0.04), and carcinoembryonic antigen ≤ 5 ng/mL before hepatectomy (RR 2.96, P = 0.017) independently predicted the performance of repeat surgery. For patients who did not present any factors, the probability of repeat surgery was 19.6%. The addition of each subsequent factor increased the probability to 41.9, 67.8, and 84.0% (for 1, 2, and 3 factors, respectively).
Conclusions
Repeat surgery for not only intrahepatic but also extrahepatic recurrence is crucial for prolonging the survival of CRLM patients. The proposed model may help to predict the possibility of repeat surgery and provide optimal individualized treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer is the third most common form of malignancy in the world [1]. The liver is the most common site of metastasis and approximately 50% of patients with colorectal cancer develop liver metastasis at some point during their disease course [2,3,4]. Surgical resection in combination with systemic chemotherapy is currently the treatment of choice and can offer the possibility of long-term survival or a cure. However, more than half of the patients with colorectal liver metastases metastasis (CRLM) develop recurrence after hepatectomy—in the majority of such cases, recurrence takes place within 2 years [5,6,7].
Repeat surgery for recurrence after hepatectomy has been shown to carry a survival benefit [8,9,10,11,12,13], even in patients with early recurrence [14]. However, recent advances in oncosurgical approaches, consisting of aggressive surgery for intrahepatic, extrahepatic, and recurrent disease, perioperative chemotherapy and the advent of specific treatment techniques such as radiofrequency ablation (RFA), portal vein embolization, and two-stage hepatectomy—which may facilitate curative treatment in specific settings—have dramatically changed the treatment strategy for patients with recurrence after hepatectomy for CRLM. Thus, the indications and the outcomes of resection in patients with recurrent disease remain uncertain and the patients who are very probable to be able to receive repeat surgery for recurrent disease have not yet been identified.
The aim of the present study was to clarify the oncological benefit of repeat surgery for recurrence after hepatectomy for CRLM. We also identified the predictive factors and developed a predictive model for the performance of repeat surgery.
Patients and methods
Patients who underwent curative-intent hepatectomy for CRLM between September 2000 and April 2016 at Kumamoto University Hospital, Kumamoto, Japan, were retrospectively identified from a prospectively maintained database. Patients who underwent repeat surgery for CRLM were excluded. Additional information was supplemented from a review of the medical records of each patient. This study was approved by the Human Ethics Review Committee of the Graduate School of Life Sciences, Kumamoto University, Kumamoto, Japan. Written informed consent was obtained from all the patients prior to treatment.
The preoperative workup
Before hepatectomy, all patients underwent routine laboratory tests, including the measurement of the serum carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9 levels, and liver function tests, including the indocyanine retention rate at 15 min (ICG-R15) and 99mTc-galactosyl human serum albumin (GSA) scintigraphy. Routine imaging modalities, including ultrasonography (US), enhanced computed tomography (CT), and magnetic resonance imaging (MRI), were performed to determine the disease stage. Preoperative chemotherapy was administered to patients with initially unresectable or marginally resectable disease, including those with concomitant extrahepatic disease in a conversion setting, or to patients with disease that was thought to be highly malignant (including those who were diagnosed synchronously, patients with a greater number of tumors, and patients with higher of tumor marker levels) in a neoadjuvant setting [15]. The response to chemotherapy was evaluated by CT, according to the Response Evaluation Criteria in Solid Tumors (RECIST) [16, 17].
Surgery
The objective of surgery was to remove all detectable lesions with a tumor-free margin. The type of hepatectomy was based on the preoperative imaging findings, intraoperative US, and the liver functional reserve [15]. In principal, non-anatomical partial hepatectomy was selected if the tumor location allowed. Portal vein embolization was performed if the future remnant liver was too small. RFA during hepatectomy was performed to treat unresectable or tumors that were located deep within the remnant liver to spare the liver parenchyma (in principle for metastatic tumors of ≤ 2 cm in size) [18]. In case with concomitant extrahepatic disease, sequential resection was considered when both sites were deemed to be resectable.
The postoperative workup
After treatment, all patients underwent regular follow-up examinations with imaging and their tumor marker levels were estimated. Postoperative chemotherapy was usually recommended.
The treatment strategy for recurrence after hepatectomy was basically same with the first hepatectomy; that is, recurrent disease was treated surgically only when the overall strategy was considered to be potentially curative, often in combination with chemotherapy. For intrahepatic recurrence, as with the first hepatectomy, hepatectomy with a tumor-free margin was indicated as a first-line choice. RFA was used for the treatment of unresectable or deeply located tumors which required extended resection. For extrahepatic recurrence, surgical resection was considered when the tumors were deemed to be resectable and controllable under chemotherapy.
Statistical analysis
Continuous variables were expressed as the median (range) and were compared using the Mann–Whitney U test. Categorical variables were compared using the χ2 test. Survival analyses were performed using the Kaplan–Meier method and the results were compared using the log-rank test. Overall survival (OS) was calculated from the date of initial hepatectomy until death or last follow-up. Disease-free survival (DFS) was calculated from the date of initial hepatectomy or the last potentially curative surgery for concomitant extrahepatic disease (if present) until the date of recurrence or death. For the univariate analysis of the factors that predicted repeat surgery, the optimal cutoff values of the continuous variables for differentiation between the groups were determined based on a receiver operating characteristics (ROC) analysis. For CEA, CA19-9, and ICG-R15, the upper limit of the normal range (CEA 5 ng/mL, CA19-9 37 U/mL, ICG-R15 10%) was used as a cutoff value because the cutoff values that were determined by the ROC analysis were lower than their upper limit of the normal range. Variables for which the P value in the univariate analysis was < 0.10 were included in a subsequent multivariate logistic regression analysis using a stepwise backward elimination procedure. A predictive model was then created based on the results of the multivariate logistic analysis. To identify independent prognostic factors, multivariate cox regression analysis was performed using a stepwise backward elimination procedure. All the statistical analyses were performed using the JMP (SAS institute, Cary, NC, USA) and R (version 3.1.1; http://www.r-project.org) software programs. P values of < 0.05 were considered to indicate statistical significance.
Results
Among the 193 patients who underwent hepatectomy for CRLM during the study period, 179 patients underwent initial hepatectomy and were eligible for inclusion in the present study (whole cohort). The demographic and clinical characteristics of study patients are summarized in Table 1. There were 117 male patients and 62 female patients; the median age of the patients was 64 (range 25–94) years. The primary tumor was located in the colon in 108 patients and in the rectum in 71 patients. Liver metastases were diagnosed synchronously (before, during or within 6 months after colorectal resection) in 105 (58.7%) patients and bilobar distribution was observed in 70 (39.3%) patients. The median number of tumors was 2 (1–19) and the median tumor size was 28 (3–160) mm. Concomitant extrahepatic disease was present in 14 (7.8%) patients; the locations included the lung (n = 9), lymph nodes (n = 3), colorectal local (n = 1), and lung + bone (n = 1). Preoperative chemotherapy was administered to 96 patients (53.6%), with a median number of 1 line (range 1–3) and 6 cycles (range 2–38). Oxaliplatin-based chemotherapy was administered in most of the patients who received preoperative chemotherapy (89.6%). Biologic agents were used for 58 patients (60.4%).
The median length of follow-up was 43.0 (3–147) months after the diagnosis of CRLM and 36.5 (0.4–146) months after hepatectomy. Nine patients could not undergo curative surgery because their concomitant extrahepatic disease was not resected. The cumulative OS at 1, 3, and 5 years was 94.7, 69.1 and 51.9%, respectively, while the cumulative DFS was 45.0, 30.3 and 21.4% (Fig. 1).
Repeat surgery for recurrence
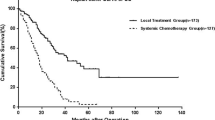
Of the 179 patients who underwent first hepatectomy for CRLM, 170 patients underwent potentially curative surgery. Among them, 113 patients (66.5%) developed recurrence after hepatectomy during the study period. Even in patients with recurrence, the 5-year OS rate after hepatectomy was 38.4%. The sites of the first recurrence are summarized in Table 2. One hundred patients (88.5%) developed single site recurrence [liver (n = 61), lung (n = 22), lymph node (n = 9), and other (n = 8)], while 13 patients developed multiple site recurrence. Numbers of recurrent tumor in total, liver, and lung were 2 (1–37), 2 (1–24), and 2 (1–37), and sizes of recurrent tumor in total, liver, and lung were 17 mm (3–130), 18 mm (5–65), and 7 mm (3–40), respectively. CEA and CA19-9 levels at recurrence were 5.3 ng/mL (0.5–490) and 17.6 U/mL (0.1–1200), respectively. For the first recurrence, potentially curative repeat surgery could be performed in 54 patients (47.8%). The detailed information regarding the procedures of repeat surgery is summarized in Table 3. Among the patients who underwent repeat surgery for recurrence, 38 patients (70.4%) received perioperative chemotherapy, including before (28 patients, 51.9%) and/or after (20 patients, 37.0%) repeat surgery. The OS in patients who underwent repeat surgery was significantly better than that in those who did not (5-year OS rate 60.7 vs. 19.5%; P < 0.0001, Fig. 2). Multivariate cox regression analyses identified repeat surgery for recurrence as one of the independent prognostic factors for OS in patients who developed recurrence (hazard ratio: 0.17, 95% confidence interval 3.41–10.96, P < 0.0001) (Supplementary Table 1).
Predictive factors for repeat surgery
Table 4 shows the results of the univariate and multivariate analyses of the factors related to the performance of repeat surgery for recurrence. According to the univariate analysis, age ≤60 years, a primary N-negative status, CEA at hepatectomy ≤ 5 (ng/mL), CA19-9 at hepatectomy ≤ 37 (U/mL), ICG-R15 ≤ 10 (%), the uptake ratio of the liver to the liver plus heart at 15 min (LHL15; determined by 99mTc-GSA scintigraphy) > 0.92, non-early recurrence (> 8 months [14]), and single site recurrence were associated with the performance of repeat surgery (P < 0.10). The multivariate logistic regression analysis identified a primary N-negative status [relative risk (RR) 2.93, P = 0.017], ICG-R15 ≤ 10 (%) (RR 2.49, P = 0.04), and CEA ≤ 5 (ng/mL) at hepatectomy (RR 2.96, P = 2.96) as independent predictive factors for the performance of repeat surgery. These factors were available before hepatectomy for CRLM.
The predictive model for the performance of repeat surgery
We subsequently created a predictive model for predicting the performance of repeat surgery for recurrence based on the 3 independent predictive factors that were identified in the multivariate logistic regression analysis. For patients without any factors, the probability of performing repeat surgery was 19.6%. The addition of each predictive factor increased the probability to 41.9% for 1 factor, 67.8% for 2 factors, and 84.0% for 3 factors (Table 5). The c-index, a measure of model discrimination represented by the area under the ROC curve, was 0.72. According to the number of predictive factors present, the 5-year OS rates after hepatectomy were 71.1% for a score of 0, 37.3% for a score of 1, 48.0% for a score of 2, and 10.8% for a score of 3.
Discussion
In the current study, of the 179 patients who underwent initial hepatectomy for CRLM, 170 patients could undergo potentially curative surgery. Among them, 113 patients (66.5%) experienced recurrence. Among them, potentially curative repeat surgery could be performed in 54 patients (47.8%) and their OS was significantly better than that in the patients in whom repeat surgery could not be performed. A primary N-negative status, normal ICG-R15 and a CEA value within the normal range before hepatectomy were identified as independent predictive factors for the performance of repeat surgery.
Complete resection is obviously the treatment of choice for resectable CRLM. However, the majority of patients with CRLM will experience recurrence after hepatectomy, mainly in the liver and lung [6, 7, 19]. Repeat hepatectomy for recurrence has been reported to be associated with an equivalent long-term outcome to initial hepatectomy, with a similarly low surgical risk [9,10,11, 20,21,22,23,24,25]; thus, its role in the treatment of metastatic colorectal cancer has recently been established. Nevertheless, the analyses of these studies only included patients undergoing repeat hepatectomy for intrahepatic recurrence. Considering the fact that recurrence after hepatectomy for CRLM can develop outside of the remnant liver, repeat surgery for extrahepatic recurrence should also be taken into account when investigating the long-term outcomes after hepatectomy for CRLM.
Some previous studies have reported that repeat surgery for recurrence (including extrahepatic recurrences) after hepatectomy provides favorable long-term outcomes and identified it as a prognostic factor in patients with CRLM [12, 13, 26,27,28]. In their studies, the rates of repeat surgery for intra- and extrahepatic recurrence after initial hepatectomy were reported to be ranged from 27 to 67%, and the 5-year OS rate ranged from 45 to 70%. Similarly, in the present study, repeat surgery could be performed in approximately half of the patients with recurrence and their 5-year OS reached 60.7%—which was significantly better than that of those who did not undergo repeat surgery. In the present study, repeat surgery was performed for recurrence in the liver (59.3%), lung (22.2%), other sites (13.0%), and multiple sites (5.6%). Our first choice of treatment strategy for metastatic disease from colorectal cancer was to perform surgical resection in combination with perioperative chemotherapy. Indeed, 78.2% of the patients in the whole cohort received perioperative chemotherapy before and/or after hepatectomy, and 70.4% of the patients who developed recurrence after hepatectomy received repeat surgery with perioperative chemotherapy. Although the prognostic role of perioperative chemotherapy remains controversial, these findings suggest that an aggressive oncosurgical approach can achieve a high repeat surgery rate and prolonged long-term survival.
The current study identified three independent predictive factors for the performance of repeat surgery: a primary N-negative status, ICG-R15 ≤ 10 (%), and CEA ≤ 5 (ng/mL) at hepatectomy (Table 4). Interestingly, the factors related to recurrence such as early recurrence, liver-only recurrence, and the number of site of recurrence were not independent predictive factors. Likewise, the administration of adjuvant chemotherapy after hepatectomy did not significantly affect the performance of repeat surgery. All three of the factors identified in this study were available before hepatectomy and the predictive model revealed that the presence of these three factors was associated with an increasing probability of repeat surgery; repeat surgery was performed in 84% of the patients in whom all three factors were present (Table 5). In the modern era, in addition to the current disease state of patients with metastatic colorectal cancer, future disease should be anticipated and subsequent recurrence should be taken into account when determining the treatment strategy. Thus, the proposed predictive model may be useful for providing optimal individualized treatment for patients who are expected to be able to undergo repeat surgery. We should consider the further treatment for recurrent disease which is strongly expected after curative surgery throughout the course of this disease.
In the current study, in addition to the primary N stage and CEA, the ICG-R15 value was significantly associated with the performance of repeat surgery. Although it is difficult to describe clearly, one of the possible reasons for this is that an impaired liver function after major hepatectomy or prolonged chemotherapy might limit the ability to perform subsequent repeat surgery. Obviously, the main site of recurrence after hepatectomy is the liver. Indeed, in the present study, a total of 72 out of 113 patients (63.7%) had intrahepatic recurrence (Table 2). Mise et al. recently reported that parenchyma-sparing hepatectomy increased the likelihood of repeat hepatectomy for liver recurrence and improved survival in patients with CRLM [29]. Although the current study failed to demonstrate the association between the performance of major hepatectomy and repeat surgery, the effort to preserve liver parenchyma during hepatectomy may be crucial for enabling repeat surgery in patients with CRLM. Another consideration is that impaired liver function might be due to prolonged chemotherapy prior to hepatectomy. The patients who required prolonged chemotherapy would have more extensive and/or chemotherapy-resistant diseases. These might be associated with lower rate of repeat surgery.
The present study is associated with some limitations, namely the retrospective nature of the study, the small sample size and the fact that it was performed in a single institution. In addition, the follow-up period was relatively short. In the modern chemotherapy era, the survival of patients with metastatic colorectal cancer is gradually increasing; thus, patients with metastatic colorectal cancer now have a greater chance of undergoing repeat surgery during their disease course, even after previous repeat surgery or prolonged chemotherapy. A validation study using an external cohort will be necessary to confirm the usefulness of the proposed predictive model. Finally, a selection bias may exist due to its retrospective nature of this study. However, the policy for the treatment of metastatic disease from colorectal cancer has not significantly changed during the study period. At this time, it would be very difficult to establish the patient selection criteria for repeat surgery after hepatectomy for CRLM. Further prospective large-size studies would be required for the development of appropriate treatment strategy for recurrence after hepatectomy for CRLM.
Conclusion
Repeat surgery for not only intrahepatic recurrence but also extrahepatic recurrence is crucial for prolonging the survival of CRLM patients after initial hepatectomy. The proposed model may help to predict the possibility of repeat surgery and enable the provision of optimal individualized treatment.
References
Jemal A, Bray F, Center MM et al (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90. https://doi.org/10.3322/caac.20107
House MG, Ito H, Gonen M et al (2010) Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg 210(5):744–752. https://doi.org/10.1016/j.jamcollsurg.2009.12.040
Manfredi S, Lepage C, Hatem C et al (2006) Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 244(2):254–259. https://doi.org/10.1097/01.sla.0000217629.94941.cf
Leporrier J, Maurel J, Chiche L et al (2006) A population-based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br J Surg 93(4):465–474. https://doi.org/10.1002/bjs.5278
Tomlinson JS, Jarnagin WR, DeMatteo RP et al (2007) Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol 25(29):4575–4580. https://doi.org/10.1200/JCO.2007.11.0833
de Jong MC, Pulitano C, Ribero D et al (2009) Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg 250(3):440–448. https://doi.org/10.1097/SLA.0b013e3181b4539b
Mise Y, Imamura H, Hashimoto T et al (2010) Cohort study of the survival benefit of resection for recurrent hepatic and/or pulmonary metastases after primary hepatectomy for colorectal metastases. Ann Surg 251(5):902–909. https://doi.org/10.1097/SLA.0b013e3181c9868a
Butte JM, Gonen M, Allen PJ et al (2015) Recurrence after partial hepatectomy for metastatic colorectal cancer: potentially curative role of salvage repeat resection. Ann Surg Oncol 22(8):2761–2771. https://doi.org/10.1245/s10434-015-4370-1
Kulik U, Bektas H, Klempnauer J et al (2013) Repeat liver resection for colorectal metastases. Br J Surg 100(7):926–932. https://doi.org/10.1002/bjs.9132
Andreou A, Brouquet A, Abdalla EK et al (2011) Repeat hepatectomy for recurrent colorectal liver metastases is associated with a high survival rate. HPB (Oxford) 13(11):774–782. https://doi.org/10.1111/j.1477-2574.2011.00370.x
Wicherts DA, de Haas RJ, Salloum C et al (2013) Repeat hepatectomy for recurrent colorectal metastases. Br J Surg 100(6):808–818. https://doi.org/10.1002/bjs.9088
Oba M, Hasegawa K, Shindoh J et al (2016) Survival benefit of repeat resection of successive recurrences after the initial hepatic resection for colorectal liver metastases. Surgery 159(2):632–640. https://doi.org/10.1016/j.surg.2015.09.003
Saiura A, Yamamoto J, Koga R et al (2014) Favorable outcome after repeat resection for colorectal liver metastases. Ann Surg Oncol 21(13):4293–4299. https://doi.org/10.1245/s10434-014-3863-7
Imai K, Allard MA, Benitez CC et al (2016) Early recurrence after hepatectomy for colorectal liver metastases: what optimal definition and what predictive factors? Oncologist 21(7):887–894. https://doi.org/10.1634/theoncologist.2015-0468
Beppu T, Miyamoto Y, Sakamoto Y et al (2014) Chemotherapy and targeted therapy for patients with initially unresectable colorectal liver metastases, focusing on conversion hepatectomy and long-term survival. Ann Surg Oncol 21(Suppl 3):S405–S413. https://doi.org/10.1245/s10434-014-3577-x
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Bogaerts J, Ford R, Sargent D et al (2009) Individual patient data analysis to assess modifications to the RECIST criteria. Eur J Cancer 45(2):248–260. https://doi.org/10.1016/j.ejca.2008.10.027
Mima K, Beppu T, Chikamoto A et al (2013) Hepatic resection combined with radiofrequency ablation for initially unresectable colorectal liver metastases after effective chemotherapy is a safe procedure with a low incidence of local recurrence. Int J Clin Oncol 18(5):847–855. https://doi.org/10.1007/s10147-012-0471-z
D’Angelica M, Kornprat P, Gonen M et al (2011) Effect on outcome of recurrence patterns after hepatectomy for colorectal metastases. Ann Surg Oncol 18(4):1096–1103. https://doi.org/10.1245/s10434-010-1409-1
Petrowsky H, Gonen M, Jarnagin W et al (2002) Second liver resections are safe and effective treatment for recurrent hepatic metastases from colorectal cancer: a bi-institutional analysis. Ann Surg 235(6):863–871
Adair RA, Young AL, Cockbain AJ et al (2012) Repeat hepatic resection for colorectal liver metastases. Br J Surg 99(9):1278–1283. https://doi.org/10.1002/bjs.8845
Nanji S, Tsang ME, Wei X et al (2016) Outcomes after repeat hepatic resection for recurrent metastatic colorectal cancer: a population-based study. Am J Surg. https://doi.org/10.1016/j.amjsurg.2016.08.014
Ali MA, Di Sandro S, Lauterio A et al (2015) Repeat hepatectomy for recurrent colorectal liver metastases: is it worth the challenge? J Gastrointest Surg 19(12):2192–2198. https://doi.org/10.1007/s11605-015-2939-4
Luo LX, Yu ZY, Huang JW et al (2014) Selecting patients for a second hepatectomy for colorectal metastases: an systemic review and meta-analysis. Eur J Surg Oncol 40(9):1036–1048. https://doi.org/10.1016/j.ejso.2014.03.012
Battula N, Tsapralis D, Mayer D et al (2014) Repeat liver resection for recurrent colorectal metastases: a single-centre, 13-year experience. HPB (Oxford) 16(2):157–163. https://doi.org/10.1111/hpb.12096
Takahashi M, Hasegawa K, Oba M et al (2015) Repeat resection leads to long-term survival: analysis of 10-year follow-up of patients with colorectal liver metastases. Am J Surg 210(5):904–910. https://doi.org/10.1016/j.amjsurg.2015.01.026
Hof J, Wertenbroek MW, Peeters PM et al (2016) Outcomes after resection and/or radiofrequency ablation for recurrence after treatment of colorectal liver metastases. Br J Surg 103(8):1055–1062. https://doi.org/10.1002/bjs.10162
Matsuda K, Hotta T, Uchiyama K et al (2007) Repeat reduction surgery after an initial hepatectomy for patients with colorectal cancer. Oncol Rep 18(1):189–194
Mise Y, Aloia TA, Brudvik KW et al (2016) Parenchymal-sparing hepatectomy in colorectal liver metastasis improves salvageability and survival. Ann Surg 263(1):146–152. https://doi.org/10.1097/SLA.0000000000001194
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Imai, K., Yamashita, Yi., Miyamoto, Y. et al. The predictors and oncological outcomes of repeat surgery for recurrence after hepatectomy for colorectal liver metastases. Int J Clin Oncol 23, 908–916 (2018). https://doi.org/10.1007/s10147-018-1273-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-018-1273-8