Abstract
Purpose
The purpose of this work was to compare toxicity and cancer control between patients with prostate cancer treated using three-dimensional conformal radiotherapy (3D-CRT) and those treated using intensity-modulated radiation therapy (IMRT).
Methods and materials
A total of 553 patients with prostate cancer were treated with 3D-CRT 70–74 Gy (3D-CRT 70, 3D-CRT 74) or IMRT 78–82 Gy (IMRT 78, IMRT/SIB 82). Late toxicity was scored according to FC-RTOG/LENT criteria. Biochemical failure was defined using the Phoenix and ASTRO definitions.
Results
The 5-year risk of grade 2–4 genitourinary toxicity was 26.3 % (3D-CRT 70), 27.2 % (3D-CRT 74), 17.3 % (IMRT 78), and 25.1 % (IMRT/SIB 82) without statistical differences. The 5-year risk of grade 2–4 gastrointestinal toxicity was 19.4 % (3D-CRT 70), 42.1 % (3D-CRT 74), 20.5 % (IMRT 78), and 26.6 % (IMRT/SIB 82). The differences between 3D-CRT 74 and 3D-CRT 70 and between 3D-CRT 74 and IMRT 78 were statistically significant (log rank p = 0.03). The 5-year Phoenix PSA relapse-free survival (PSA-RFS) in low-risk, intermediate-risk, and high-risk patients treated using 3D-CRT were 89.4, 65.5, and 57.8 %, respectively. Patients treated with IMRT achieved the following results: 90.9, 89.4, and 83.9 %. Clinical relapse-free survival (C-RFS) in patients treated using 3D-CRT vs. IMRT for the aforementioned groups were 94.7 vs. 100 %, 86.8 vs. 98.6 %, and 84.4 vs. 94.5 %. Disease-free survival (DFS) for patients treated using 3D-CRT were 83.1, 70.9, and 71.5 %. The IMRT group reached 95.8, 89.1, and 87.6 %. The PSA-RFS for intermediate- and high-risk patients were statistically significant, while C-RFS and DFS were marginally better.
Conclusion
Dose escalation with IMRT was associated with improved cancer control in intermediate- and high-risk patients in comparison with 3D-CRT, without compromising toxicity.
Zusammenfassung
Zielsetzung
Es erfolgte ein Vergleich von Toxizität und Tumorkontrolle bei Patienten mit Prostatakarzinom nach der Behandlung mit dreidimensionaler konformaler Strahlentherapie (3D-CRT) und intensitätsmodulierter Strahlentherapie (IMRT).
Patienten und Methodik
Es wurden 553 Patienten mit Prostatakarzinom mit 3D-CRT 70–74 Gy (3D-CRT 70, 3D-CRT 74) oder IMRT 78–82 Gy (IMRT 78, IMRT/SIB 82) behandelt. Späte Toxizität wurde gemäß FC-RTOG/LENT-Kriterien bewertet. Biochemisches Versagen wurde unter Verwendung der Phoenix- und ASTRO-Definition festgelegt.
Ergebnisse
Das 5-Jahres-Risiko einer Urogenitaltoxizität Grad 2–4 lag bei 26,3 % (3D-CRT 70), 27,2 % (3D-CRT 74), 17,3 % (IMRT 78) und 25,1 % (IMRT/SIB 82) mit keinem statistischen Unterschied. Das 5-Jahres-Risiko einer Gastrointestinaltoxizität Grad 2–4 betrug 19,4 % (3D-CRT 70), 42,1 % (3D-CRT 74), 20,5 % (IMRT 78) und 26,6 % (IMRT/SIB 82). Die Differenz zwischen 3D-CRT 74 und 3D-CRT 70 bzw. zwischen 3D-CRT 74 und IMRT 78 ist statistisch signifikant (Logrank-Test p = 0,03). Die 5-Jahres-Überlebensrate ohne PSA-Relaps (Phoenix PSA-RFS) bei Patienten mit niedrigem, mittlerem und hohem Risiko lag nach 3D-CRT bei 89,4, 65,5 und 57,8 %. Nach IMRT lag die entsprechende Überlebensrate bei 90,9, 89,4 und 83,9 %. Das klinische rezidivfreie Überleben (C-RFS) nach 3D-CRT vs. IMRT bei den oben genannten Risikogruppen betrug jeweils 94,7 vs. 100 %, 86,8 vs. 98,6 % und 84,4 vs. 94,5 %. Das krankheitsfreies Überleben (DFS) betrug nach der Behandlung mit 3D-CRT 83,1, 70,9 und 71,5 %, nach der IMRT Behandlung 95,8, 89,1 und 87,6 %. Bei Mittel- und Hochrisikopatienten ist die PSA-RFS-Differenz statistisch signifikant, und C-RFS und DFS zeigen geringfügig bessere Resultate.
Schlussfolgerung
Bei Mittel- und Hochrisikopatienten mit Prostatakarzinom führt eine Dosiseskalation mit IMRT gegenüber der 3D-CRT zu besserer Tumorkontrolle ohne kompromittierende Toxizität.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
For a long time, three-dimensional conformal radiotherapy (3D-CRT) was the standard treatment technique for prostate irradiation [1–5]. In recent years, new technologies such as intensity-modulated radiation therapy (IMRT) and image-guided radiotherapy (IGRT) have been introduced [6–15]. IMRT makes it possible to minimize the volume of normal tissue irradiated to high doses by producing steeper dose gradients [16–18]. The need to reduce toxicity is enhanced by the growing evidence of prostate cancer dose dependence. Recently, four randomized controlled trials have focused on the role of dose levels in conformal radiotherapy with photons. All trials have confirmed improved biochemical results in patients treated with higher radiation doses compared with lower doses, but unfortunately even higher toxicity in escalated arms [19–22].
IMRT offers not only critical structure sparing, but also irradiation of different targets at different dose levels within a single treatment session. This treatment strategy, called simultaneous integrated boost (SIB), has become established in many anatomical sites. This technique has also been used in prostate cancer treatment for combined irradiation of prostate and pelvic nodes in high-risk patients or for dose escalation to the prostate only or to intraprostatic lesions [23–26].
Although a body of literature has demonstrated the ability of IMRT to reduce radiation doses to organs at risk compared with 3D-CRT, there is a relative lack of studies directly comparing patient outcomes, including morbidity and cancer control. Therefore, it is necessary to establish whether these dosimetric benefits can translate into improved patient outcomes. [27].
Patients and methods
Between December 1997 and February 2008, a total of 553 patients with biopsy-proven localized prostate cancer were primarily treated with curative radiation therapy using 3D-CRT and IMRT. Pretreatment diagnostic evaluation consisted of physical examination (digital rectal examination included), PSA, Gleason score (GS) in the biopsy specimen, computed tomography of pelvis, skeletal scintigraphy and transrectal ultrasonography of the prostate. Patients were separated into three recurrence risk groups according to the National Comprehensive Cancer Network guidelines. 3D-CRT was used in 320/553 patients (57.9 %) and IMRT in 233/553 patients (42.1 %).
Radiation technique
The radiation treatment technique used was described previously [24, 28]. Briefly, patients were planned and treated in a supine position and they were advised to have a comfortably full bladder. A vacuum cushion for knee and foot support (VacLok/Dual Leg Positioner Cushion, MED-TEC) was used for immobilization.
In the 3D-CRT group, the clinical target volume (CTV) included the prostate and the base of the seminal vesicles. In cases with seminal vesicle invasion, the prostate and all of the seminal vesicles were covered. The planning target volume (PTV) was created by adding an isotropic 10 mm margin. Organs at risk (rectum and bladder) were delineated in CT slices 10 mm superior and inferior of the slices containing the PTV. Patients were treated with four wedged fields (30 °, 90 °, 270 °, 330 °) shaped with a 52-leaf multileaf collimator (MLC), 10 mm width per leaf. The prescribed dose was 70 Gy in a daily fraction of 2 Gy, 5 times a week for 229 patients primarily with stage T1–T2 (3D-CRT 70) and 74 Gy in 37 fractions for 91 patients with T3 tumors (3D-CRT 74). Treatment verification was performed by using the electronic portal imaging device (PortalVision 3.8. Varian) once a week with accepted inaccuracy of 6 mm.
Two IMRT techniques were used. IMRT with a dose of 78 Gy in 39 fractions to the prostate and seminal vesicles (IMRT 78) in 160 patients and simultaneous integrated boost with a dose of 82 Gy in 41 fractions to the prostate and concurrently 73.8 Gy in 41 fractions to the seminal vesicles (IMRT/SIB 82) in 73 patients without seminal vesicle invasion. The PTV was generated by an isotropic 10 mm expansion of the CTV. The dose was prescribed at the isocenter. The treatment technique included five coplanar fields (45 °, 100 °, 180 °, 260 °, 315 °), the intensity-modulated beams were delivered with a dynamic MLC, using the sliding window technique. The following constraints for PTV and organs at risk were observed: at least 95 % of the PTV received 95 % of the prescribed dose; no more than 25 % of the rectal volume and 30 % of the bladder volume could receive a dose of 70 Gy; no more than 15 % or 15 cm³ of the rectal volume in absolute could receive a dose of 75 Gy [24, 28]. For treatment plan calculation, the planning system CadPlan R 6.3.6 Helios/Eclipse 7.3 was used, and treatment was delivered by Linac 600C linear accelerator using 52-leaf MLC Mark with a width of 10 mm per leaf (Varian Medical Systems, Palo Alto, CA). Treatment verification was identical as for the 3D-CRT cohort.
Hormonal treatment
In the 3D-CRT cohort, hormonal treatment was only administered in patients with clinically locally advanced stage (T3). Hormonal treatment consisted of a combination of antiandrogen and luteinizing hormone-releasing hormone for 4–6 months before and during radiotherapy. Patients with GS 8–10 were treated using long-term hormonal treatment for 2–3 years.
In the IMRT era, hormonal treatment was administered in all patients with high-risk cancer with an analogous treatment regimen. Patients with GS 2–7 were treated using neoadjuvant and concomitant hormonal treatment for 4–6 months before and during radiotherapy and patients with GS 8–10 were treated using long-term adjuvant treatment for 2–3 years.
Follow-up
All patients were continuously followed during and after radiotherapy. The patients were scheduled to be seen every week during radiotherapy, 1 month after the end of treatment, every 3 months for the first two years, every 6 months for the third to fifth year and once a year thereafter. Late gastrointestinal (GI) and genitourinary (GU) symptoms were recorded at each visit, using Fox Chase (FC) modification of the Radiation Therapy Oncology Group (RTOG) and Late Effects Normal Tissue Task Force (LENT) toxicity criteria [29]. The routine DRE and PSA were performed at each visit. Definition of event for the disease-free survival was as follows: clinical recurrence or death from any cause, whichever comes first. Clinical relapse-free survival was defined as survival without clinical recurrence (local/regional recurrence, distant metastases).
Statistics
The Kaplan–Meier product–limit method was selected to determine the risk of late toxicity development over time, the PSA relapse-free survival, the clinical relapse-free survival, the cancer-specific survival and overall survival. Treatment techniques (3D-CRT 70, 3D-CRT 74, IMRT 78, IMRT/SIB 82) were compared using a log-rank test. The Cox–Mantel test was employed to calculate the hazard ratio of late toxicity for particular treatment techniques.
Results
Patient distribution concerning age, T stage, GS, pretreatment PSA, risk group, additional hormonal therapy, history of urologic surgery, volume of prostate, and maximal proportion of tumor is shown in detail in Table 1. The median follow-up for 3D-CRT and IMRT was 104 (range 6–180) and 60 (range 7–110) months.
Acute toxicity
We observed acute grade 2–4 genitourinary toxicity in 30.1, 31.9, 33.1, and 28.8 % of patients in the 3D-CRT 70, 3D-CRT 74, IMRT 78, and IMRT/SIB 82 group, respectively. All cases of grade 4 toxicity (1.3, 6.6, 3.7, and 4.2 %) were caused by urethral obstruction requiring placement of a urinary catheter. Acute grade 1 genitourinary toxicity was noticed in 38.4 % (3D-CRT 70), 36.3 % (3D-CRT 74), 35.0 % (IMRT 78), and 36.9 % (IMRT/SIB 82) of patients. Symptoms of acute genitourinary toxicity persisted for more than 1 month in 24.0 % (3D-CRT 70), 27.5 % (3D-CRT 74), 36.2 % (IMRT 78), and 32.9 % of patients in the IMRT/SIB 82 group. Symptoms were present for more than 12 weeks after treatment in 6.1 % (3D-CRT 70), 8.8 % (3D-CRT 74), 13.1 % (IMRT 78), and 10.9 % (IMRT/SIB 82) of patients.
Overall, no fatal toxicity or grade 3–4 acute gastrointestinal toxicity was observed. Acute grade 2 gastrointestinal toxicity was noticed in 32.3 % (3D-CRT 70), 34.1 % (3D-CRT 74), 14.3 % (IMRT 78), and 9.5 % (IMRT/SIB 82) of patients. Acute gastrointestinal toxicity grade 1 was observed in 41.9 % (3D-CRT 70), 37.4 % (3D-CRT 74), 38.1 % (IMRT 78), and 36.9 % (IMRT/SIB 82) of patients. Acute gastrointestinal toxicity continued for more than 4 weeks in 25.3 % (3D-CRT 70), 29.7 % (3D-CRT 74), 26.8 % (IMRT 78), and 23.3 % of patients from IMRT/SIB 82 group.
Late toxicity
The proportion of patients suffering from severe late toxicity was low. No fatal late GI/GU toxicity and one case of grade 4 toxicity were observed. The only patient with grade 4 toxicity underwent cystectomy for intractable hematuria 4.5 years after 3D-CRT to a dose of 70 Gy. He also suffered from coagulopathy due to liver cirrhosis. The 5-year risk of grade 2–4 GU toxicity was 26.3 % (3D-CRT 70), 27.2 % (3D-CRT 74), 17.3 % (IMRT 78), and 25.1 % (IMRT/SIB 82) without statistical differences. The median time to the development of grade ≥ 2 GU toxicity was 36 months (range 6–156 months).
The 5-year risk of grade 2–3 GI toxicity was 19.4 % (3D-CRT 70), 42.1 % (3D-CRT 74), 20.5 % (IMRT 78), and 26.6 % (IMRT/SIB 82). The differences between 3D-CRT 74 and 3D-CRT 70 and also between 3D-CRT 74 and IMRT 78 were statistically significant (log rank p = 0.03). The median time to the development of grade 2–3 GI toxicity was 18 months (range 6–96 months). Detailed analysis of late toxicity is shown in Table 2 and Fig. 1.
Cancer control
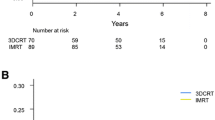
The 5-year ASTRO PSA relapse-free survival, Phoenix PSA relapse-free survival and 5-year disease-free survival rates for intermediate- and high-risk patients treated using dose-escalated IMRT were significantly better in comparison with 3D-CRT. The high-risk patients treated using IMRT achieved also significant improvement in terms of clinical relapse-free survival, cancer-specific survival, and overall survival at 5 years. Detailed analysis is presented in Table 3, Figs. 2, 3.
Discussion
Although a body of literature has demonstrated the ability of IMRT to reduce radiation doses to organs at risk compared with 3D-CRT, there is a relative lack of studies directly comparing patient outcomes, including morbidity and cancer control.
We report the long-term results of 553 patients with prostate cancer who underwent primary radiation therapy using 3D-CRT or IMRT. We observed improved 5-year PSA relapse-free survival and disease-free survival in patients at intermediate and high risk, treated using dose-escalated IMRT 78–82 Gy in comparison with 3D-CRT 70–74 Gy without compromising toxicity results. Conversely, patients treated with higher doses using IMRT experienced less GI toxicity grade 3 compared with patients treated using 3D-CRT 74 Gy.
Our results compare favorably with previously published data. Zelefsky et al. [30] reported a fivefold reduction in 2-year actuarial incidence of late grade 2 and 3 rectal bleeding for patients treated with 81 Gy IMRT compared with 81 Gy 3D-CRT. In subset analysis of a Dutch trial (70 Gy vs 78 Gy), IMRT was associated with reduced 5-year late grade ≥ 2 GI toxicity, without statistical significance (p = 0.16) [31]. Similar results were obtained in subset analysis of the RTOG 0126 study, comparing 491 patients treated to the dose 79.2 Gy using 3D-CRT to 257 patients treated to the same dose level using IMRT [32]. The cumulative incidence of GI toxicity grade ≥ 2 at 3 years was 22 vs 15.1 % (p = 0.039). Retrospective studies and studies using the Surveillance, Epidemiology, and End Results (SEER)-Medicare database have demonstrated similar findings [33–35].
As in the present article, most studies have not found a reduction in late GU toxicity from IMRT compared with 3D-CRT [31, 32, 34]. These findings were consistent with the results of two SEER-Medicare studies [35]. Conversely, Zelefsky et al. [33] reported that IMRT to 81 Gy was associated with a higher rate of late GU toxicity grade ≥ 2 in comparison with 3D-CRT 66–81 Gy.
There is now growing evidence supporting a benefit of dose escalation. Four randomized controlled trials have confirmed improved biochemical control outcomes with higher radiation dose levels compared with lower doses [19–22]. Recently, higher radiation dose levels were consistently associated with reduction in distant metastases [4]. Increasing use of dose escalation with 3D-CRT techniques leads to higher morbidity, especially long-term rectal toxicity. The assumption that IMRT will improve the oncological outcome without affecting toxicity was confirmed by Vora et al. [36]. In this retrospective study patients were treated with 3D-CRT to 68.4 Gy or by IMRT to 75.6 Gy. The patients in the IMRT group had improved 5-year biochemical control without significant difference in GI or GU toxicity. Our results are consistent with this data. Moreover, the improvement in PSA relapse-free survival was translated into significantly better clinical relapse-free survival in intermediate- and high-risk patients.
The presented study can be influenced by retrospective bias. The median of follow-up for patients treated using 3D-CRT was 104 months vs 60 months in patients receiving IMRT. The results of high-risk patients were also related to changes in hormonal treatment policies. Whereas all patients with high risk in the IMRT cohort underwent hormonal treatment, in the 3D-CRT cohort, only patients with locally advanced T3 tumors were treated using hormonal therapy. Nevertheless, we are convinced that improved results for intermediate-risk patients in our series are mainly due to dose escalation using IMRT with relative large CTV–PTV margins. First, contouring and margins remained identical since the beginning of the 3D-CRT era. Second, treatment verification was identical for all patients. Third, no patients with intermediate risk in either subgroup were treated with hormonal therapy. Fourth, treatment staging was identical for both groups (transrectal ultrasonography was performed in all patients by only two radiologists, MRI was not part of staging procedure during this period). And finally, all patients were followed prospectively and DRE with PSA were performed at each visit. To minimize the retrospective bias by eliminating the pathologic migration, we compared patients with PSA value 8–18 μg/l without any hormonal treatment according to the treatment technique. The 5-year DFS for 3D-CRT cohort was 82.9 % in comparison with 95.6 % for patients treated using IMRT (p = 0.03). This result is consistent with the explorative analysis of a Dutch trial for identical subgroups of patients published recently by Heemsbergen et al. [37].
We believe that a possible explanation of the improved outcome in patients with intermediate risk is combination of dose escalation and using of relatively large CTV–PTV margins. The mean dose in this subgroup was 80.2 Gy for IMRT group while all patients with intermediate risk in the 3D-CRT group were treated to 70 Gy. In the MRC RT01 trial, no margins were used after 64 Gy. For patients treated in the Dutch trial, a 5 mm margin was used after 68 Gy, except for the interface between CTV and rectal wall where no margin was taken to spare rectum. In a French trial the PTV was obtained by adjunction of a 10 mm margin in all directions except posterior, where the margin was reduced to 5 mm. The largest margins were used in MD Anderson, where the PTV included a margin of 10 mm superiorly and inferiorly, 10–12.5 mm anteriorly, and 7.5 mm posteriorly.
These results can be improved by the use of image-guided radiotherapy [9, 38, 39]. Targeted irradiation and reduction of the safety margin can lead to a further decrease of toxicity and better biochemical tumor control [14].
Conclusion
In our retrospective study, the use of IMRT with dose escalation to 78–82 Gy was associated with improved PSA relapse-free survival and clinical relapse-free survival at 5 years in patients at intermediate and high risk in comparison with 3D-CRT 70–74 Gy without compromising toxicity results. These results should be confirmed in prospective study to exclude retrospective bias.
References
Hanks GE, Hanlon AL, Epstein B et al (2009) Dose response in prostate cancer with 8–12 years’ follow-up. Int J Radiat Oncol Biol Phys 54:427–435
Goldner G, Bombosch V, Geinitz H et al (2009) Moderate risk-adapted dose escalation with three-dimensional conformal radiotherapy of localized prostate cancer from 70–74 Gy. First report on 5-year morbidity and biochemical control from a prospective Austrian–German multicenter phase II trial. Strahlenther Onkol 185:94–100
Goldner G, Wachtner S, Wachtner-Gerstner N et al (2006) Long-term results in three-dimensional conformal radiotherapy of localized prostate cancer at moderate dose (66 Gy). Strahlenther Onkol 182:537–542
Zelefsky MJ, Pei X, Chou JF et al (2011) Dose escalation for prostate cancer radiotherapy: predictors of long-term biochemical tumor control and distant metastases-free survival outcomes. Eur Urol 60:1133–1139
Zelefsky MJ, Cowen D, Fuks Z et al (1999) Long term tolerance of high dose three-dimensional conformal radiotherapy in patients with localized prostate carcinoma. Cancer 85:2460–2468
Cahlon O, Hunt M, Zelefsky MJ (2008). Intensity-modulated radiation therapy: supportive data for prostate cancer. Semin Radiat Oncol 18:48–57
Spratt DE, Pei X, Yamada J et al (2013) Long-term survival and toxicity in patients treated with high-dose intensity modulated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 85:686–692
Gershkevitsh E, Clark CH, Staffurth J et al (2005) Dose to bone marrow using IMRT techniques in prostate cancer patients. Strahlenther Onkol 181:172–178
Kok D, Gill S, Bressel M et al (2013) Late toxicity and biochemical control in 554 prostate cancer patients treated with and without dose escalated image guided radiotherapy. Radiother Oncol 107:140–146
Guckenberger M, Mayer J, Wilbert J et al (2007) Precision of image-guided radiotherapy (IGRT) in six degrees of freedom and limitations in clinical practice. Strahlenther Onkol 183:307–313
Guckenberger M, Flentje M (2007). Intensity-Modulated Radiotherapy (IMRT) of localized prostate cancer. Strahlenther Onkol 183:57–62
Guckenberger M, Lawrenz I, Flentje M (2014). Moderately hypofractionated radiotherapy for localized prostate cancer: long-term outcome using IMRT and volumetric IGRT. Strahlenther Onkol 190:48–53
Pinkawa M, Purch-Lee M, Asadpour B et al (2008) Image-guided radiotherapy for rostate cancer. Implementation of ultrasound-based prostate localization for the analysis of inter- and intrafraction organ motion. Strahlenther Onkol 184:679–685
Zelefsky MJ, Kollmeier M, Cox B et al (2012) Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 84:125–129
Schulze D, Liang J, Yan D et al (2009) Comparison of various online IGRT strategies: the benefits of online treatment plan re-optimization. Radiother Oncol 90:367–376
Bauman G, Rumble RB, Chen J et al (2012) Intensity-modulated radiotherapy in the treatment of prostate cancer. Clin Oncol (R Coll Radiol) 24:461–473
Perna L, Fiorino C, Cozzarini C et al (2009) Sparing the penile bulb in the radical irradiation of clinically localised prostate carcinoma: a comparison between MRI and CT prostatic apex definition in 3DCRT, Linac-IMRT and Helical Tomotherapy. Radiother Oncol 93:57–63
Oh CE, Antes K, Darby M et al (1999) Comparison of 2D conventional, 3D conformal, and intensity-modulated treatment planning techniques for patients with prostate cancer with regard to target-dose homogeneity and dose to critical, uninvolved structures. Med Dosim 24:255–263
Al-Mamgani A, van Putten WLJ, Heemsbergen WD et al (2008) Update of the Dutch multicenter dose escalation trial of radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 72:980–988
Dearnaley DP Jovic G Syndikus I et al (2014) Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol 15:464–473
Kuban DA, Tucker SL, Dong L et al (2008) Long-term results of the M.D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys 70:67–74
Zietman AL, DeSilvio ML, Slater JD et al (2005) Comparison of conventional-dose vs high dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA 294: 1233–1239
Alongi F, Fogliata A, Navarria P et al (2012) Moderate hypofractionation and simultaneous integrated boost with volumetric modulated arc therapy (RapidArc) for prostate cancer. Report of feasibility and acute toxicity. Strahlenther Onkol 188:990–996
Dolezel M, Odrazka K, Vaculikova M et al (2010) Dose escalation in prostate radiotherapy up to 82 Gy using simultaneous integrated boost: direct comparison of acute and late toxicity with 3D-CRT 74 Gy and IMRT 78 Gy. Strahlenther Onkol 186:197–202
Pinkawa M, Holy R, Piroth MD et al (2010) Intensity-modulated radiotherapy for prostate cancer implementing molecular imaging with 18F-choline PET-CT to define a simultaneous integrated boost. Strahlenther Onkol 186:600–606
Fonteyne V, Villeirs G, Speleers B et al (2008) Intensity-modulated radiotherapy as primary therapy for prostate cancer: report on acute toxicity after dose escalation with simultaneous integrated boost to intraprostatic lesion. Int J Radiat Oncol Biol Phys 72:799–807
Pearlstein KA, Chen RC (2013). Comparing dosimetric, morbidity, quality of life, and cancer control outcomes after 3D conformal, intensity-modulated, and proton radiation therapy for prostate cancer. Semin Radiat Oncol 23:182–190
Odrazka K, Zouhar M, Petera J et al (2005) Comparison of rectal dose-volume constraints for IMRT prostate treatment planning. Phys Med 21:129–135
Hanlon AL, Schultheiss TE, Hunt MA et al (1997) Chronic rectal bleeding after high-dose conformal treatment of prostate cancer warrants modification of existing morbidity scales. Int J Radiat Oncol Biol Phys 38:59–63
Zelefsky MJ, Fuks Z, Happersett L et al (2000) Clinical experience with intensity modulated radiation therapy (IMRT) in prostate cancer. Radiother Oncol 55:241–249
Al-Mamgani A Heemsbergen WD Peeters ST et al (2009) Role of intensity-modulated radiotherapy in reducing toxicity in dose escalation for localized prostate cancer. Int J Radiat Oncol Biol Phys 73:685–691
Michalski JM, Yan Y, Watkins-Bruner D et al (2013) Preliminary toxicity analysis of 3-dimensional conformal radiation therapy versus intensity modulated radiotherapy on the high-dose arm of the RTOG 0126 prostate cancer trial. Int J Radiat Oncol Biol Phys 87:932–938
Zelefsky MJ Levin EJ Hunt M et al (2008) Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 70:1124–1129
Sharma NK, Li T, Chen DY et al (2011) Intensity-modulated radiotherapy reduces gastrointestinal toxicity in patients treated with androgen deprivation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 80:437–444
Sheets NC, Goldin GH, Meyer AM et al (2012) Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA 307:1611–1620
Vora SA, Wong WW, Schild SE et al (2007) Analysis of biochemical control and prognostic factors in patients treated with either low-dose three-dimensional conformal radiation therapy or high-dose intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 68:1053–1058
Heemsbergen WD, Al-Mamgani A, Slot A et al (2014) Long-term results of the Dutch randomized prostate cancer trial: impact of dose-escalation on local, biochemical, clinical failure, and survival. Radiother Oncol 110:104–109
Kupelian PA, Langen KM, Willoughby TR et al (2008) Image-guided radiotherapy for localized prostate cancer: treating a moving target. Semin Radiat Oncol 18:58–66
Paluska P, Hanus J, Sefrova J et al (2012) Utilization of cone-beam CT for offline evaluation of target volume coverage during prostate image-guided radiotherapy based on bony anatomy alignment. Reports of Practical Oncology and Radiotherapy 17:134–140
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Dolezel, K. Odrazka, M. Zouhar, M. Vaculikova, J. Sefrova, J. Jansa, P. Paluska, T. Kohlova, J. Vanasek and J. Kovarik state that there are no conflicts of interest.
All studies on humans described in the present manuscript were carried out with the approval of the responsible ethics committee and in accordance with national law and the Helsinki Declaration of 1975 (in its current, revised form). Informed consent was obtained from all patients included in studies.
Rights and permissions
About this article
Cite this article
Dolezel, M., Odrazka, K., Zouhar, M. et al. Comparing morbidity and cancer control after 3D-conformal (70/74 Gy) and intensity modulated radiotherapy (78/82 Gy) for prostate cancer. Strahlenther Onkol 191, 338–346 (2015). https://doi.org/10.1007/s00066-014-0806-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-014-0806-y