Abstract
Background
We analyzed the treatment outcomes after curative surgery for stage IV colorectal cancer to develop outcome-based follow-up protocols and treatment strategies.
Methods
This study was a multi-institutional retrospective analysis of treatment outcomes in patients who underwent R0 surgery for stage IV colorectal cancer.
Results
A total of 1133 patients, of whom 837 had recurrence, were included in this study. Recurrence occurred within 12 and 24 months after R0 surgery in 452 (54.0 %) and 652 (77.9 %) patients, respectively. Surgical resection was performed less frequently for recurrence within 12 months of R0 surgery than for recurrence after more than 12 months (p = 0.003). Prognosis was significantly better in patients who had recurrence more than 24 months after R0 surgery than in those who had recurrence within 24 months; this was not only for all patients but also specifically for patients with resection for recurrent disease. Recurrence was less frequent in patients who received preoperative chemotherapy than in patients who did not receive preoperative chemotherapy (p = 0.04). Of significance, fewer patients who received preoperative chemotherapy (57.5 %) had recurrence within 24 months compared with patients who did not receive preoperative chemotherapy (79.8 %) (p = 0.00001).
Conclusions
Intensive follow-up for at least 24 months was considered appropriate for monitoring disease recurrence after R0 surgery for stage IV colorectal cancer. In addition, preoperative chemotherapy contributed to improved outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third leading cause of cancer-related death in Japan and this rate has been increasing. Approximately 15–20 % of patients with CRC are diagnosed with synchronous distant metastases despite widespread screening for early detection [1, 2]. However, surgical resection has been established for some metastatic CRC lesions [3], and cure can even be expected in some patients.
To date, the optimal management of stage IV CRC after R0 surgery remains to be established. There are some reports that suggest the efficacy of adjuvant chemotherapy after R0 surgery for stage IV CRC [4, 5]. Although the efficacy of repeated surgery has been reported for recurrent disease after R0 surgery for stage IV CRC [6, 7], the efficacies of postoperative adjuvant chemotherapy as well as repeated surgery for recurrent disease are still controversial. Recently, the beneficial effect of preoperative chemotherapy for stage IV CRC has been reported [8].
Follow-up after R0 surgery has traditionally been performed for the early detection of recurrence. However, recommendations are not consistent. The national comprehensive cancer network (NCCN) guideline recommends intensive follow-up for 2 years after curative surgery in cases of stage IV CRC, following a similar protocol to that used for patients with stage II/III CRC [3]. In contrast, the European Society for Medical Oncology (ESMO) guideline recommends a more intensive follow-up protocol for patients with stage IV CRC compared with stage II/III CRC, indicating that an intensive follow-up period of 3 years is appropriate after R0 surgery [9]. However, the prognostic efficacies of these protocols are not yet proven and, to date, no evidence-based follow-up protocol has been established for stage IV CRC.
This study was conducted to facilitate the development of evidence-based follow-up protocols and treatment strategies informed by treatment efficacy. We analyzed the treatment outcomes after R0 surgery for stage IV CRC, particularly with regard to the disease-free period, in a multicenter retrospective study of Japanese patients referred to hospitals in the Japanese Study Group for Postoperative Follow-up of Colorectal Cancer.
Patients and methods
Patients and study design
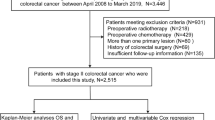
The data of 1133 patients (675 males and 458 females; median age 63.0 years; age range 18–93 years) with stage IV CRC who underwent R0 resection between January 1997 and December 2007 were collected from the databases of all 20 referral hospitals of the Japanese Study Group for Postoperative Follow-up of Colorectal Cancer. R0 surgery for multiple organ metastases was performed in 69 patients (liver and lungs in 26, liver and peritoneal in 14, liver and lymph node in 11, liver and other organs in 5, lungs and peritoneum in 2, peritoneum and lymph node in 7, peritoneum and other organs in 1, liver, peritoneum, and lymph node in 2, liver, lungs, and other in 1). Their treatment outcomes were then analyzed.
Definitions and diagnosis
Clinical and pathological data were recorded according to the Japanese Society for Cancer of the Colon and Rectum (JSCCR) classification system [10]. Tumor depth of invasion and lymph node metastases were classified according to the seventh edition of the TNM classification system [11]. The cut-off values for carcinoembryonic antigen (CEA) and CA19-9 were 5.0 ng/mL and 37.0 U/mL, respectively. The cut-off values for the number of dissected lymph nodes in the analyses of factors affecting recurrence were determined using receiver operating characteristic curve analyses. Intensive chemotherapy was defined as any regimen that contained fluorouracil or fluorouracil derivatives and irinotecan or oxaliplatin.
Follow-up assessment
After R0 surgery, postoperative surveillance was performed according to the JSCCR guidelines for the treatment of CRC [12]. Follow-up consisted of a physical examination, serum CEA, and CA19-9 measurements every 3 months for the first 3 years and every 6 months for the following 2 years; abdominal imaging (ultrasonography and/or computed tomography) and chest computed tomography were performed every 6 months for 5 years; and a barium enema or colonoscopy was performed every 1–2 years for 5 years. Patients were observed for at least 5 years after surgery.
Recurrences were confirmed either histologically or radiologically. The grade of metastasis was classified into three subgroups according to extent and size, using the Japanese classification of colorectal carcinoma by the JSCCR [10] as follows—liver metastasis (H1, 1–4 metastatic tumors all of which are ≤5 cm in maximum diameter; H2, not H1 or H3; and H3, ≥5 metastatic tumors, of which at least one is >5 cm in maximum diameter), lung metastasis (LM1, metastasis limited to one lobe; LM2, metastasis in more than one lobe, but restricted to one lung; and LM3, metastasis in both lungs or the presence of lymphangitis carcinomatosis, pleuritis carcinomatosis, or hilar node metastasis), and peritoneal metastasis (P1, metastasis localized to adjacent peritoneum; P2, limited metastasis to distant peritoneum; and P3, diffuse metastasis to distant peritoneum).
Statistical analysis
The Mann–Whitney U test or an independent t test was used for statistical analysis. Categorical variables were analyzed using the chi-squared test or Fisher’s exact probability test. Factors affecting recurrence were analyzed using binomial logistic regression analyses. Survival rates were calculated according to the Kaplan–Meier method and compared using the log-rank test. Multiple comparisons were performed with Bonferroni adjustment. A p-value of <0.05 was considered statistically significant. Data were statistically analyzed using JMP 11 software (SAS Institute Inc., Cary, NC, USA). All data are expressed as numbers of patients and frequencies (%).
Results
Patients
Tumors were located in the colon in 816 patients (72.0 %) and the rectum in 317 patients (28.0 %). The median follow-up period was 45.7 months (range 0.2–201.7 months).
Factors affecting recurrence after R0 surgery
Recurrence occurred in 837 patients (73.9 %) after R0 surgery and was significantly associated with multiple organ metastases (p = 0.005), peritoneal metastases (p = 0.02), lymph node metastases (p = 0.00001), preoperative chemotherapy (p = 0.04), and venous invasion (p = 0.04) by univariate analysis (Table 1). Logistic regression analyses revealed that, of these factors, multiple organ metastases and lymph node metastases independently affected recurrence (Table 1).
Perioperative chemotherapy and recurrence according to the metastatic organ
Table 2 summarizes the results for perioperative chemotherapy and recurrence according to the metastatic organ. Recurrence after R0 surgery was more frequent after the resection of liver (p = 0.04) and peritoneal (p = 0.004) metastases than after resection of lung metastases.
Preoperative chemotherapy was administered to 111 patients (9.8 %), and adjuvant chemotherapy was administered to 684 patients (60.4 %) after R0 surgery. Intensive adjuvant chemotherapy was prescribed in 76 patients (6.7 %), and there were no significant differences in the administration frequencies of intensive adjuvant chemotherapy by metastatic organ. Recurrence occurred in 73 patients receiving preoperative chemotherapy (65.8 %) and 522 patients receiving postoperative adjuvant chemotherapy (76.3 %). A combination of preoperative and postoperative chemotherapy was administered to 64 patients (5.6 %), among whom recurrence occurred in 49 (76.6 %). Neither preoperative nor postoperative chemotherapy was administered to 369 patients (32.6 %), and 273 (74.0 %) of these patients developed recurrence.
Preoperative chemotherapy was administered significantly more and adjuvant chemotherapy significantly less for lung metastases than for other metastases. Furthermore, preoperative chemotherapy was administered more frequently for multiple organ metastases than for solitary metastases (p = 0.009). Although there were no significant differences in the frequencies of recurrence between patients who did and did not receive postoperative adjuvant chemotherapy, recurrence was less frequent in those receiving preoperative chemotherapy (65.8 %) than in those who did not (74.8 %; p = 0.04), especially among patients with lung metastases (p = 0.02) and solitary metastases (p = 0.02). Recurrence occurred more frequently in patients receiving adjuvant chemotherapy after lung surgery than in those who did not (p = 0.02).
Of the 73 patients with recurrence after preoperative chemotherapy, 22 (30.1 %) had recurrence within 12 months after R0 surgery, and 42 (57.5 %) had recurrence within 24 months. Recurrence rates within 12 or 24 months after R0 surgery were significantly reduced for patients who received preoperative chemotherapy than for those who did not (12 months = 56.3 %; 24 months = 79.8 %) (p = 0.00001). Recurrence was found in 53 of the 76 patients who received intensive adjuvant chemotherapy (69.7 %) and in 784 of the 1057 patients who did not receive intensive adjuvant chemotherapy (74.2 %). The recurrence rate was significantly lower after 2005 (70.2 %; 276/393), when oxaliplatin was approved for use in CRC in Japan, than before (75.8 %; 561/740) (p = 0.04).
Treatment for disease recurrence
Treatment for first recurrence after R0 surgery is shown in Table 3 according to the organ in which recurrence developed. Surgical resection for recurrent disease was performed more frequently for liver recurrence than for other recurrences (p = 0.0001) but was performed less frequently for recurrent disease in multiple organs than for solitary organs (p = 0.03). The frequencies of recurrence after repeated resections for solitary (65.3 %) and multiple organ recurrences (77.3 %) were not significantly different (p = 0.27).
The treatments for recurrent disease are shown in Table 4 according to the disease-free period. Of the 837 patients who had recurrence, 452 (54.0 %) patients developed recurrence within 12 months, and 652 (77.9 %) within 24 months. Of the 791 patients whose disease-free period was known, 652 (82.4 %) developed recurrence within 24 months, and 759 (96.0 %) developed recurrence within 48 months. Recurrence occurred within 12 months for 452 patients, and 149 of these (33.0 %) underwent surgical resection, whereas it occurred after 12 months in 339 patients, and 147 of these (44.0 %) underwent surgical resection. With regard to the disease-free period, surgical resection was performed less frequently for recurrent disease that occurred within 12 months after R0 surgery than for one that occurred more than 12 months after R0 surgery (p = 0.003).
The surgical resection rate for recurrent disease is shown in Table 5 according to the organ of recurrence and disease-free period. Similar to the results for treatment by disease-free period alone, surgical resection was performed more frequently for recurrent lesions beyond 12 months after R0 surgery than before 12 months after R0 surgery in both liver (p = 0.00001) and local recurrences (p = 0.007). Surgical resection was performed more frequently for recurrent lesions beyond 12 months after R0 surgery than for recurrence within 12 months after R0 surgery, not only in solitary organ recurrence (p = 0.003) but also in multiple organ recurrence (p = 0.004).
Prognosis after recurrence
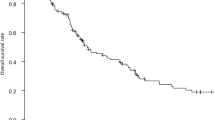
The survival curve after recurrence according to the disease-free period in stage IV CRC with R0 surgery is shown in Fig. 1a. The survival curve after recurrence according to the disease-free period in stage IV CRC in patients who underwent surgical resection for recurrent disease after R0 surgery is shown in Fig. 1b. Prognosis was significantly better in patients who had recurrence more than 24 months after R0 surgery than in those who had recurrence within 24 months; this was not only for all patients but also specifically for patients with resection for recurrent disease.
a Prognosis after recurrence according to disease-free period after curative surgery in all stage IV patients with colorectal cancer. b Prognosis after recurrence according to disease-free period after curative surgery in stage IV patients with colorectal cancer who underwent surgery for recurrent diseases
Discussion
According to data in the JSCCR guidelines for the treatment of CRC, synchronous distant metastases typically occur in the following order of decreasing prevalence—liver metastasis (55.8 %), peritoneal metastasis (22.9 %), and lung metastasis (12.2 %) [12]. In this study of 1133 patients, 69.7, 17.4, 10.4, and 7.5 % underwent R0 surgery for liver, peritoneal, lung, and distant lymph node metastases, respectively. Although some patients with distant metastases are suitable candidates for curative surgery, criteria for R0 resectability have not been standardized. Therefore, treatment typically depends on the experience of the multidisciplinary team. In this study, recurrence occurred after R0 surgery in 73.9 % of all patients with stage IV CRC.
There have been some retrospective reports of the factors affecting recurrence after R0 surgery in stage IV CRC [13–16]. In patients with liver metastases, associated lymph node metastasis, associated extrahepatic disease, pre-surgical serum CEA level, disease-free interval, and the largest tumor diameter in the liver have each been related to recurrence [13, 14]. In patients with peritoneal metastases, the presence of lymph node metastasis was an important factor affecting recurrence [14], whereas in patients with lung metastases, the number and distribution of pulmonary tumors were associated with disease-free survival [16].
In the present study, we showed that multiple organ metastases and lymph node metastases were independent factors affecting recurrence. The postoperative recurrence rate was significantly lower for patients with lung metastases than for those with either liver or peritoneal metastases, although recurrence was high regardless of the organ in which metastasis developed. Some reports have documented the treatment outcomes after R0 surgery for multiple organ metastases, but most of these reports on treatment outcomes for synchronous two-organ metastases, mainly involve the liver and lungs [17–19]. There are no clear resection criteria for multiple organ metastases, and treatment outcomes after R0 surgery are controversial even for cases with two-organ metastases. Indeed, R0 surgery for liver and lung metastases accounted for only 38 % of all cases of multiple organ metastases treated with R0 surgery in the present study. No recurrence was found in >10 % of cases with multiple organ metastases after R0 surgery, despite the recurrence rate being significantly higher than those with solitary organ metastasis. Therefore, aggressive surgery may be expected to improve treatment outcomes, especially in cases of two-organ metastases.
For recurrent disease after R0 surgery in stage IV CRC, a few reports suggest the utility of repeated surgery to improve the prognosis, although repeated resection is challenging for recurrent disease [6, 7]. Resection was performed in 37 % of patients with recurrence in this study, and the resection rate differed by the organ affected. Notably, rates were higher in liver recurrence and lower in lymph node recurrence than in other organs. Although the resection rate was significantly lower in cases with recurrence in multiple organs than that in cases with recurrence in a solitary organ, there was no significant difference in the recurrence rates. Moreover, repeated aggressive resection was performed in 12 % of patients with recurrence in multiple organs, and almost 34 % of patients with repeated resection for recurrent disease had no recurrence. Thus, the efficacy of repeated resection for recurrent disease after R0 surgery for stage IV CRC was suggested.
In stage IV CRC, the efficacies of preoperative chemotherapy and postoperative adjuvant chemotherapy are controversial. This study did not support the efficacy of adjuvant chemotherapy to decrease recurrence, but it must be noted that we were limited to data collected between 1997 and 2007. This could have resulted in bias because of the tendency to reserve adjuvant chemotherapy for more advanced cases or to use intensive chemotherapy less frequently at that time. Indeed, compared with the period before 2005, the recurrence rate was significantly reduced after 2005 when oxaliplatin was approved for use in CRC in Japan. In contrast, recurrence was less frequent in patients receiving preoperative chemotherapy than in those who did not, especially among those with lung metastases. Therefore, our data indicate that preoperative chemotherapy contributes to improved treatment outcomes in stage IV CRC.
The ESMO guideline recommends that intensive follow-up is needed for at least 3 years to identify recurrence after R0 surgery for stage IV CRC [9], whereas the NCCN guideline recommends 2 years [3]. In this study, intensive follow-up was performed for at least 3 years, consistent with the JSCCR guidelines [12]. Among patients whose disease-free period was known, >80 % of recurrences were found within 2 years of R0 surgery in the present study. The American Society of Clinical Oncology recommend intensive follow-up for 3 years after R0 surgery in patients with stage II and III CRC because 80 % of recurrences occur in the first 2.5 years [20]. Thus, intensive follow-up for at least 2 years may be appropriate. The NCCN guideline recommends post-treatment surveillance for 5 years for patients with stage II and III CRC because 95 % of recurrences occurred in the first 5 years. Among patients whose disease-free period was known, 96 % of recurrences were found within 4 years of R0 surgery in this study. Thus, follow-up for at least 4 years may be appropriate for patients with stage IV CRC. However, this study shows that the resection rate was low among patients who had recurrence within 12 months and that the prognosis was poor among those who had recurrence within 24 months. In other words, our results may indicate that intensive follow-up offers no benefit to treatment outcomes, although it was useful in identifying recurrence. The outcomes of this study suggest that extending the period between R0 surgery and recurrence would contribute to the improvement of the resection rate and prognosis. Furthermore, recurrence within 24 months was significantly less among patients who received preoperative chemotherapy, indicating that this may effectively extend the disease-free interval and improve both the resection rate and prognosis. Intensive follow-up beyond 2 years after R0 surgery may only be significant in patients receiving preoperative chemotherapy.
Notably, this study was retrospective and included only a small number of patients who received preoperative chemotherapy. Therefore, our observations warrant further evaluation and validation in a larger series of patients with stage IV CRC. Some clinical trials on the efficacy of intensive adjuvant chemotherapy after R0 surgery or preoperative chemotherapy are ongoing for patients with stage IV CRC, and it is expected that these will provide validated criteria for the administration of preoperative chemotherapy and adjuvant chemotherapy after R0 surgery in stage IV CRC.
In conclusion, intensive follow-up for at least 2 years is probably appropriate for discovering recurrent disease after R0 surgery in stage IV CRC. However, after R0 surgery, the rate of additional resection was low in this study for recurrence within 12 months, and the prognosis was poor for recurrence within 24 months. The data also indicate that preoperative chemotherapy may contribute to prognostic improvement in stage IV CRC by extending survival from R0 surgery to recurrence. Thus, we consider that intensive follow-up beyond 2 years after R0 surgery would be appropriate if preoperative chemotherapy is administered. It is expected that a postoperative follow-up protocol will be developed in the future that can improve prognosis and that appropriate guidance will be developed for the administration of preoperative and postoperative chemotherapy in patients with stage IV CRC requiring R0 surgery.
References
Nitzkorski JR, Farma JM, Watson JC et al (2012) Outcome and natural history of patients with stage IV colorectal cancer receiving chemotherapy without primary tumor resection. Ann Surg Oncol 19:379–383
Watanabe T, Itabashi M, Shimada Y et al (2015) Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 20:207–239
Network NCC (2016) Clinical practice guidelines in oncology: Colon Cancer Version 2 2016. http://www.nccn.org. Accessed 1 May 2016
Mitry E, Fields AL, Bleiberg H et al (2008) Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol 26:4906–4911
Parks R, Gonen M, Kemeny N et al (2007) Adjuvant chemotherapy improves survival after resection of hepatic colorectal metastases: analysis of data from two continents. J Am Coll Surg 204:753–761
Morise Z, Sugioka A, Fujita J et al (2006) Does repeated surgery improve the prognosis of colorectal liver metastases? J Gastrointest Surg 10:6–11
Oba M, Hasegawa K, Shindoh J et al (2016) Survival benefit of repeat resection of successive recurrences after the initial hepatic resection for colorectal liver metastases. Surgery 159:632–640
Nordlinger B, Sorbye H, Glimelius B et al (2013) Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol 14:1208–1215
Van Cutsem E, Cervantes A, Nordlinger B et al (2014) Metastatic colorectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 25(Suppl 3):iii1–iii9
Japan Society for Cancer of the Colon and Rectum (ed) (2009) Japanese classification of colorectal carcinoma, 2nd English edition edn. Kanehara & Co, Tokyo
Sobin L, Gospondarowicz M, Wittekind C, UICC International Union Against Cancer (2009) TNM classification of malignant tumors, 7th edn. Wiley, New York, pp 100–105
Japan Society for Cancer of the Colon and Rectum (ed) (2014) Japanese classification of colorectal carcinoma, 2nd English edition edn. Kanehara & Co, Tokyo
Wei AC, Greig PD, Grant D et al (2006) Survival after hepatic resection for colorectal metastases: a 10-year experience. Ann Surg Oncol 13:668–676
Fong Y, Fortner J, Sun RL et al (1999) Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer. Ann Surg 230:309–321
Sato H, Maeda K, Kotake K et al (2015) Factors affecting recurrence and prognosis after R0 resection for colorectal cancer with peritoneal metastasis. J Gastroenterol 51:465–472
Watanabe K, Nagai K, Kobayashi A et al (2009) Factors influencing survival after complete resection of pulmonary metastases from colorectal cancer. Br J Surg 96:1058–1065
Ambiru S, Miyazaki M, Ito H et al (1998) Resection of hepatic and pulmonary metastases in patients with colorectal carcinoma. Cancer 82:274–278
Shah SA, Haddad R, Al-Sukhni W et al (2006) Surgical resection of hepatic and pulmonary metastases from colorectal carcinoma. J Am Coll Surg 202:468–475
Miller G, Biernacki P, Kemeny NE et al (2007) Outcomes after resection of synchronous or metachronous hepatic and pulmonary colorectal metastases. J Am Coll Surg 205:231–238
Meyerhardt JA, Mangu PB, Flynn PJ et al (2013) Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol 31:4465–4470
Acknowledgments
This paper was presented on behalf of the project team of the Japanese Study Group for Postoperative Follow-up of Colorectal Cancer. The authors thank the following institutional investigators for collecting the patient data—Masamichi Yasuno (Tokyo Medical and Dental University), Ichiro Takemasa (Sapporo Medical University), Kenichi Hakamada (Hirosaki University), Hitoshi Kameyama (Niigata University), Kazuo Hase (National Defense Medical University), Kenjiro Kotake (Tochigi Cancer Center), Toshiaki Watanabe (Tokyo University), Keiichi Takahashi (Tokyo Metropolitan Cancer and Infectious diseases Center Komagome Hospital), Yukihide Kanemitsu (National Cancer Center Hospital), Michio Itabashi (Tokyo Women’s Medical University), Hideo Yano (National Center for Global Health and Medicine), Masamichi Yasuno (Tokyo Medical and Dental University), Hirotoshi Hasegawa (Keio University), Yojiro Hashiguchi (Teikyo University), Tadahiko Masaki (Kyorin University), Masahiko Watanabe (Kitasato University), Koji Komori (Aichi Cancer Center Hospital), Yoshiharu Sakai (Kyoto University), and Masayuki Ohue (Osaka Medical Center for Cancer and Cardiovascular Diseases).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there is no conflict of interest in this manuscript.
Additional information
Disclaimers: This study has not been presented in part elsewhere. We certify that no portion of this manuscript has been previously published, and is not under consideration by another journal.
About this article
Cite this article
Sato, H., Maeda, K., Morise, Z. et al. Clinical outcomes of stage IV colorectal cancer after R0 resection: a multi-institutional retrospective analysis. Int J Clin Oncol 22, 297–306 (2017). https://doi.org/10.1007/s10147-016-1043-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-016-1043-4