Abstract
Background
Intensity-modulated radiation therapy (IMRT) reduces the dose delivered to organs at risk. However, there have been few direct comparisons of IMRT with conventional three-dimensional conformal radiotherapy (3DCRT). The aim of this study was to evaluate the clinical benefit of IMRT in terms of toxicity and biochemical control.
Methods
The medical records of 203 consecutive patients with localized to non-metastatic (stage T1a–T3bN0M0) prostate cancer between 2007 and 2011 were retrospectively reviewed. The prescribed dose was 76 Gy delivered in 38 fractions in both the 3DCRT and IMRT treatment groups. The frequency of grade 2 or greater late gastrointestinal (GI) and genitourinary toxicity and biochemical control were estimated by the log-rank test and Cox proportional hazards model with and without adjustment by the propensity score for treatment choice.
Results
A total of 159 patients were included in the study (3DCRT: 70 patients, IMRT: 89 patients). The median follow-up period was 4.7 years. The estimated 5-year cumulative risk of late GI toxicity was significantly lower in the IMRT group than in the 3DCRT group (3.6 vs 13.2%, respectively, p = 0.022). After adjustment by propensity score, IMRT remained associated with a lower risk of late GI toxicity (hazard ratio 0.22; 95% confidence interval 0.058–0.85; p = 0.028). The 5-year biochemical failure-free rate was 93.2% in the 3DCRT group and 95.4% in the IMRT group (non-significant difference).
Conclusions
The incidence of late GI toxicity was significantly lower in the IMRT group than in the 3DCRT group, while the biochemical control rates were no different between the two groups. These clinical data suggest the benefit of IMRT in the reduction of late GI toxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several clinical trials performed in the 1990s confirmed the safety and efficacy of dose-escalated radiotherapy for the treatment of localized prostate cancer [1–6]. However, an escalated prescription dose was also found to result in higher risks of late toxicities [1, 2, 4–7], which led in turn to the development and implementation of highly conformal dose delivery to lower these toxicities. The 1990s also saw developments in treatment technology, first with three-dimensional conformal radiation therapy (3DCRT) replacing two-dimensional treatment [8], followed at the end of the 1990s by the emergence of intensity-modulated radiation therapy (IMRT) as an evolutionary form of 3DCRT [9]. IMRT is a relative new technology in radiation therapy that can deliver a dose distribution around a complex and irregular target volume. Planning studies have demonstrated that IMRT can reduce the dose to surrounding tissue without reducing planning target volume (PTV) coverage [10, 11].
Although the implementation of IMRT into a wide variety of treatment programs has been rapid and widespread, there have been few studies of the modality itself in prospective clinical trials. We hypothesized that the use of IMRT is associated with less toxicity but that it may impair coverage of the prostate and consequently harm tumor control. To evaluate this hypothesis, we retrospectively evaluated the outcomes of patients who received definitive radiation therapy as either 3DCRT or IMRT and compared the toxicity and biochemical control outcomes of these two patient cohorts.
Methods and materials
In March 2007, IMRT was initiated at our institution for the treatment of localized prostate cancer as part of the routine treatment program together with conventional 3DCRT. From this time onward, the choice of treatment was left to the patients.
Patient population
A retrospective review of the medical records of patients with localized (stage T1–T3N0M0) and pathologically proven prostate cancer who had received external beam radiation therapy between March 2007 and December 2011 at our institution identified 203 consecutive patients. The inclusion criteria were: (1) no previous treatment for prostate cancer, with the exception of neo-adjuvant androgen deprivation therapy (ADT); (2) ≥1 year of follow-up; (3) prostate-confined radiation therapy. Ultimately, a total of 159 patients were included in this analysis.
Patient-related characteristics including age, National Comprehensive Cancer Network (NCCN) risk classification, Gleason score, pre-treatment serum prostrate-specific antigen (PSA) values, and status of neoadjuvant and adjuvant ADT were recorded [12]. Use of anticoagulant agents and presence of co-existing diabetes mellitus were also documented, both of which are known risk factors for gastrointestinal (GI) and genitourinary (GU) toxicities [7, 13, 14].
Radiation and androgen deprivation therapy
Clinical target volume (CTV) was defined as the entire prostate for low-risk patients and as the entire prostate and the proximal seminal vesicles for intermediate- to high-risk patients. The PTV included the CTV with a 10-mm margin except posteriorly, where a 5-mm margin was used. Elective pelvic nodal irradiation was not applied to any patients in this study. For patients treated with 3DCRT, the seminal vesicles were excluded from the CTV after the cumulative dose reached 66 Gy. The prescribed dose to the prostate was 76 Gy in 38 fractions and was delivered as the mean dose to the PTV in IMRT treatment and to the isocenter in 3DCRT.
Radiation therapy was delivered by 10-MV photons in both treatment groups, and both groups were subject to the same dose–volume constraints for normal tissues. For each treatment the patient was in the prone position. Preceding each treatment the patient underwent a bladder and bowel preparation protocol; stool control was encouraged and patients were restricted from urinating 1 h before treatment. A belly board was applied to reduce respiratory-induced target motion in the prone position [15].
For set-up verification and correction, on-line bony anatomy matching with megavoltage electronic portal imaging was obtained in the first five treatment fractions and once a week after that.
For patients classified in the intermediate- to high-risk group, neoadjuvant ADT was given 3 months prior to radiotherapy. Adjuvant ADT was given to high-risk patients. A luteinizing hormone-releasing hormone agonist was used and combined with an anti-testosterone agent for the first 2 weeks to suppress the flare reaction.
Follow-up and post-treatment periods
After treatment, the patients were followed in the clinic every 3 months to check serum PSA levels and physical findings. No additional treatment was performed unless the patients developed biochemical failure or clinical failure.
Definitions and endpoints
Late toxicities were defined as those which occurred >90 days after initiation of radiation therapy. These toxicities were evaluated by two radiation oncologists following the Common Toxicity Criteria for Adverse Events (CTCAE) version 4.0 of the National Cancer Institute of the National Institutes of Health (Bethesda, MD). The frequency of grade 2 or higher late GI and GU toxicities at 5 years after the initiation of radiation therapy was the main objective of this analysis. The biochemical failure-free (BFF) rate was also evaluated. Biochemical failure was defined as a serum PSA rise of ≥2 ng/ml above the nadir.
Statistical analysis
The balance of baseline characteristics between the two cohorts was tested by the Mann–Whitney U test and Chi-square test. Comparisons of the toxicity and biochemical control between the two groups were done by log-rank test.
To minimize the effect of potential selection bias between the two groups, multivariate logistic regression analysis was performed by calculating patients propensity scores for treatment choice on the basis of age, risk group, use of anticoagulant agents, co-existing diabetes mellitus, and the prescribed radiation dose. As the propensity scores did not have normal distribution, they were logit-transformed for analysis. The association of the treatment and outcome was estimated using Cox proportional hazards model, both unadjusted and adjusted for propensity score. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient and tumor characteristics
A total of 159 patients with a mean age of 71 (range 49–84) years were included in this study (70 receiving 3DCRT; 89 receiving IMRT). The median duration of the median follow-up period was 4.7 (range 1.3–7.9) years for the entire living patient cohort, 5.1 years for the 3DCRT group, and 4.5 years for the IMRT group. The main tumor-related and treatment-related characteristics of the two treatment groups are shown in Table 1. At diagnosis, five, 31, and 34 patients in the 3DCRT group and 11, 42, and 36 patients in the IMRT group were classified into the low-, intermediate- and high-risk groups, respectively, according to the NCCN criteria. The Gleason score was significantly lower in the IMRT group, while other patient characteristics, including age, T stage, NCCN risk category, use of anticoagulant agents, and diabetes, were not significantly different between the two groups. A modified dose (70–74 Gy in 2.0-Gy fractions) delivery protocol was used for 18 patients (11.3% of all patients, of whom 12 were in the 3DCRT group and 6 were in the IMRT group). The reasons for dose modification were history of abdominal surgery for colorectal cancer (6 patients), acute treatment toxicity (5 patients), clinical determination of radiation oncologist to use a lower prescription dose for low-risk prostate cancer against the institutional protocol (5 patients), and other co-existing disease (2 patients).
Treatment-related toxicity
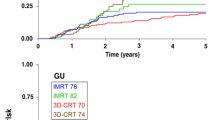
Grade 2 or greater GI adverse events were observed in nine patients (grade 2: 8 patients, grade 3: 1 patient) in the 3DCRT group and in three patients (grade 2: 2 patients, grade 3: 1 patient) in the IMRT group. Regarding GU adverse events, a total of three patients had grade 2 adverse events, of whom one patient was in the 3DCRT group and two patients were in the IMRT group; there were no grade 3 GU adverse events. The estimated 5-year cumulative risk of grade 2 or greater GI toxicity for patients in the 3DCRT and IMRT groups was 13.2 and 3.6% (p = 0.022), respectively, and that of Grade 2 or greater GU toxicity was 1.6 and 2.5% (p = 0.72), respectively (Fig. 1). The median interval from treatment to the development of grade 2 or greater toxicities was 20 (range 8.5–39) months. All grade 2 or greater adverse events are listed in Table 2.
Kaplan–Meier plot of the actuarial likelihood of late grade 2 or greater gastrointestinal toxicity events (a) and late grade 2 or greater genitourinary toxicity events (b) for patients undergoing three-dimensional conformal radiotherapy (3DCRT, blue line) and intensity-modulated radiotherapy (IMRT, yellow line)
Table 3 shows the results of the unadjusted and propensity score-adjusted Cox proportional hazards model. When adjusted by propensity scores, IMRT remained associated with less frequent GI toxicity events [hazard ratio 0.22; 95% confidence interval (CI) 0.058–0.85; p = 0.028].
Biochemical control
Biochemical failure was observed in three patients of the 3DCRT group and four patients of the IMRT group. The 5-year BFF rate was 93.2% (95% CI 85.6–100.0) in the 3DCRT group and 95.4% (95% CI 90.8–100.0) in the IMRT group (p = 0.79) (Fig. 2). The BFF rates for the low-, intermediate-, and high-risk groups were 100.0, 94.5, and 91.5%, respectively. In any sub-group of risk categories, the BFF rate was not different between the 3DCRT and IMRT groups.
Discussion
In this study, both treatment groups shared common conditions in terms of dose prescription, target definition, use of ADT, position-reiterating modality, and treatment period. We observed that late GI toxicity was significantly lower in our patients treated with IMRT. This finding may provide the answer to the simple question of whether IMRT has a clinical advantage over 3DCRT in definitive prostate therapy.
The toxicity analysis performed by Radiation Therapy Oncology Group (RTOG) 01-26 revealed that patients treated with IMRT experienced grade 2 or greater late GI toxicity events less frequently than those treated with 3DCRT (9.7 vs 15.1%) [16]. This study is the basis of one of the most reliable reports of toxicity data, since the data were prospectively collected. To the contrary, Bruner et al. in their subsequent patient-reported quality of life (QOL) outcome analysis of the same prospective study failed to show a meaningful advantage of IMRT on treatment-related QOL [17]. In a partial cohort analysis of the Dutch phase III trial, Al-Mamgani et al. reported that there was a significantly lower incidence of acute grade 2 or greater GI toxicity events in patients treated with IMRT than in those treated with 3DCRT (20 vs 61%, p = 0.001), with a moderate improvement in the acute GU and late GI and GU toxicity rates [18]. Zelefski et al. reported that the use of IMRT significantly reduced the risk of rectal toxicities compared with conventional 3DCRT (13–5%, p < 0.001) [19].
Each of these four trials [16–19] adopted a different prescription definition for IMRT. For example, the RTOG 01-26 trial [16] required that 98% of the PTV and 100% of the CTV be covered with the prescribed dose. In light of International Commission on Radiation Units and Measurement (ICRU) report 83 [20], which claims that the median absorbed dose (D50%) should be reported in IMRT, the prescription used in RTOG 01-26 was somewhat higher than the conventional prescription. Based on ICRU 83, the mean dose for the PTV was used in our study for IMRT, which can minimize the difference in the delivered dose between IMRT and 3DCRT.
Earlier trials also are marred by bias and unbalanced patient cohorts. In our study, both patient cohorts were relatively well balanced, and biases were adjusted by the propensity score. The data of our study demonstrate a clinically meaningful difference between the two treatment modalities.
Many studies have reported a correlation between dose volume histogram and toxicity. At the present time, one of the most reliable reviews of toxicity in this context is the Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC) [21]. The QUANTEC recommendations are primarily based on 3DCRT DVH analysis; the impact of IMRT and image-guided radiation therapy (IGRT) are not reflected.
At the present time, it does not appear possible to start a new randomized trial to evaluate the advantage of IMRT over 3DCRT. Analysis of the dose–toxicity relationship analysis, such as the QUANTEC criteria, planning studies that show the advantage of IMRT for DVHs, and retrospective clinical reports such as the present study are the best possible strategies to study IMRT as the standard treatment technique in EBRT for localized prostate cancer.
The accuracy of treatment may also affect treatment-related toxicity. Relatively new technologies, such as image-guidance using cone-beam computed tomography and implanted fiducial markers, further increase the precision of radiation therapy. With higher precision IGRT, the irradiated dose for organs at risk can be reduced as a result of positional correction and a reduced setup margin.
Zelefski et al. reported that implanted prostatic fiducial markers and daily kV images were associated with a lower rate of late urinary toxicity [22]. Shingh et al. also reported a lower incidence of GI toxicity with implanted prostatic fiducial markers and daily kV images [23]. Wortel et al. compared image-guided IMRT with 3DCRT and reported that the use of these technologies reduced the dose delivered to organs at risk, leading to a clinically meaningful reduction of acute toxicity levels in routine clinical prostate treatment [24]. Generally, with a more conformal treatment strategy, the greater is the concern about insufficient target coverage.
To the best of our knowledge, no prospective trial has demonstrated the non-inferiority of IMRT for biochemical control.
The two treatment groups in our study had an equally good BFF rate, although the study population was too small and too heterogeneous to detect a meaningful difference in biochemical control outcomes. A larger population-based study or carefully matched pair analysis is needed to examine this issue in more detail.
There are a number of limitations to our study. First, the selection bias of the treatment modality is undeniable. As mentioned earlier, in the early implementation period, only a limited number of patients were treated with IMRT, which may have affected treatment decisions, although the patient characteristics, including risk criteria and other known risk factors, of the two groups were relatively well balanced. Second, adverse events were retrospectively evaluated, raising the possibility that some events were missed. Nonetheless, most late GI events were clearly evident and observed within 2 years of treatment initiation, and the observation period was sufficiently long. The difference in dose constraints may also have affected the outcome. As the dose–volume data were lost, DVH analysis could not be conducted in this study. In addition, the follow-up duration is not adequate to predict the biochemical control for a longer period.
In conclusion, with the same prescribed dose and setting, late GI toxicity was significantly improved in the IMRT group, while the biochemical control rates were no different between the two groups. These data suggest that IMRT is of clinical benefit in terms of reducing the incidence of late GI toxicity in definitive localized prostate cancer treatment.
References
Hanks GE, Hanlon AL, Schultheiss TE et al (1998) Dose escalation with 3D conformal treatment: five year outcomes, treatment optimization, and future directions. Int J Radiat Oncol Biol Phys 41:501–510
Dearnaley DP, Hall E, Lawrence D et al (2005) Phase III pilot study of dose escalation using conformal radiotherapy in prostate cancer: PSA control and side effects. Br J Cancer 92:488–498
Zietman AL, DeSilvio ML, Slater JD et al (2005) Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA 294:1233–1239
Peeters ST, Heemsbergen WD, Koper PC et al (2006) Dose-response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol 24:1990–1996
Kuban DA, Tucker SL, Dong L et al (2008) Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys 70:67–74
Zelefsky MJ, Fuks Z, Hunt M et al (2001) High dose radiation delivered by intensity modulated conformal radiotherapy improves the outcome of localized prostate cancer. J Urol 166:876–881
Zelefsky MJ, Cowen D, Fuks Z et al (1999) Long term tolerance of high dose three-dimensional conformal radiotherapy in patients with localized prostate carcinoma. Cancer 85:2460–2468
Dearnaley DP, Khoo VS, Norman AR et al (1999) Comparison of radiation side-effects of conformal and conventional radiotherapy in prostate cancer: a randomised trial. Lancet 353:267–272
Sheets NC, Goldin GH, Meyer AM et al (2012) Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA 307:1611–1620
Ling CC, Burman C, Chui CS et al (1996) Conformal radiation treatment of prostate cancer using inversely-planned intensity-modulated photon beams produced with dynamic multileaf collimation. Int J Radiat Oncol Biol Phys 35:721–730
De Meerleer GO, Vakaet LA, De Gersem WR et al (2000) Radiotherapy of prostate cancer with or without intensity modulated beams: a planning comparison. Int J Radiat Oncol Biol Phys 47:639–648
National Comprehensive Cancer Network (2016) NCCN clinical practice guidelines in oncology (NCCN Guideline): Prostate cancer (version 3.2016). http://www.nccn.org/. Accessed 30 Aug 2016
Choe KS, Jani AB, Liauw SL (2010) External beam radiotherapy for prostate cancer patients on anticoagulation therapy: how significant is the bleeding toxicity? Int J Radiat Oncol Biol Phys 76:755–760
Kalakota K, Liauw SL (2013) Toxicity after external beam radiotherapy for prostate cancer: an analysis of late morbidity in men with diabetes mellitus. Urology 81:1196–1201
Terashima K, Nakamura K, Shioyama Y et al (2013) Can a belly board reduce respiratory-induced prostate motion in the prone position?—assessed by cine-magnetic resonance imaging. Technol Cancer Res Treat 12:447–453
Michalski JM, Yan Y, Watkins-Bruner D et al (2013) Preliminary toxicity analysis of 3-dimensional conformal radiation therapy versus intensity modulated radiation therapy on the high-dose arm of the Radiation Therapy Oncology Group 0126 prostate cancer trial. Int J Radiat Oncol Biol Phys 87:932–938
Bruner DW, Hunt D, Michalski JM et al (2015) Preliminary patient-reported outcomes analysis of 3-dimensional radiation therapy versus intensity-modulated radiation therapy on the high-dose arm of the Radiation Therapy Oncology Group (RTOG) 0126 prostate cancer trial. Cancer 121:2422–2430
Al-Mamgani A, Heemsbergen WD, Peeters ST et al (2009) Role of intensity-modulated radiotherapy in reducing toxicity in dose escalation for localized prostate cancer. Int J Radiat Oncol Biol Phys 73:685–691
Zelefsky MJ, Levin EJ, Hunt M et al (2008) Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 70:1124–1129
International Commission on Radiation Units and Measurement (ICRU) (2010) ICRU Report 83: prescribing, recording, and reporting photon-beam intensity-modulated radiation therapy (IMRT). J ICRU 10(1):NP. doi: 10.1093/jicru/ndq001
Michalski JM, Gay H, Jackson A et al (2010) Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys 76:S123–S129
Zelefsky MJ, Kollmeier M, Cox B et al (2012) Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 84:125–129
Singh J, Greer PB, White MA et al (2013) Treatment-related morbidity in prostate cancer: a comparison of 3-dimensional conformal radiation therapy with and without image guidance using implanted fiducial markers. Int J Radiat Oncol Biol Phys 85:1018–1023
Wortel RC, Incrocci L, Pos FJ et al (2015) Acute toxicity after image-guided intensity modulated radiation therapy compared to 3D conformal radiation therapy in prostate cancer patients. Int J Radiat Oncol Biol Phys 91:737–744
Acknowledgements
We thank Makoto Saito for his contribution to the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
About this article
Cite this article
Shimizuguchi, T., Nihei, K., Okano, T. et al. A comparison of clinical outcomes between three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for prostate cancer. Int J Clin Oncol 22, 373–379 (2017). https://doi.org/10.1007/s10147-016-1057-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-016-1057-y