Abstract
Introduction
Repeat hepatectomy (RH) is considered a valuable option for management of recurrent colorectal liver metastases (R-CLM). Here, the outcome of RH for R-CLM was compared to that of patients who underwent single hepatectomy (SH) after subdividing the later according to re-recurrence status.
Methods
Between 2001 and 2013, patients who received hepatectomy for CLM and R-CLM were included in study. Patients with non-resectable R-CLM were excluded.
Results
One hundred sixteen patients were included: 86 patients in SH group and 30 patients in RH group. Repeat hepatectomy group had more synchronous CLM (76.7 versus 50 %, p = 0.011). From the 86 patients who underwent SH, 69 patients did not have R-CLM. Survival analysis was done from the time of first hepatectomy for the no R-CLM group and the time of RH for the RH group. The 3- and 5-year survival rates for the no R-CLM group were 66.4 and 48.8%, respectively, and for the RH group were 56 and 44.8% respectively (p = 0.841). Multivariate analysis showed that larger size of R-CLM is an independent risk factor for survival after RH.
Conclusion
Repeat hepatectomy for R-CLM shows a comparable OS to non-recurrent CLM after single hepatectomy, despite the RH group had higher incidence of synchronous CLM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatic resection is now considered as the standard management of colorectal liver metastases (CLM), owing to its established survival benefit with reported 5-year survival rate up to 60 % and 10-year survival rate up to 30 %.1–4 However, around 50 % of patients undergoing resection for CLM will experience recurrence. The good outcome of primary resection of CLM and the advance in liver surgery motivated adoption of repeat hepatectomy (RH) for recurrent CLM (R-CLM).
Several reports showed good survival outcome of RH with 5-year survival rate between 31 and 50%.5–9 Few reports even showed a significant survival advantage of RH over cases that underwent single hepatectomy (SH).10,11 However, those reports included all cases that underwent SH regardless re-recurrence status. Patients undergo only SH due to different conditions: absence of re-recurrence, re-recurrence treated by modalities other than re-resection and non-resectable re-recurrence. Survival data obtained from this non-homogenous cohort may be of questionable value. Also, survival analysis usually started from the time of first hepatectomy for both groups which gave immediate survival advantage for repeat hepatectomy group, as they lived enough to get recurrence and to be treated.
In this study, we evaluated the outcome of RH for R-CLM in comparison to patients who underwent SH after subdividing the latter according to re-recurrence status. Also, evaluation of prognostic factors affecting survival after RH was performed.
Patients and methods
On retrospective analysis of our prospectively maintained database, 165 consecutive patients were found to undergo resection for CLM at the Division of General Surgery and Transplantation, Niguarda Ca’ Granda Hospital, Milan, Italy, between January 2001 and December 2013. Patients who underwent RH for R-CLM were identified. Patients with non-resectable R-CLM were excluded from the study.
Diagnosis of CLM
The diagnosis of CLM, in patients with histopathologically proven colorectal cancer, was made by detection of hepatic nodule/s at contrast enhanced ultrasonography (CEUS), CT scans and/or MRI with a radiological pattern suggestive for CLM. Radiological diagnosis using two of the three imaging modalities confirmed the diagnosis. CEA and Ca 19–9 elevations alone were not considered diagnostic for CLM, but a CEA level >200 ng/ml associated with a suggestive imaging was diagnostic. Liver biopsy has been considered just in case of discordance between two of three imaging techniques.
Chemotherapy
FOLFOX and FOLFIRI regimens were used for 6–8 cycles. They were used as neoadjuvant and adjuvant therapy. Bevacizumab and cetuximab were chosen as second line therapy in case of tumour resistance to the first line treatment.
Selection criteria for liver resection and re-resection
All cases were studied in a multidisciplinary meeting including surgeons, oncologists and radiologists. Dimensions, number and site of CLM were not considered as contraindications for liver resection. Patients with recurrence in the primary resection site or with non-resectable extrahepatic metastases were excluded from surgery. A balance between obtaining R0 margins and preserving adequate liver parenchyma was considered in every case; at least two adjacent segments, with intact biliary and vascular inflow and outflow, should be preserved. The same criteria were used for selecting patients with R-CLM when considering them for RH.
Surgical technique
Our surgical technique was mentioned elsewhere.12 Balancing the oncological outcome with parenchymal preservation was the cornerstone of choice of extent of resection. Whenever possible, parenchyma-preserving hepatectomy was our first choice to maintain a good hepatic reserve to carry out its functions and to preserve the parenchyma, making RH feasible. Major hepatectomy was defined as resection of three or more Couinaud’s segments. Intraoperative ultrasonography was performed in order to study the lesion’s relationship with vascular structures and to recognise lesions undetected preoperatively. Hepatic transection was performed with CUSA and monopolar bowie, while hemostasis was obtained with bipolar forceps and Prolene 4/0 or 5/0 stiches. Intermittent hepatic pedicle clamping was used in several cases according to preference of the surgeon.
Follow-up
Patients were followed-up at 1 month from surgery and then every 4 months, checking tumour markers (CEA and CA 19–9 blood levels), liver function tests and contrast enhancement hepatic ultrasonography for the first 3 years. Thereafter, the periodicity of follow-up changed to a 6 and a 12-month basis. CT scan was performed every 6 months in the first 5 years, then on a yearly basis. Synchronous metastases were defined as CLM diagnosed at time of resection of primary colorectal tumour or within 6 months after resection. Metachronous metastases refer to metastases diagnosed after 6 months of resection of colorectal tumour. Postoperative mortality was defined as mortality within 90 days of the procedure.
Statistical analysis
Continuous variables were expressed as medians and interquartile ranges and compared using Mann–Whitney U test. Categorical variables were expressed as numbers and percentages and compared with the χ 2 test or Fisher’s exact test whenever appropriate. Overall survival (OS) and progression-free survival (PFS) were obtained by Kaplan–Meier method, and differences in survival curves were obtained by log-rank test. Potential risk factors were analyzed for their influence on OS in RH group by cox proportional hazard model. Variables with p value <0.1 were enrolled onto multivariate analysis by Cox proportional hazard model with backward stepwise method. Overall survival was estimated from the date of first hepatectomy for the SH group without R-CLM and from the date of repeat hepatectomy for RH group. Progression-free survival was estimated from the date of RH until date of further recurrence or the date of last follow-up. Other than for enrolling variables in multivariate analysis, p value <0.05 was considered significant. Analysis was performed with SPSS program 16.0 for Windows (SPSS, Chicago, IL).
Results
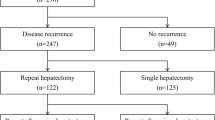
From 165 patients underwent hepatic resection for CLM, 29 patients lost follow-up or had follow-up in other hospitals, and 20 patients had non-resectable R-CLM. The study group is 116 patients divided into 2 groups: SH group (86 patients; 74.1 %) and RH group (30 patients; 25.9 %). Baseline characteristics, features of primary colorectal tumor and features of the first CLM are shown in Table 1. There was no significant difference as for gender and age at time of resection of primary CLM. Most of cases in RH group had Duke D primary colorectal tumor (82.6 %), which was significantly more than SH group (45.9 %, p = 0.008). Repeat hepatectomy group had significantly more synchronous CLM than SH group (76.7 versus 50 %, p = 0.011). In the first hepatectomy, major resection was performed significantly more often in SH group than RH group (52.3 versus 26.7 %, p = 0.015). Also, in SH group, median diameter of the largest resected nodule was significantly larger than RH group.
Table 2 demonstrates the operative criteria of primary and repeated resections. Age at time of first and repeat hepatectomy was nearly equal. Repeat hepatectomy had more cases with multiple metastases than the first hepatectomy (60 versus 46.4%). Repeated resection was not found to be associated with significantly prolonged operative time. As regards the extent of hepatectomy, 40% of repeated resections were major resections, which was comparable to the first resection (52.3%). There was no postoperative mortality in both groups.
Survival analysis
From the 86 patients who underwent SH, 69 patients were found not to have R-CLM. Survival analysis was done from the time of management of 2 groups, the time of first hepatectomy of the no R-CLM group and the time of RH for the RH group (Fig. 1). The 3- and 5-year survival rates for the no R-CLM group were 66.4 and 48.8 %, respectively, and for the RH group were 56 and 44.8 % respectively, and there was no significant difference between them (p = 0.841).
Survival analysis starting from the time of repeat hepatectomy
Median follow up after RH was 25.2 months (range, 6–97.5 months). Survival analysis after RH was also estimated. The 3- and 5-year OS rate after RH (Fig. 2) was 56 and 44.8 %, respectively, while 3- and 5-year PFS rate (Fig. 2) was 36.1 and 36.1 %, respectively.
Predictors of outcome after repeat hepatectomy
Univariate analysis for risk factors adversely affecting the OS after RH (Table 3) showed that bilateral distribution and multiple metastases in the initial metastasis, shorter progression-free interval after first hepatectomy and larger size of metastasis in RH has a negative impact on OS. On multivariate analysis, only larger size of second metastasis was proved to be an independent risk factor negatively impacting OS in RH group. When setting the median diameter of the biggest nodule in R-CLM as a cutoff value, cases with diameter <3.5 cm had a 5-year survival of 87.5 % and the others with diameter ≥3.5 cm had a 5-year survival of 20.8 % (p = 0.016).
Discussion
Hepatic resection for CLM was proven to carry a significant survival benefit, mandating resection for all resectable CLMs.1,13,14 For R-CLM, several reports showed that patients subjected to RH have comparable long-term outcome with those who underwent SH.5,15,16 Few reports even demonstrated significant advantage of RH over patients who were subjected only to SH.10,11 In addition, a recent meta-analysis showed a significant survival advantage of RH over SH.17 Concluding that RH provides longer overall survival when compared to single hepatectomy may be misleading because all those reports compared RH cases to the whole cohort of cases that underwent SH. The reasons for which patients undergo only a SH are diverse, and including this non-homogenous cohort of diverse conditions make the results non-conclusive. Another factor is that starting the time of enrollment for survival analysis from the time of first hepatectomy for all patients provides immediate survival advantage to the RH group because they lived long enough to have recurrence and to be treated. We excluded non-resectable R-CLM and sub-classified patients who underwent SH according to re-recurrence status. We started survival analysis from the date of last treatment: the first hepatectomy for non-recurrent CLM and the management of R-CLM in RH. Our results showed that RH offers a comparable survival outcome to patients who did not experience re-recurrence after first resection of CLM. Those results support the concept of RH for R-CLM, given the comparable survival with cases without recurrence.
Synchronous CLM is considered as a poor prognostic factor for recurrence and survival.18,19 In this study, RH group had significantly more synchronous CLM than SH group. Despite the fact that more than 75 % of the patients had synchronous metastases, RH showed a good survival. Also, synchronous metastases were not found to be a poor prognostic after repeat hepatectomy. This shows that RH can provide those high risk patients a better survival outcome.
On comparing the tumor features in first and second hepatectomy, we found that multiple tumors were found more in the second hepatectomy than the first hepatectomy (60 versus 46.4 %). The same finding was also noted by Shaw et al.10 In addition, the median diameter was equal in both groups. Resection of CLM went further beyond the size and number concept. Remnant liver volume, ranging between 20 and 30 %, is currently the main determinant for opting patients for CLM resection.20–23 This concept has been inherited by RH,9,24 despite the fact that increasing the size and number of R-CLM is associated with unfavorable survival.17,24,25 Our results showed that larger size of R-CLM is considered as an independent risk factor for OS, but tumor number failed to show the same negative impact. Despite those findings, RH should still be offered to large resectable R-CLM. Surgical resection is considered as the only hope of cure for those patients, owing to the limited value of RFA in big tumors and the palliative nature of chemotherapy. Other factors like shorter PFS after first hepatectomy and bilobar involvement that have shown a negative impact on survival17 failed to show the same impact in our study.
Repeat hepatectomy usually face intraoperative difficulties due to adhesions, remnant liver rotation due to regeneration and unclear hilar anatomy when hilar dissection is performed. Those difficulties increase in cases underwent major primary hepatectomy because hilar dissection is usually performed and smaller remnant liver volume have a higher rate of regeneration in the vacant sub-phrenic space. In addition, if recurrence occurs, smaller remnant liver may hinder RH. In our series, RH group had significantly fewer major primary hepatectomy than SH group, which may be attributed for the aforementioned causes. Major resection was performed in the second hepatectomy in a comparable frequency to the first hepatectomy. This was feasible because parenchyma spared in the first hepatectomy facilitated further major resections. More than 50 % of cases underwent first resection for CLM will experience recurrence.7,26 Owing to that high incidence of recurrence, resection of the first CLM should be kept to a minimum, avoiding hilar dissection whenever possible, to preserve hepatic parenchyma if further resections are needed and to technically facilitate RH. A questionable issue in this context is the fear of increasing the recurrence rate when non-anatomical resections with smaller resection margins are used. Our previous results showed that the extent of resection in the primary resection of CLM has no impact on survival or recurrence.12 Kokudo et al., with the use of genetic and histological assessment of surgical margins in resected CLM, found that anticipated narrow resection margin should not hinder resection of CLM.27 Furthermore, non-anatomical and parenchyma-sparing procedures do not have a negative oncological impact.28–30 According to the previous discussion, we recommend opting parenchyma-preserving hepatectomy without hilar dissection whenever possible in surgical management of CLM, whether primary or repeat hepatectomy.
This study is not free from limitations, namely the retrospective nature, the small sample size of RH cases and the selection bias. However, we believe that sub-classifying SH group according to the re-recurrence status and comparing the outcome of more homogenous sub-groups with that of patients who underwent RH gives a clearer image of the survival advantage of RH.
Conclusion
Repeat hepatectomy for R-CLM shows a comparable overall survival to non-recurrent CLM after single hepatectomy, despite the RH group had higher incidence of synchronous CLM. Parenchyma-preserving hepatectomy in the first resection of CLM increases the patient’s chance to receive further hepatectomy when recurrence occurs.
Abbreviations
- CLM:
-
Colorectal liver metastases
- RH:
-
Repeat hepatectomy
- R-CLM:
-
Recurrent colorectal liver metastasis
- SH:
-
Single hepatectomy
- RFA:
-
Radiofrequency ablation
- CEUS:
-
Contrast enhanced ultrasonography
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- CEA:
-
Carcino-embryonic antigen
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
References
Fong Y, Fortner J, Sun RL, Brennan MF and Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999; 230:309–318.
Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 2004; 239:818–825.
Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg 1995; 19:59 –71.
Wei AC, Greig PD, Grant D, Taylor B, Langer B, Gallinger S. Survival after hepatic resection for colorectal metastases: A 10-year experience. Ann Surg Oncol, 2006; 13:668–676.
Sa Cunha A, Laurent C, Rault A, Couderc P, Rullier E, Saric J. A second liver resection due to recurrent colorectal liver metastases. Arch Surg 2007; 142:1144–1149.
Suzuki S, Sakaguchi T, Yokoi Y, et al. Impact of repeat hepatectomy on recurrent colorectal liver metastases. Surgery 2001; 129:421–428.
Adam R, Bismuth H, Castaing D, et al. Repeat hepatectomy for colorectal liver metastases. Ann Surg 1997; 225:51–60.
Nishio H, Hamady ZZ, Malik HZ, Fenwick S, Rajendra Prasad K, Toogood GJ, Lodge JP. Outcome following repeat liver resection for colorectal liver metastases. Eur J Surg Oncol 2007; 33:729–734.
Neeff HP, Drognitz O, Holzner P, Klock A, Bronsert P, Hopt UT, Makowiec F. Outcome after repeat resection of liver metastases from colorectal cancer. Int J Colorectal Dis 2013; 28:1135–1141.
Shaw IM, Rees M, Welsh FK, Bygrave S, John TG. Repeat hepatic resection for recurrent colorectal liver metastases is associated with favourable long-term survival. Br J Surg 2006; 93:457–464.
Wicherts DA, de Haas RJ, Salloum C, et al. Repeat hepatectomy for recurrent colorectal metastases. Br J Surg 2013; 100:808–818.
De Carlis L., Di Sandro S., Giacomoni A, et al. Colorectal liver metastases: Hepatic pedicle clamping during hepatectomy reduces the incidence of tumor recurrence in selected patients. Case-matched analysis. Eur J Surg Oncol 2013; 39:726–733.
D’Angelica M, Brennan MF, Fortner JG, Cohen AM, Blumgart LH, Fong Y. Ninety-six five year survivors after liver resection for metastatic colorectal cancer. J Am Coll Surg 1997; 185:554–559.
Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg 2002; 235:759–766.
Jonsson K, Grondahl G, Salo M, Tingstedt B, Andersson R. Repeated Liver Resection for Colorectal Liver Metastases: a Comparison with Primary Liver Resections concerning Perioperative and Long-Term Outcome. Gastroenterol Res Pract 2012; 2012:568214.
Takahashi S, Inoue K, Konishi M, Nakagouri T, Kinoshita T. Prognostic factors for poor survival after repeat hepatectomy in patients with colorectal liver metastases. Surgery 2003; 133:627–634.
Luo LX, Yu ZY, Huang JW, Wu H. Selecting patients for a second hepatectomy for colorectal metastases: An systemic review and meta-analysis. Eur J Surg Oncol 2014; 40:1036–1048.
Tsai MS, Su YH, Ho MC, Liang JT, Chen TP, Lai HS, Lee PH. Clinicopathological features and prognosis in resectable synchronous and Metachronous colorectal liver metastasis. Ann Surg Oncol 2007; 14:786–794.
Ghiringhelli F, Hennequin A, Drouillard A, Lepage C, Faivre J, Bouvier AM. Epidemiology and prognosis of synchronous and metachronous colon cancer metastasis: a French population-based study. Dig Liver Dis 2014; 46: 854–858.
Adam R, De Gramont A, Figueras J, et al. The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. The Oncologist 2012; 17:1225–1239.
Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med 2007; 356:1545–1559.
Misiakos EP, Karidis NP, Kouraklis G. Current treatment for colorectal liver metastases. World J Gastroenterol 2011; 17:4067– 4075.
Charnsangavej C, Clary B, Fong Y, Grothey A, Pawlik TM, Choti MA. Selection of patients for resection of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol 2006; 13:1261–1268.
Pessaux P, Lermite E, Brehant O, Tuech JJ, Lorimier G, Arnaud JP. Repeat hepatectomy for recurrent colorectal liver metastases. J Surg Oncol 2006; 93:1–7.
Petrowsky H, Gonen M, JarnaginW, et al. Second liver resections are safe and effective treatment for recurrent hepatic metastases from colorectal cancer: a bi-institutional analysis. Ann Surg 2002; 235:863–871.
de Jong MC, Pulitano C, Ribero D, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg 2009; 250:440–448.
Kokudo N., Miki Y, Sugai S, et al. Genetic and histological assessment of surgical margins in resected liver metastases from colorectal carcinoma: minimum surgical margins for successful resection. Arch Surg 2002; 137:833–840.
Lalmahomed ZS, Ayez N, van der Pool AE, Verheij J, IJzermans JN, Verhoef C. Anatomical versus nonanatomical resection of colorectal liver metastases: is there a difference in surgical and oncological outcome? World J Surg 2011; 35:656–661.
Gold JS, Are C, Kornprat P, et al. Increased use of parenchymal-sparing surgery for bilateral liver metastases from colorectal cancer is associated with improved mortality without change in oncologic outcome: trends in treatment over time in 440 patients. Ann Surg 2008; 247:109–117
Kokudo N, Tada K, Seki M et al. Anatomical major resection versus nonanatomical limited resection for liver metastases from colorectal carcinoma. Am J Surg 2001; 181:153–159.
Conflict of interest
No conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ali, M.A., Di Sandro, S., Lauterio, A. et al. Repeat Hepatectomy for Recurrent Colorectal Liver Metastases: Is it Worth the Challenge?. J Gastrointest Surg 19, 2192–2198 (2015). https://doi.org/10.1007/s11605-015-2939-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-015-2939-4