Abstract
Background
Clinical benefits of presurgical axitinib therapy for renal cell carcinoma (RCC) extending into the inferior vena cava (IVC) remain unclear. We aimed to investigate surgical benefits and pathological antitumor effects of presurgical axitinib therapy for RCC with IVC thrombus.
Methods
Of 56 consecutive RCC patients with IVC thrombus between January 1994 and December 2016, 41 patients who underwent radical nephrectomy (RN) were categorized as upfront RN (Upfront group) or presurgical axitinib followed by RN (Presurgical group). We retrospectively evaluated safety, radiologic tumor responses, and Ki-67 proliferation index before and after axitinib administration in the Presurgical group. Surgical outcomes, postoperative complications, and fibrosis within the IVC thrombus were compared between the Upfront and Presurgical groups.
Results
The number of patients in the Upfront and Presurgical groups was 31 and 10, respectively. Major presurgical axitinib-related adverse events were grade 2 or 3 hypertension (50%). The median radiological tumor response in the renal tumor, IVC thrombus length, and IVC thrombus volume were −19%, −21 mm, and −54%, respectively. The fibrosis within the IVC thrombus was significantly higher in the Presurgical group (10%) than in the Upfront group (3.4%). The Ki-67 proliferation index was significantly decreased in RN specimens (7.3%) versus needle biopsy specimens (23%) in the Presurgical group. Blood loss and operative duration were significantly lower and shorter, respectively, in the Presurgical group than in the Upfront group.
Conclusions
Presurgical axitinib therapy enhanced tumor reduction accompanied by fibrosis and may contribute to surgical risk reduction for selected patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surgical management of renal cell carcinoma (RCC) with tumor thrombus extending to the inferior vena cava (IVC) is challenging. Advances in molecular-targeting agents have allowed significantly prolonged survival in metastatic RCC over the past decade. Corresponding to the accumulating evidence for efficacy and safety, the interest in presurgical therapy using molecular-targeting agents has been increasingly reported for non-metastatic disease [1], large unresectable tumors or metastatic disease [2,3,4], and venous tumor thrombus extending to the IVC [5, 6]. Among molecular-targeting agents, tyrosine kinase inhibitors (TKIs) including sunitinib [6] and axitinib [7,8,9] have been proposed as potential candidates in presurgical settings for RCC with IVC thrombus. Conversely, several studies have suggested the limited efficacy of presurgical therapy for IVC thrombus due to limited thrombus shrinkage and disease progression during the presurgical periods [10,11,12]. Because no level 1 evidence has identified the role of presurgical therapy for advanced RCC, the use of presurgical targeted therapy to shrink RCC is not recommended in European Association of Urology guidelines [13]. Although the clinical benefits of presurgical TKI therapy in RCC patients with IVC thrombus remain unclear, the potential benefits of cytoreductive effects and surgical risk reduction for carefully selected patients need to be investigated. Here, we investigated the surgical benefits and pathological antitumor effects of presurgical axitinib therapy for RCC with IVC thrombus.
Patients and methods
Design and ethics statement
This retrospective, single-center study on the use of axitinib prior to radical nephrectomy (RN) in RCC patients with IVC thrombus was conducted in accordance with the ethical standards of the Declaration of Helsinki. It was approved by the Ethics Committee of the Hirosaki University Graduate School of Medicine (authorization number 2012-099).
Patient selection
Of 56 consecutive RCC patients with IVC thrombus between January 1994 and December 2016, 41 patients who underwent RN were identified and categorized as upfront RN (Upfront group) or presurgical axitinib with RN (Presurgical group). We retrospectively evaluated radiologic tumor responses and IVC thrombus levels before and after axitinib in the Presurgical group. We underwent ultrasound-guided needle biopsies of renal mass (three to five cores) before presurgical treatment to confirm the histology in the Presurgical group. Patients with presurgical therapy received standard dose of axitinib (5 mg BID). Surgical outcomes (operative duration and blood loss), postoperative complications, proliferation marker (Ki67), and fibrosis within IVC thrombus were compared between the Upfront and Presurgical groups.
Evaluation of variables
The variables analyzed were age, sex, Eastern Cooperative Oncology Group performance status (ECOG PS), presence of cardiovascular disease (CVD), presence of diabetes mellitus (DM), age-adjusted Charlson comorbidity index (CCI) [14], clinical stage, IVC thrombus level (Mayo staging system) [15], and metastatic volume [low volume (defined as the presence of small lung metastases <5 lesions within 2 cm, single bone lesion, or involvement of <2 lymph nodes) vs. high volume]. Toxicity was prospectively recorded based on the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Radiographical assessment in the Presurgical group
Contrast-enhanced computed tomography of the chest, abdomen, and pelvis was performed before and after presurgical therapy with axitinib. The tumor response in the renal mass was analyzed using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [16]. In addition, responses of IVC thrombus, such as shrinkage in length (mm) and volume (mm3), were separately evaluated. Volumes of IVC thrombus were measured using a formula: using width × depth × height × 1/2 (Fig. 1).
Tumor volume evaluation of inferior vena cava (IVC) thrombus. Tumor volume of IVC thrombus were measured using a formula: using width × depth × height × 1/2. Representative images of coronal (a) and sagittal (b) planes were shown between before and after presurgical axitinib therapy. Tumor volume reduction of IVC thrombus was 52% in this case
Surgical intervention
Open procedures were performed in patients with RN. Operative duration (min), blood loss (g), use of extracorporeal circulation with cardiopulmonary bypass, and postoperative complications (Clavien-Dindo classification) were reviewed.
Planned surgery in the Presurgical group
We planned surgical intervention within 3–6 months after presurgical therapy initiation. Indications for cytoreductive nephrectomy for metastatic disease were a low volume or controllable metastatic lesions after presurgical axitinib therapy. Axitinib was discontinued 48–72 h before RN. Axitinib was continuously administered in patients with residual metastatic masses after surgery.
Surgical outcomes comparison between the Upfront and Presurgical groups
We compared surgical outcomes (operative duration and blood loss), postoperative complications, and fibrosis within IVC thrombus between the Upfront and Presurgical groups.
Immunohistochemistry of Ki67 proliferation index in the Presurgical group
To evaluate the Ki67 proliferation marker, immunohistochemistry for Ki67 proliferation index was performed using 3-μm slices from paraffin-embedded specimens and the Histofine immunostaining kit (Nichirei Co. Ltd., Tokyo, Japan). The monoclonal antibody against Ki67 (MIB1; Dako, Denmark) was used at the optimal dilution of 1:50. In all cases, 5–10 high-power fields (400×) were selected; at least 1000 cells were independently evaluated by two authors (SH and YH). The number of Ki67-positive cells per 100 clear cell carcinoma cells was designated as the labeling index (Ki-67 proliferation index). The Ki-67 proliferation index was compared between the pretreatment biopsy and RN specimens in the presurgical group.
Interstitial fibrosis measurement in the Presurgical group
To evaluate the tumor response within tumor thrombus, we measured the interstitial fibrosis rate using Azan staining with Image J software. In all cases, 5–10 high-power fields (100×) were independently evaluated by two authors (YT and YH).
Statistical analysis
Statistical analyses were performed using SPSS ver. 24.0 (SPSS, Inc., Chicago, IL, USA), GraphPad Prism 5.03 (GraphPad Software, San Diego, CA, USA), and R 3.3.2 (The R Foundation for Statistical Computing, Vienna, Austria). Categorical variables were compared using the Fisher’s exact or χ 2 test. Quantitative variables were expressed as means with a standard deviation or medians with an interquartile range (IQR). The differences between the groups were compared using the t test for normal distributions or the Mann–Whitney U test for non-normal distributions.
Results
Baseline characteristics
Of 56 RCC patients with IVC thrombus, the number of patients in the Upfront and Presurgical groups were 31 and 10, respectively. Two patients in the presurgical axitinib therapy group did not have indications for RN due to uncontrollable high-volume metastases. There were no significant differences in the baseline patient characteristics before RN between the Upfront and Presurgical groups except for age-adjusted CCI (Table 1). All tumor biopsies showed clear cell carcinoma in the presurgical group. Tumor histologies between biopsy and nephrectomy specimens were same. Mean and median relative dose intensity of presurgical axitinib were 97 and 100%, respectively.
Radiological response
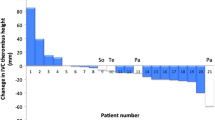
To evaluate tumor response, we used RECIST for the primary tumor and length (mm) and volume (mm3) for IVC thrombus among 12 patients with presurgical axitinib treatment. The median radiological tumor response per RECIST was −19% (partial response: 33%, stable disease: 67%), and no patients experienced progressive disease in the presurgical group (Fig. 2a). The median tumor reduction of IVC thrombus in length (Fig. 2b) and volume (Fig. 2c) were −21 mm and −54%, respectively. The IVC thrombus levels were significantly decreased before and after presurgical axitinib therapy (P = 0.0172, Fig. 2d). The number of patients with IVC thrombus level reduction and mean downstaging were five (5/12, 42%) and 0.42 ± 0.49, respectively, in the Presurgical group.
Radiologic response and thrombus levels after presurgical axitinib therapy. The median radiological tumor response as per RECIST was −19% (partial response: 33%, stable disease: 67%), and no patients experienced progressive disease in the Presurgical group (a). Median reduction of IVC thrombus in length was −21 mm (b). Median reduction of IVC thrombus volume was −54% (c). The IVC thrombus levels were significantly decreased before and after presurgical axitinib therapy (d)
Adverse events related to presurgical axitinib therapy
Axitinib-related adverse events were tolerable and controllable. The majority of adverse events were grade 2 or 3 (Table 2, upper rows). Grade 2 or 3 hypertension was the major adverse event during presurgical TKI administration (50%). No grade 4 or 5 events were observed.
Postoperative complications between the Upfront and Presurgical groups
The incidence of postoperative complications was not significantly different between the Upfront and Presurgical groups (32 vs. 50%, respectively, P = 0.724); however, grade 4 or 5 events were significantly higher in the Upfront group (6/10 events: 60%) than that in the Presurgical group (0/5 events, P = 0.044). Two patients in the Upfront group experienced grade 5 postoperative complications (pulmonary embolism and gastrointestinal hemorrhage; Table 2, lower rows).
Surgical and pathological outcomes between the Upfront and Presurgical groups
Operative procedure was changed in four patients (4/10, 40%) including clamp of portal vein (level 3–2, n = 3) and complete clamp of IVC (level 1–0, n = 1), whereas IVC manipulations were not changed in one patient (level 2–1) among the patients with IVC thrombus reduction. Blood loss (Fig. 3a) and operative duration (Fig. 3b) were significantly lower and shorter in the Presurgical group than those in the Upfront group. The Ki-67 proliferation index was significantly decreased in RN specimens (7.3%) versus needle biopsy specimens (23%) in the Presurgical group (Fig. 3c). One patient with an IVC thrombus (level 1) developed stage pT0 tumor after presurgical axitinib. Furthermore, the interstitial fibrosis rate within the IVC thrombus was significantly higher in the Presurgical group (10%) than that in the Upfront group (3.4%; Fig. 3d). Representative pictures of interstitial fibrosis (red area) of the Upfront (Fig. 3e) and Presurgical (Fig. 3f) groups are shown.
Surgical and pathological outcomes. Blood loss (a) and operative duration (b) were significantly lower and shorter in the Presurgical group than those in the Upfront group. The Ki-67 proliferation index was significantly decreased in RN specimens (7.3%) versus needle biopsy specimens (23%) in the Presurgical group (c). The interstitial fibrosis rate within the IVC thrombus was significantly higher in the Presurgical group (10%) than that in the Upfront group (3.4%; d). A representative picture shows interstitial fibrosis in the Upfront (e) and Presurgical (f) groups. Blue area in the azan stating (left) was highlighted in red (right), and measured by Image J
Discussion
The clinical implications of presurgical therapy with molecular-targeting agents for RCC patients with IVC thrombus are debatable. Although the role of presurgical therapy for IVC thrombus is not clearly established, several studies have suggested a survival benefit [1,2,3,4,5,6, 17, 18]. However, other studies failed to prove the benefit of presurgical therapy in terms of tumor shrinkage [10,11,12]. Therefore, to confirm the efficacy of presurgical therapy, we compared not only tumor shrinkage but also surgical outcomes and pathological outcomes between patients with and without presurgical therapy. The essential finding of the present study is that presurgical therapy for RCC patients with IVC thrombus is feasible regarding toxicity and surgical and pathological outcomes. It should be noted that one patient with IVC thrombus (level 1) developed stage pT0 tumor after presurgical axitinib. Although the median radiological response using RECIST was not significant (only −19%), a significantly higher extent of tumor decreases in IVC thrombus volume (−54%) was observed in the Presurgical group. In addition, the median interstitial fibrosis rate of IVC thrombus was significantly higher in the Presurgical group than that in the Upfront group. Increased fibrosis and decreased Ki-67 proliferation index suggest an antitumor effect of axitinib, inducing a sclerotic change in the thrombus, and decreasing the risk of potentially challenging surgery. Indeed, significantly better surgical outcomes (time of operation and blood loss) without grade 4–5 complications were observed in the Presurgical group. As a result, operative procedure was minimized in 4 patients (40%) in the presurgical group.
The optimal agent for presurgical therapy remain undetermined. Although a randomized phase III trial for 1st line axitinib failed to prove the clinical efficacy, axitinib arm revealed numerically longer progression-free survival (median, 10.1 vs. 6.5 months; hazard ratio, 0.77; 1-sided P = 0.038) and significantly higher objective response rate (32 vs. 15%; 1-sided P = 0.0006) than those of sorafenib [19]. In addition, axitinib is effective for several months in most of all patients with clear cell carcinoma [18]. Based on our clinical experience for the 1st line axitinib [17, 20], we believe disadvantages for short-term presurgical axitinib before elective surgery are limited. Therefore, we enrolled all RCC patients with IVC thrombus for presurgical therapy after August 2013. Although we could not definitively conclude that there was a surgical benefit because of selection biases in the present study, presurgical axitinib therapy enhanced tumor sclerotic change accompanied by fibrosis and may offer the potential to minimize surgical risk for selected patients.
The role of presurgical therapy and cytoreductive nephrectomy for metastatic RCC is also debatable. Because of the sample size limitation, we could not clearly show the benefit of presurgical axitinib and cytoreductive nephrectomy in the present study. Two patients in our study did not proceed with elective surgery after presurgical axitinib therapy. However, these two cases were challenging given the presence of unresectable high volume metastases. Therefore, the clinical benefit of presurgical axitinib therapy might be limited to patients with low volume, manageable metastatic lesions. Two ongoing prospective, randomized, phase III trials (CARMENA and SURTIME) evaluating the role of cytoreductive nephrectomy prior to targeted therapy in metastatic RCC [21] will hopefully provide insight for difficult cases such as these.
Prognostic benefits of presurgical therapy on RCC with IVC thrombus have not yet been fully explored. Due to the sample size and selection bias, we could not address prognostic benefits of presurgical therapy on RCC with IVC thrombus in the present study. Currently, no recommendation exists for the role of presurgical therapy for RCC with IVC thrombus. Although it remains difficult to make conclusions about the clinical benefits of presurgical therapy from the present study, our results may support the potential benefit of presurgical axitinib therapy (via enhancing tumor reduction accompanied by fibrosis, and contributing to surgical risk reduction in selected patients). The optimal selection for presurgical therapy requires further prospective investigation.
This study has several limitations. The number of patients (the upfront and presurgical groups was 31 and 10, respectively) were too small to draw important conclusions. Furthermore, the backgrounds of the two cohorts are apparently different. The retrospective design also prevents definitive conclusions regarding the benefits of presurgical therapy on outcomes. The feasibility of the fibrosis within the IVC thrombus and Ki-67 proliferation index as a surrogate marker for tumor response remains unclear. It has not generally been used as an endpoint, and is unclear whether these parameters predict the better clinical outcomes. Furthermore, we were unable to control for selection bias and other unmeasurable confounding factors. Therefore, we were unable to definitively assess the oncological benefit of presurgical therapy due to the selection biases. In addition, because contemporary RCC patients with IVC thrombus received presurgical treatment, we could not exclude the influence of technical development in our institute on surgical outcomes including amount of blood loss and shorter operation time. Despite these limitations, our results suggest that presurgical axitinib therapy enhances tumor sclerotic change accompanied by fibrosis and contributes to surgical risk reduction.
In conclusion, presurgical axitinib therapy enhanced tumor sclerotic change accompanied by fibrosis and may contribute to surgical risk reduction for selected patients. Further, large-scale studies are necessary to identify the indications, clinical benefits, and standard protocols for presurgical therapy.
References
Karam JA, Devine CE, Urbauer DL et al (2014) Phase 2 trial of neoadjuvant axitinib in patients with locally advanced nonmetastatic clear cell renal cell carcinoma. Eur Urol 66(5):874–880. doi:10.1016/j.eururo.2014.01.035
Thomas AA, Rini BI, Lane BR et al (2009) Response of the primary tumor to neoadjuvant sunitinib in patients with advanced renal cell carcinoma. J Urol 181(2):518–523. doi:10.1016/j.juro.2008.10.001 (discussion 523)
Cowey CL, Amin C, Pruthi RS et al (2010) Neoadjuvant clinical trial with sorafenib for patients with stage II or higher renal cell carcinoma. J Clin Oncol 28(9):1502–1507. doi:10.1200/jco.2009.24.7759
Rini BI, Garcia J, Elson P et al (2012) The effect of sunitinib on primary renal cell carcinoma and facilitation of subsequent surgery. J Urol 187(5):1548–1554. doi:10.1016/j.juro.2011.12.075
Kondo T, Hashimoto Y, Kobayashi H et al (2010) Presurgical targeted therapy with tyrosine kinase inhibitors for advanced renal cell carcinoma: clinical results and histopathological therapeutic effects. Jpn J Clin Oncol 40(12):1173–1179. doi:10.1093/jjco/hyq150
Cost NG, Delacroix SE Jr, Sleeper JP et al (2011) The impact of targeted molecular therapies on the level of renal cell carcinoma vena caval tumor thrombus. Eur Urol 59(6):912–918. doi:10.1016/j.eururo.2011.02.032
Sassa N, Kato M, Funahashi Y et al (2014) Efficacy of pre-surgical axitinib for shrinkage of inferior vena cava thrombus in a patient with advanced renal cell carcinoma. Jpn J Clin Oncol 44(4):370–373. doi:10.1093/jjco/hyu014
Yuki H, Kamai T, Kubota K et al (2014) Axitinib for preoperative downstaging of renal cell carcinoma with sarcomatoid differentiation and direct invasion of the duodenum and inferior vena cava: a case report. Onco Targets Ther 7:289–295. doi:10.2147/ott.s58089
Komatsubara M, Yamazaki M, Fujisaki A et al (2016) Tumor thrombus of renal cell carcinoma extending into the inferior vena cava, ovarian vein, and ureter treated with neoadjuvant axitinib. Urology 95:e3–e4. doi:10.1016/j.urology.2016.05.057
Schrader AJ, Steffens S, Schnoeller TJ et al (2012) Neoadjuvant therapy of renal cell carcinoma: a novel treatment option in the era of targeted therapy? Int J Urol 19(10):903–907. doi:10.1111/j.1442-2042.2012.03065.x
Bigot P, Fardoun T, Bernhard JC et al (2014) Neoadjuvant targeted molecular therapies in patients undergoing nephrectomy and inferior vena cava thrombectomy: is it useful? World J Urol 32(1):109–114. doi:10.1007/s00345-013-1088-1
Ujike T, Uemura M, Kawashima A et al (2016) Clinical and histopathological effects of presurgical treatment with sunitinib for renal cell carcinoma with inferior vena cava tumor thrombus at a single institution. Anticancer Drugs 27(10):1038–1043. doi:10.1097/cad.0000000000000422
Ljungberg B, Bensalah K, Bex A et al (2016) Guidelines on renal cell carcinoma. http://uroweb.org/wp-content/uploads/10-Renal-Cell-Carcinoma_LR1.pdf. Accessed 20 Mar 2017
Charlson M, Szatrowski TP, Peterson J et al (1994) Validation of a combined comorbidity index. J Clin Epidemiol 47(11):1245–1251
Hatakeyama S, Yoneyama T, Hamano I et al (2013) Prognostic benefit of surgical management in renal cell carcinoma patients with thrombus extending to the renal vein and inferior vena cava: 17-year experience at a single center. BMC Urol 13:47. doi:10.1186/1471-2490-13-47
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216. doi:10.1093/jnci/92.3.205
Koie T, Ohyama C, Okamoto A et al (2013) Presurgical therapy with axitinib for advanced renal cell carcinoma: a case report. BMC Res Notes 6:484. doi:10.1186/1756-0500-6-484
Rini BI, Plimack ER, Takagi T et al (2015) A phase II study of pazopanib in patients with localized renal cell carcinoma to optimize preservation of renal parenchyma. J Urol 194(2):297–303. doi:10.1016/j.juro.2015.03.096
Hutson TE, Lesovoy V, Al-Shukri S et al (2013) Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised open-label phase 3 trial. Lancet Oncol 14(13):1287–1294. doi:10.1016/s1470-2045(13)70465-0
Hosogoe S, Hatakeyama S, Kusaka A et al (2017) Contrast media enhancement reduction predicts tumor response to presurgical molecular-targeting therapy in patients with advanced renal cell carcinoma. Oncotarget. doi:10.18632/oncotarget.17930
Chery LJ, Karam JA, Wood CG (2016) Cytoreductive nephrectomy for metastatic renal cell carcinoma. Clin Adv Hematol Oncol 14(9):696–703
Acknowledgements
We thank Yuki Fujita, Yukie Nishizawa, Kaname Higuchi, Satomi Sakamoto, and Masako Isono for their invaluable help with the data and sample collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Source of funding
This work was supported by a Grant-in-Aid for Scientific Research (nos. 15H02563 15K15579, 17K11118, 17K11119, 17K16768, 17K16770, and 17K16771) from the Japan Society for the Promotion of Science.
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study formal consent is not required. Pursuant to the provisions of the ethics committee and the ethic guideline in Japan, written consent was not required in exchange for public disclosure of study information in the case of retrospective and/or observational study using a material such as the existing documentation. The study information was open for the public consumption at http://www.med.hirosaki-u.ac.jp/~uro/html/IRB/IRBdoc.html.
About this article
Cite this article
Tanaka, Y., Hatakeyama, S., Hosogoe, S. et al. Presurgical axitinib therapy increases fibrotic reactions within tumor thrombus in renal cell carcinoma with thrombus extending to the inferior vena cava. Int J Clin Oncol 23, 134–141 (2018). https://doi.org/10.1007/s10147-017-1169-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-017-1169-z