Abstract
Background
Primary tumor resection (PTR) before commencing systemic chemotherapy in patients with stage IV colorectal cancer and unresectable metastases (mCRC) remains controversial. This study aimed to assess whether PTR before systemic chemotherapy is associated with mortality in mCRC patients, after adjusting for confounding factors, such as the severity of the primary tumor and metastatic lesions.
Methods
We analyzed hospital-based cancer registries from nine designated cancer hospitals in Fukushima Prefecture, Japan. Patients were divided into two groups (PTR and non-PTR), based on whether PTR was performed as initial therapy for mCRC or not. The primary outcome was all-cause mortality. Kaplan–Meier survival analysis was performed, and survival estimates were compared using the log-rank test. Adjusted hazard ratios were calculated using Cox regression to adjust for confounding factors. All tests were two-sided; P-values < 0.05 were considered statistically significant.
Results
Between 2008 and 2015, 616 mCRC patients were included (PTR: 414 [67.2%]; non-PTR: 202 [32.8%]). The median follow-up time was 18.0 (interquartile range [IQR]: 8.4–29.7) months, and 492 patients (79.9%) died during the study period. Median overall survival in the PTR and non-PTR groups was 23.9 (IQR: 12.2–39.9) and 12.3 (IQR: 6.2–23.8) months, respectively (P < 0.001, log-rank test). PTR was significantly associated with improved overall survival (adjusted hazard ratio: 0.51; 95% confidence interval: 0.42–0.64, P < 0.001).
Conclusions
PTR before systemic chemotherapy in patients with mCRC was associated with improved survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among colorectal cancer (CRC) cases, the proportion of patients with stage IV CRC has been estimated to be around 15–25% [1,2,3]. Although complete resection of the primary tumor and metastatic lesions is a radical treatment for these patients, almost all patients have unresectable distant metastases and therefore receive systemic chemotherapy[4]. Despite progress in systemic chemotherapy, which now includes targeted molecular agents, improvements in survival rates have been unsatisfactory[5].
Primary tumor resection (PTR) before systemic chemotherapy in patients with stage IV CRC with unresectable metastases (mCRC) is controversial. PTR improves the quality of life (QOL) and reduces the side effects of systemic chemotherapy as well as the risk of complications, such as bleeding, obstruction, and perforation that may occur due to the primary tumor[6, 7]. However, PTR prolongs the introduction of systemic chemotherapy with further delays if complications arise[8, 9].
Several randomized controlled trials (RCTs) have been conducted to investigate the effects of PTR in mCRC patients. However, one study required a reduction in the sample size as managing accumulating cases became difficult, and to date, all RCTs remain incomplete [10,11,12,13,14,15]. Moreover, the overall condition of these participants is better, with limited progression of the primary lesion and severity of distant metastases. These cases are, therefore, different from the cases in clinical practice where it is difficult to decide whether PTR should be performed. Although some observational studies have been conducted, these studies could not adjust for some important confounding factors, such as symptoms from the primary tumor, depth of tumor invasion, severity of regional lymph node metastases, and pattern and severity of distant metastases[16, 17].
This study aimed to assess whether PTR before systemic chemotherapy is associated with mortality in patients with mCRC, when adjusted for confounding factors, including severity of the primary tumor and metastatic lesions.
Materials and methods
Study design and cohort development
This was a multicenter, retrospective cohort study. All nine designated cancer hospitals across Fukushima Prefecture, Japan, participated. First, we extracted data of patients with stage IV CRC, defined based on the International Classification of Diseases for Oncology, Third Edition (ICD-O3) topographical codes: C18.0, C18.2–C18.9, C19.9, C20.9, from each hospital-based cancer registry. We extracted data on age, sex, primary tumor site, and degree of differentiation. Second, we extracted data from medical records and administrative data on patients’ clinical and demographic characteristics, including the Charlson comorbidity index (CCI), clinical symptoms, clinical staging (cTNM stage) (based on the TNM classification system (version 7) of the American Joint Committee on Cancer), Barthel index (a measure of activities of daily living (ADLs)), and type of treatment. Two gastrointestinal surgeons (MH and HK), who were blinded to the survival outcome, reviewed the medical records and computed tomography (CT) images before initial treatment in this cohort and made the diagnosis based on cTNM staging, metastatic pattern, and clinical symptoms of the primary tumor. Anonymized datasets acquired from individual hospitals were merged into a single dataset.
Patients were included if they fulfilled the following criteria: consecutive adult patients (≥ 18 years old) seen between 2008 and 2015, with histologically confirmed colorectal adenocarcinomas or intraoperatively diagnosed with stage IV CRC.
The exclusion criteria were as follows: metastasectomy for metastatic disease, best supportive care in main treatment for mCRC, perforation due to the primary tumor, emergency surgery, and absence of data on treatment strategy.
Definition of PTR and non-PTR
Patients who underwent PTR as initial treatment were classified into the PTR group, and the rest of the patients comprised the non-PTR group. Patients who underwent palliative surgery, such as colostomy and bypass, as initial treatment were also classified into the non-PTR group.
Outcomes
The primary endpoint was overall survival (OS), which was calculated as the number of days from the date of CRC diagnosis until death. Patients were censored if they were lost to follow-up or were still alive on December 31, 2017.
Covariates and categorization
Several demographic and clinical variables were included in the analysis, such as sex, age at diagnosis (< 75, ≥ 75 years), period of diagnosis (2008–2009, 2010–2012, 2013–2015), primary tumor site (right colon cancer, RCC: tumors located in the cecum, ascending colon, hepatic flexure, or transverse colon; left colon cancer, LCC: tumors located within the splenic flexure, descending colon, sigmoid colon or recto-sigmoid junction; rectal cancer: tumor located between rectum and anus), and degree of tumor differentiation (well or moderately differentiated, poorly or undifferentiated). The CCI was used to assess patients’ comorbidities at first admission for CRC-related hospitalization and was classified into three categories based on the CCI scores (0, 1–2, ≥ 3)[18]. The Barthel index was used to measure patients’ ADLs on admission and discharge after the first hospitalization. This index uses a scale of 0–100 and is classified into two categories (0–60, 61–100) for analysis [19]. Patients with obstruction or melena due to the primary tumor were defined as having clinical symptoms. Melena was defined as anemia requiring transfusion or bleeding that required medical intervention. Obstruction was defined as the inability of a colonoscope to pass through the primary lesion and/or the presence of obstructive symptoms (fullness, nausea, or vomiting).
Based on the 7th edition of the TNM classification [20], we classified patients into two categories (T1–3, and T4a or T4b) in terms of the T factor, and two categories (N0–1, N2) for the N factor. Based on the Japanese Classification of Colorectal Carcinoma [21], we distinguished the following types of metastases: liver metastases (hepatic tumors [HT], H1: ≤ 5 HT and HT size ≤ 5 cm; H2: ≥ 5 HT or HT size ≥ 5 cm; H3: ≥ 5 HT and HT size ≥ 5 cm) and pulmonary metastases (lung tumors [LT], PUL1: < 3 LT in one lung or two LTs in both lungs; PUL2: ≥ 3 LTs in both lungs; carcinomatous pleurisy; or mediastinal lymph node metastases). Additionally, we described the remaining metastatic patterns as follows: peritoneal dissemination (presence or absence), distal lymph node metastases (presence or absence), other organ metastases: bone, brain, ovary, and others (presence or absence), and number of metastatic organs (1 or ≥ 2).
Regarding treatment, in addition to the presence or absence of PTR, we described the number of days from the date of diagnosis to the introduction of systemic chemotherapy and classified the systemic chemotherapy regimen as follows: 5-fluorouracil (5-FU) (none, oral, or infusion), oxaliplatin (OX) or irinotecan (IRI) (none, OX, or IRI), and molecular target drug (none, anti-vascular endothelial growth factor [VEGF] drug, or anti-epidermal growth factor receptor [EGFR] drug).
Statistical analyses
Patient characteristics are reported as descriptive statistics, with continuous variables expressed as medians and interquartile ranges (IQR) and categorical variables expressed as counts and percentages. Univariate analyses were employed to compare the variables between the two groups, in which categorical variables were compared with the Fisher’s exact test, and continuous variables were compared with the Mann–Whitney U test. We described missing values and applied complete case analysis in the main analysis. For sensitivity analysis, we applied multiple imputations using the chained equation method for participants with one or more missing covariates. Twenty multiple imputed datasets were created, and the estimates from each dataset were combined using Rubin’s rule [22].
Survival analysis was performed using the Kaplan–Meier method, and survival estimates were compared using the log-rank test. The association between PTR and overall survival (OS) was analyzed using Cox proportional hazards regression models for all-cause mortality, adjusted for confounding and prognostic factors (age at diagnosis, sex, CCI, period of diagnosis, ADLs, clinical symptoms due to primary tumor, degree of tumor differentiation, T-stage, N-stage, liver metastases, lung metastases, peritoneal dissemination, distal lymph node metastases, other organ metastases, and number of metastatic organs). We carried out the following subgroup analyses: clinical symptoms due to the primary tumor (absence or presence) and the location of the primary tumor (colon cancer or rectal cancer). Adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. All significance tests were 2-sided, and P values < 0.05 were considered statistically significant. Statistical analyses were performed using STATA version 16.0 software (STATA Corporation, College Station, TX, USA).
Results
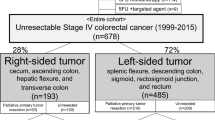
We identified 1,262 patients diagnosed with stage IV CRC, between 2008 and 2015, and excluded 478 patients based on the exclusion criteria. Moreover, 168 patients were excluded because of missing covariates: 109 without Barthel index data, 43 without data on the degree of differentiation, and 16 lacked data concerning the primary tumor site. As a result, 616 patients were included in the main analysis (Fig. 1). The PTR group had 414 patients (67.2%), and the non-PTR group had 202 (32.8%) patients. The median follow-up time was 18.0 months (IQR, 8.4–29.7), and 492 patients (79.9%) died during the study period. In the PTR group, 61 patients (14.7%) did not receive systematic chemotherapy following PTR. Twenty-seven patients (13.4%) in the non-PTR group did not receive systematic chemotherapy following palliative surgery.
Patient demographics and clinical characteristics are summarized in Table 1. The median age was 69 (IQR: 60–76) years. A total of 394 patients (64.0%) exhibited clinical symptoms from the primary tumor. A higher percentage of patients exhibited clinical symptoms in the PTR group (PTR: 69.8%; non-PTR group: 52.0%). Only one metastatic organ was more frequently reported in the PTR group (264 [63.8%]) than in the non-PTR group (103 (51.0%)). The incidences of various parameters in the non-PTR and PTR groups were as follows: rectal cancer (non-PTR: 74 [36.6%], PTR: 73 [17.6%]); T4 stage (non-PTR: 139 [68.8%], PTR: 265 [64.0%]); H3 or severe liver metastases (non-PTR: 73 [36.1%], PTR: 83 [20.0%]); PUL2 or severe lung metastases (non-PTR: 57 [28.2%], PTR: 77 [18.6%]); distal lymph node metastases (non-PTR: 76 [37.6%], PTR: 93 [22.5%]); other organ metastases (non-PTR: 17 [8.4%], PTR: 15 [3.6%]); and peritoneal dissemination (non-PTR: 21.8%, PTR: 28.3%).
Table 2 shows the distribution of systemic chemotherapy regimens in the two groups and surgical treatment in the non-PTR group. The median number of days from diagnosis to introduction of systemic chemotherapy was 49 and 28 days in the PTR and non-PTR groups, respectively. Among the non-PTR group, 97 patients (48.0%) underwent palliative surgery before commencing systemic chemotherapy, and 23 patients (11.4%) underwent primary tumor resection during systemic chemotherapy.
OS analysis was performed using the Kaplan–Meier method (Fig. 2). The median OS was 23.9 (IQR: 12.2–39.9) months and 12.3 (IQR: 6.2–23.8) months for the PTR and non-PTR groups, respectively (P < 0.001, log-rank test).
The effect of PTR on survival benefit was estimated using Cox proportional hazards regression models for all-cause mortality with complete case analysis (Table 3). The adjusted HR of the PTR group was 0.51 (95% CI: 0.42–0.64, P < 0.001), compared with the non-PTR group. Sensitivity analysis with multiple imputation methods revealed similar results for the PTR (HR = 0.49; 95% CI: 0.41–0.59, P < 0.001) (Supplemental material).
Figure 3 shows adjusted HRs for OS from the subgroup analysis performed on clinical symptoms due to primary tumor and the location of primary tumor. In each aspect, the PTR group was associated with better prognosis when compared with the non-PTR group.
Discussion
This retrospective cohort study evaluated the effectiveness of PTR before systemic chemotherapy in mCRC patients, and adjusted for confounding factors, including severity of the primary tumor and metastatic lesions. PTR before systemic chemotherapy for mCRC was associated with improved prognosis. A previous report from Japan revealed an association between PTR and prognosis in mCRC patients (HR: 0.46; 95% CI: 0.32–0.66), which demonstrated similar results to those found in our study [16]. It is noteworthy that molecular targeted drugs contribute to improved prognosis in mCRC patients [23], and the former study included patients diagnosed between 1997 and 2007, before molecular targeted drugs had been introduced in Japan. Meanwhile, our study included patients who could already receive molecular targeted drugs as standard treatment. Similar results obtained in our study suggest that PTR contributes to improved prognosis in mCRC patients regardless of the systemic chemotherapy regimen employed. The reason for the favorable prognosis associated with PTR may be the prevention of potential complications associated with the primary tumor during systemic chemotherapy.
Our study classified the severity of liver and lung metastases before treatment using CT scans, based on the Japanese Classification of Colorectal Carcinoma [21]. Additionally, our study investigated T and N factors with CT scans before treatment, which was lacking in a previous report [24]. In the non-PTR group, a higher percentage of patients had rectal cancer and T4b, for which it is difficult to perform PTR. Additionally, a higher percentage of non-PTR patients had H3, PUL2, distal lymph node metastases, and other organ metastases, which are poorer prognostic factors, as compared to PTR patients. These factors were biased between the groups, contributing to the need for decision-making for treatment and to the overall prognosis. Therefore, they could be important confounding factors.
Postoperative complications or delays in introducing systemic chemotherapy due to PTR could also be important factors that significantly impact prognosis. In our study, the time taken from making a diagnosis to introduction of systemic chemotherapy was 21 days longer in the PTR group than that in the non-PTR group. Additionally, 14.7% of the PTR group did not receive systemic chemotherapy. These results could be influenced by postoperative complications, which were lacking in our study. The incidence of postoperative complications in CRC is reported as 8–10% [25]. In particular, stage IV CRC had a higher incidence of postoperative complications than other stages of CRC, owing to the overall poor condition of patients [26]. In our study, a certain proportion of postoperative complications occurred in the PTR group. Despite this, our study showed an association between PTR and improved prognosis. In the future, it will be important to identify patients at high risk of postoperative complications and select low-risk surgical procedures for such patients.
To our knowledge, this is the first retrospective cohort study to assess whether PTR before systemic chemotherapy is associated with mortality in mCRC patients, after adjusting for confounding factors, such as severity of the primary tumor and metastatic lesion (based on the 7th edition of TNM classification and Japanese Classification of Colorectal Carcinoma). Although some RCTs are currently in progress, these have not been completed because of difficulties in gathering cases. Since patients with mCRC are often treated across various clinical departments, i.e., surgery, oncology, palliative care, etc., it is difficult to extract data of patients with stage IV CRC. Hospital-based cancer registries, which include almost all patients treated in the hospital and the registries of all designated cancer hospitals are in the same format, are useful to extract the data of patients with stage IV CRC and to conduct real-world and multicenter cohort studies. Additionally, the combination of hospital-based cancer registries and clinical records helped to overcome the limitations of depending on hospital-based cancer registries alone, which could lack certain information, such as severity of the primary tumor and metastatic lesions. Therefore, the results of our study could help clinicians to decide whether PTR before systemic chemotherapy is associated with mortality in mCRC patients.
There are several limitations to our study. First, unmeasured confounding factors could exist as a result of the doctor’s preferences and are difficult to measure. Second, although patients were enrolled from 2008 to 2015, treatment strategies, including intensive chemotherapeutic regimens and molecular analysis (RAS, BRAF, and MSI), have changed significantly. Thus, our study may not be fully reflective of the current medical practice. Third, there were missing data of covariates in 21.4% of cases. We described the missing values and applied multiple imputation methods to compensate for them in the sensitivity analysis. The findings in the main analysis and the sensitivity analysis were similar. Fourth, measurement bias could exist in the evaluation of peritoneal dissemination, since a higher percentage of patients in the PTR group had peritoneal dissemination, although the difference was not statistically significant. In mCRC, peritoneal dissemination is the poorest prognostic factor in the metastatic pattern [27]. A small amount of peritoneal dissemination that could not be identified in the images was identified during intraoperative findings in all patients in the PTR group.
Conclusions
PTR before systemic chemotherapy for unresectable mCRC was associated with improved survival. Pragmatic clinical trials involving mCRC patients, for whom surgeons would find it difficult to determine whether to perform PTR in clinical practice, are required.
References
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Miller KD, Siegel RL, Lin CC et al (2016) Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 66:271–289
Hori M, Matsuda T, Shibata A et al (2015) Cancer incidence and incidence rates in Japan in 2009: a study of 32 population-based cancer registries for the monitoring of cancer incidence in Japan (MCIJ) project. Jpn J Clin Oncol 45:884–891
de Haas RJ, Wicherts DA, Andreani P et al (2011) Impact of expanding criteria for resectability of colorectal metastases on short- and long-term outcomes after hepatic resection. Ann Surg 253:1069–1079
Van Cutsem E, Cervantes A, Adam R et al (2016) ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 27:1386–1422
Ruo L, Gougoutas C, Paty PB et al (2003) Elective bowel resection for incurable stage IV colorectal cancer: prognostic variables for asymptomatic patients. J Am Coll Surg 196:722–728
Cummins ER, Vick KD, Poole GV (2004) Incurable colorectal carcinoma: the role of surgical palliation. Am Surg 70:433–437
Benoist S, Pautrat K, Mitry E et al (2005) Treatment strategy for patients with colorectal cancer and synchronous irresectable liver metastases. Br J Surg 92:1155–1160
Poultsides GA, Servais EL, Saltz LB et al (2009) Outcome of primary tumor in patients with synchronous stage IV colorectal cancer receiving combination chemotherapy without surgery as initial treatment. J Clin Oncol 27:3379–3384
Kim CW, Baek JH, Choi GS et al (2016) The role of primary tumor resection in colorectal cancer patients with asymptomatic, synchronous unresectable metastasis: study protocol for a randomized controlled trial. Trials 17:34
Biondo S, Frago R, Kreisler E et al (2017) Impact of resection versus no resection of the primary tumor on survival in patients with colorectal cancer and synchronous unresectable metastases: protocol for a randomized multicenter study (CR4). Int J Colorectal Dis 32:1085–1090
Cotte E, Villeneuve L, Passot G et al (2015) GRECCAR 8: impact on survival of the primary tumor resection in rectal cancer with unresectable synchronous metastasis: a randomized multicentre study. BMC Cancer 15:47
Rahbari NN, Lordick F, Fink C et al (2012) Resection of the primary tumour versus no resection prior to systemic therapy in patients with colon cancer and synchronous unresectable metastases (UICC stage IV): SYNCHRONOUS–a randomised controlled multicentre trial (ISRCTN30964555). BMC Cancer 12:142
t Lam-Boer J, Mol L, Verhoef C et al (2014) The CAIRO4 study: the role of surgery of the primary tumour with few or absent symptoms in patients with synchronous unresectable metastases of colorectal cancer–a randomized phase III study of the dutch colorectal cancer group (DCCG). BMC Cancer 14:741
Moritani K, Kanemitsu Y, Shida D et al (2019) A randomized controlled trial comparing primary tumour resection plus chemotherapy with chemotherapy alone in incurable stage IV colorectal cancer: JCOG1007 (iPACS study). Jpn J Clin Oncol 50(1):89–93
Ishihara S, Nishikawa T, Tanaka T et al (2015) Benefit of primary tumor resection in stage IV colorectal cancer with unresectable metastasis: a multicenter retrospective study using a propensity score analysis. Int J Colorectal Dis 30:807–812
van Rooijen KL, Shi Q, Goey KKH et al (2018) Prognostic value of primary tumour resection in synchronous metastatic colorectal cancer: individual patient data analysis of first-line randomised trials from the ARCAD database. Eur J Cancer 91:99–106
Charlson ME, Pompei P, Ales KL et al (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Shah S, Vanclay F, Cooper B (1989) Improving the sensitivity of the Barthel index for stroke rehabilitation. J Clin Epidemiol 42:703–709
Edge SB, Compton CC (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17:1471–74
Japanese Society for Cancer of the C, Rectum (2019) Japanese classification of colorectal, appendiceal, and anal carcinoma: the 3d edition [secondary publication]. J Anus Rectum Colon 3:175–195
Rubin DB (1987) Multiple imputation for nonresponse in surveys. John Wiley and Sons, New York
Recondo G Jr, Díaz-Cantón E, de la Vega M et al (2014) Advances and new perspectives in the treatment of metastatic colon cancer. World J Gastrointest Oncol 6:211–224
‘t Lam-Boer J, Van der Geest LG, Verhoef C et al (2016) Palliative resection of the primary tumor is associated with improved overall survival in incurable stage IV colorectal cancer: a nationwide population-based propensity-score adjusted study in the Netherlands. Int J Cancer 139:2082–2094
Kakeji Y, Takahashi A, Udagawa H et al (2018) Surgical outcomes in gastroenterological surgery in Japan: report of national clinical database 2011–2016. Ann Gastroenterol Surg 2:37–54
van Leersum NJ, Aalbers AG, Snijders HS et al (2014) Synchronous colorectal carcinoma: a risk factor in colorectal cancer surgery. Dis Colon Rectum 57:460–466
Lemmens VE, Klaver YL, Verwaal VJ et al (2011) Predictors and survival of synchronous peritoneal carcinomatosis of colorectal origin: a population-based study. Int J Cancer 128:2717–2725
Acknowledgements
We would like to express our gratitude to Seria Sato, Koji Uehara, Nobuko Kanno, Mika Yusa, Kazuhira Saito, Tomoko Oya, Yosinobu Yamazaki, Yoko Endo, Chieko Tairako, Yumi Inaba for their contribution to data collection.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki and relevant local laws and regulations. The study protocol was approved by the institutional review board of all participating hospitals (UMIN000033718).
Informed consent
The institutional review board waived the requirement for informed consent in accordance with the Japanese government’s Ethical Guidelines for Medical and Health Research Involving Human Subjects, which allow for the opt-out approach.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kawamura, H., Ogawa, Y., Yamazaki, H. et al. Impact of Primary Tumor Resection on Mortality in Patients with Stage IV Colorectal Cancer with Unresectable Metastases: A Multicenter Retrospective Cohort Study. World J Surg 45, 3230–3239 (2021). https://doi.org/10.1007/s00268-021-06233-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-021-06233-x