Abstract
Background
Although palonosetron (PALO) and NK1 receptor antagonist both reduce chemotherapy-induced nausea and vomiting, no comparison trial in moderately emetogenic chemotherapy (MEC) had been reported. The purpose of this study was to find out which drug combinations are preferable for patients receiving MEC.
Methods
Chemotherapy-naive patients receiving MEC were randomized to two groups; group A first received PALO therapy [PALO plus 1-day dexamethasone (DEX)], and group B first received fosaprepitant (FAPR) therapy [FAPR, granisetron (GRAN), and DEX]. Patients were re-allocated to the other therapy, respectively, for the second cycle of chemotherapy. We administered intravenous PALO (0.75 mg) and DEX (9.9 mg) to the PALO therapy group, and FAPR (150 mg), DEX (4.95 mg), and GRAN (3 mg) to the FAPR therapy group, on Day 1. Complete response (CR) was the primary endpoint; complete control (CC), total control (CT), and the therapy chosen by the patients for their third and following cycles of antiemetic therapy were the secondary endpoints. We evaluated CR, CC, and TC in the acute phase, in the delayed phase, and over the whole period.
Results
A total of 35 patients and 70 cycles of therapy was evaluable for analysis. No significant difference was found at all evaluation points. Overall CR rates for PALO and FAPR therapy were 74 vs 69 % (P = 0.567), CC rates 66 vs 69 % (P = 0.521), and TC rates 46 vs 60 % (P = 0.235), respectively. Patients also showed no clear preference for their third and following cycles of chemotherapy, choosing both regimens almost equally often (PALO 10 vs FAPR 13).
Conclusions
PALO and 1-day DEX is almost equivalent to FAPR, GRAN, and DEX for MEC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemotherapy-induced nausea and vomiting (CINV) is a non-hematological toxicity which accompanies chemotherapy. Despite significant progress in its management, CINV continues to be among the most feared side effects in cancer patients receiving chemotherapy [1]. Uncontrolled CINV limits the efficacy of cancer therapy and also reduces a patient’s quality of life [2], which means that control of CINV is very important for the success of chemotherapy.
The risk of CINV depends on several factors, the most predictive being the emetogenic potential of the chemotherapeutic agent [3]. Intravenously administered antineoplastic agents are divided into high, moderate, low, and minimal emetic risk groups. Cytotoxic agents such as oxaliplatin, irinotecan, carboplatin, cyclophosphamide (<1500 mg/m2), doxorubicin, and epirubicin are defined as moderately (30–90 %) emetogenic [4]. Current antiemetic guidelines recommend the two-drug combination of palonosetron (PALO) and dexamethasone (DEX) as an antiemetic therapy with moderately emetogenic chemotherapy (MEC) [5–7]. American guidelines recommend the three-drug combination of 5-HT3 receptor antagonist (RA), DEX, plus aprepitant as an option without PALO or with any higher emetogenic risk drugs, such as irinotecan, carboplatin doxorubicin, or epirubicin [6, 7].
Unlike the first-generation 5-HT3 RAs, like granisetron (GRAN), PALO, a second-generation 5-HT3 RA, reduces delayed CINV [8]. PALO with 1-day DEX showed non-inferiority to PALO with 3-day DEX in MEC [9, 10]. On the other hand, fosaprepitant (FAPR), a prodrug of NK1 RA, aprepitant, can also reduce delayed CINV after a single administration [11]. It might be unnecessary to administer DEX on Days 2 and 3 if FAPR is added to 5-HT3 RA for MEC. No data comparing PALO with NK1 RA in MEC have been published, and the efficacy of both drugs has not been confirmed yet. We thus planned a prospective randomized crossover study to find out which drug combinations are prefered by patients receiving MEC.
Patients and methods
Study population
Chemotherapy-naive adults with histologically or cytologically confirmed solid malignant tumor receiving MEC were eligible for inclusion. Patients were required to have acceptable hematological, hepatic, and renal functions for administration of chemotherapy, and an adequate Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0, 1, or 2.
Exclusion criteria included: known hypersensitivity to 5-HT3 RA, FAPR, or DEX; central nervous system malignancy; and any other organic cause of nausea and vomiting unrelated to chemotherapy administration. Radiotherapy within 30 days before chemotherapy initiation or during the study period, and unrelated nausea or vomiting within 24 h prior to initiation of chemotherapy also led to exclusion. Patients were ineligible if they had active infection or were unable to understand or cooperate with study procedures. Pregnant or nursing women were also ineligible. All patients provided written informed consent before entering the study.
Study design
This prospective, single-blind, randomized, crossover study was conducted at Tonan Hospital in Sapporo, Japan. Patients were randomized to two groups; group A first received PALO and 1-day DEX (PALO therapy), and group B first received FAPR, GRAN, and DEX (FAPR therapy). Patients were then given the other therapy for the second cycle of chemotherapy. We administered intravenous PALO (0.75 mg) and DEX (9.9 mg) for PALO therapy, and FAPR (150 mg), DEX (4.95 mg), and GRAN (3 mg) for FAPR therapy, on Day 1. PALO, DEX, and GRAN were administered 30 min before chemotherapy initiation as a bolus over 30 s. FAPR was administered 60 min before chemotherapy initiation as a drip over 30 min. After chemotherapy, rescue medication, including DEX and metoclopramide for CINV, was permitted as needed. Whether the patients continued to receive PALO or FAPR therapy after the second cycle of chemotherapy was decided based on a questionnaire investigating patient preference. The Institutional Review Board of Tonan Hospital approved the study protocol.
Study objectives and efficacy endpoints
The aim of this study was to compare the efficacy of PALO and FAPR antiemetic therapies in MEC. The efficacy was evaluated based on data collected with the Japanese version of MASCC Antiemesis Tool (MAT), and on the monitored frequency of use of rescue medication. Patients recorded the incidence and severity of CINV on Days 2 and 5. The severity of nausea was recorded using a numeric rating scale (NRS) of MAT.
The primary endpoint was the complete response (CR), defined as no vomiting and no rescue therapy. Secondary endpoints were: complete control (CC), defined as CR with no more than mild nausea (NRS ≤ 3), total control (TC), defined as no nausea, and the therapy chosen by patients for their third and following cycles of antiemetic therapy. We evaluated CR, CC, and TC in the acute phase (Day 1), delayed phase (Days 2–5), and over the whole period (Days 1–5).
Statistical analysis
Patients who met the inclusion criteria were stratified by chemotherapy regimen (oxaliplatin base, irinotecan base, or other), sex, and age (≥50 years or <50 years), using the minimization method.
We analyzed the per-protocol cohort including all patients who received the study medication and completed the follow-up period (5 days after the second cycle of chemotherapy initiation) without any protocol deviation. This study was conducted as a preliminary assessment of which of the therapies, PALO or FAPR, appears to be superior, and by how much. For efficacy analysis, CR, CC, and TC rates during the acute, delayed, and overall intervals were compared between the two therapies. We used the Cochran–Mantel–Haenszel tests. All P values were two-sided, and a P value < 0.05 was considered statically significant. TC rates at all intervals were analyzed with the 95 % confidence interval of the difference between the two therapies. We used SPSS Statistics 22 (IBM, Armonk, NY, USA) for all analyses.
Results
Patient characteristics
From April 2013 to November 2014, 39 chemotherapy-naive, adult, and solid malignant tumor patients receiving MEC were randomized to group A or B. Two patients declined to receive study treatment (Fig. 1). Two more patients were not able to complete chemotherapy treatment, one in group A due to septic shock, and one in group B due to tumor bleeding. Therefore, 35 patients and 70 treatments were available for analysis.
Baseline clinical characteristics of the per-protocol cohort were similar between the two groups (Table 1). However, there were more patients with ECOG PS 0 and with a history of vomiting during pregnancy in group B. 80 % of the study population was older than 49 years of age. 21 of 35 patients (60 %) received an oxaliplatin-based regimen, 10 of 35 patients (29 %) received docetaxel plus cyclophosphamide, and 4 of 35 patients (11 %) received another regimen. Almost all oxaliplatin-based regimens were mFOLFOX6, except for one, XELOX. Three patients receiving a carboplatin-based regimen were all in group A. One patient receiving an irinotecan-based regimen (FOLFIRI) was in group B. Most patients [31 of 35 patients (89 %)] were diagnosed with colorectal or breast cancer. The majority of patients [24 of 35 (69 %)] received chemotherapy as postoperative adjuvant therapy.
Efficacy
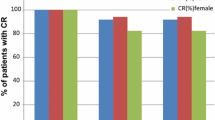
The proportions of patients achieving CR and CC in the acute, delayed, and overall time intervals after MEC are presented in Figs. 2 and 3. No significant difference was found at any evaluation point. Good CR and CC rates (≥90 %) were found in the acute phases of both therapies. The proportion of patients achieving TC is presented Fig. 4. No significant difference was found for any time interval, but FAPR therapy showed higher TC rates in this graph; the difference was about 8, 15, and 15 %, in the acute phase, delayed phase, and overall period, respectively. Table 2 shows the therapy chosen by patients for the third and following cycles of chemotherapy. There was no clear preference, both regimens being chosen almost equally frequently. One patient selected PALO therapy in group A and nine in group B. Seven patients selected FAPR therapy in group A and six in group B. Generally, patients in both groups preferred to continue with their second cycle antiemetic therapy.
Safety
No patients had grade 3 or 4 toxicity related to the study drugs. Four patients (11 %) receiving PALO therapy and three patients (9 %) receiving FAPR therapy had constipation, an adverse event commonly associated with 5-HT3 RA therapy. Our hospital almost always uses a central venous port for chemotherapy so no infusion site adverse events were recorded.
Discussion
In this study, we made an important clinical observation that PALO and 1-day DEX was almost equivalent to FAPR, GRAN, and DEX in MEC. Both therapies showed no significant difference in CR or CC rates. One other trial had evaluated the utility of NK1 RA in MEC consisting of an anthracycline and cyclophosphamide (AC)-based regimen [12]. Adding aprepitant to first-generation 5-HT3 RA achieved about 9 % improvement in CR rate in the acute phase. That trial also showed about 10 % improvement in CR rate with aprepitant in the delayed phase compared with 5-HT3 RA. In our study, both therapies showed no significant difference in CR or CC rates. The earlier trial, which included both AC, a higher emetogenic regimen as recommended by current antiemetic guidelines [6, 7], and a non-AC regimen, also found no significant difference in CR rates, as in our study. Additionally, the prior study had a high proportion of women with breast cancer [12]. Being female is a high risk factor for CINV, and the efficacy of NK1 RA was reported to be higher in females than in males [13]. Our results suggest that PALO and 1-day DEX has almost the same efficacy as FAPR, GRAN, and DEX in MEC, excluding an AC regimen.
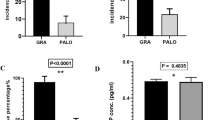
Regarding an antiemetic therapy of MEC, a three-drug combination including NK1 RA might have some advantages when compared with two-drug combinations including PALO, especially for delayed CINV of MEC. In our study, FAPR therapy showed a non-significant but about 5 % higher CR rate in the acute phase than PALO therapy. This difference corresponds to the non-AC based regimen results of the previous trial [12]. Additionally, FAPR therapy also showed non-significant but improved TC rates, and a larger number of cases seem to show a superior TC rate during all intervals, but especially in the delayed phase. One meta-analysis showed that NK1 RA improved CINV control of MEC during all intervals [14], and substance P concentration in plasma significantly increased only in patients with delayed CINV [15]. On the other hand, another meta-analysis showed no significant difference in the efficacy of 5-HT3 RA, even first-generation, for delayed CINV [16]. Therefore, our results suggest that FAPR might have slightly better efficacy than PALO in preventing CINV during all intervals, but especially in the delayed phase.
Our study suggests that the PALO with 1-day DEX regimen is generally suitable for antiemetic therapy of MEC. PALO with 1-day DEX showed non-inferiority to PALO with 3-day DEX, the standard therapy at the moment in MEC [5–7, 9, 10]. 1-Day DEX dosing is likely to reduce many steroid-related complications, especially in diabetic mellitus patients. Compared with FAPR, patients can avoid vascular pain and excessive drug costs. The slight difference in TC rate results in few clinical benefits affecting the patients’ selection of antiemetic therapy for the third chemotherapy cycle in our study. Approximately the same number of patients selected both therapies. Nausea at NRS 1 or 2, which affects TC rate, might in reality not impact patients’ quality of life significantly. However, in patients with high risk factors for CINV, like being female, no drinking habit, and being young, the effects of NK1 RA are favorable [13]. Clinicians should consider a three-drug combination therapy including FAPR for CINV high-risk patients. On the other hand, PALO exhibits unique pharmacological profiles, a long half-life (about 40 h), strong receptor affinity [17], and crosstalk inhibition compared with first-generation 5-HT3 RA. PALO also inhibits substance P response by crosstalk inhibition of 5-HT3 and NK1 receptors [18]. These profiles and PALO (a 0.75-mg dose is covered by insurance in Japan) might be the reason why a two-drug combination including PALO is not inferior to a three-drug combination including FAPR. Additionally, GRAN (a 3-mg dose is also covered by insurance in Japan) is not effective for delayed CINV.
Our study has two limitations. First, our study was preliminary, with a small number of cases, whose characteristics were not uniform. The number of cases may have been insufficient to detect a statistical difference; also, group B may have contained more patients with a high risk of CINV because of their history of vomiting during pregnancy. However, the crossover design alleviated these problems, and our study shows that patients in both groups preferred to continue with their second-cycle antiemetic therapy. Both therapies were efficient enough and the difference did not seem to be significant. We do need further study to clarify whether PALO and 1-day DEX is truly equivalent to FAPR, GRAN, and DEX in MEC.
Second, we used a very simple questionnaire for evaluation by patients. Many studies evaluate CINV using the patients’ records for every day, 1 through 5. Although the original MAT is confirmed to be superior to other available assessment tools [19], the Japanese version of MAT is a simple assessment tool, and patients need to fill it out only twice for each cycle of chemotherapy. Whether we can reliably evaluate the various kinds of CINV using the Japanese version of MAT is unclear. However, we were able to achieve a relatively high collection rate and a low number of withdrawals in this study.
In conclusion, we found that PALO and 1-day DEX were almost equivalent to FAPR, GRAN, and DEX in MEC. Our study also suggests that a three-drug combination including NK1 RA might have some advantages over a two-drug combination including PALO, especially for delayed CINV in MEC. Clinicians should consider a three-drug combination therapy including NK1 RA for CINV in high-risk patients, like those who are female, young, and abstinent.
References
Sun CC, Bodurka DC, Weaver CB et al (2005) Rankings and symptom assessments of side effects from chemotherapy: insights from experienced patients with ovarian cancer. Support Care Cancer 13:219–227
Oo TH, Hesketh PJ (2005) Drug insight: new antiemetics in the management of chemotherapy-induced nausea and vomiting. Nat Clin Pract Oncol 2:196–201
Hesketh PJ (2008) Chemotherapy-induced nausea and vomiting. N Engl J Med 358:2482–2494
Roila F, Hesketh PJ, Herrstedt J (2006) Prevention of chemotherapy- and radiotherapy-induced emesis: results of the 2004 Perugia International Antiemetic Consensus Conference; Antiemetic Subcommitte of the Multinational Association of Supportive Care in Cancer. Ann Oncol 17:20–28
Gralla RJ, Roila F, Tonato M et al (2013) Title of subordinate document. In: MASCC/ESMO antiemetic guideline 2013. Multinational Association of Supportive Care in Cancer. http://www.mascc.org/assets/Guidelines-Tools/mascc_guidlines_english_2014.pdf. Accessed Dec 2014
Basch E, Prestrud AA, Hesketh PJ et al (2011) Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 29:4189–4198
Ettinger DS, Berger MJ, Armstrong DK et al (2014) Title of subordinate document. In: NCCN Version 2.2014, Antiemesis. National Comprehensive Cancer Network. http://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed Mar 2015
Aapro MS, Grunberg SM, Manikhas GM et al (2006) A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol 17:1441–1449
Aapro M, Fabi A, Nolè F et al (2010) Double-blind, randomised, controlled study of the efficacy and tolerability of palonosetron plus dexamethasone for 1 day with or without dexamethasone on days 2 and 3 in the prevention of nausea and vomiting induced by moderately emetogenic chemotherapy. Ann Oncol 21:1083–1088
Celio L, Frustaci S, Denaro A et al (2011) Palonosetron in combination with 1-day versus 3-day dexamethasone for prevention of nausea and vomiting following moderately emetogenic chemotherapy: a randomized, multicenter, phase III trial. Support Care Cancer 19:1217–1225
Grunberg S, Chua D, Maru A et al (2011) Single-dose fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with cisplatin therapy: randomized, double-blind study protocol—EASE. J Clin Oncol 29:1495–1501
Rapoport BL, Jordan K, Boice JA et al (2010) Aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with a broad range of moderately emetogenic chemotherapies and tumor types: a randomized, double-blind study. Support Care Cancer 18:423–431
Hesketh PJ, Aapro M, Street JC et al (2010) Evaluation of risk factors predictive of nausea and vomiting with current standard-of-care antiemetic treatment: analysis of two phase III trials of aprepitant in patients receiving cisplatin-based chemotherapy. Support Care Cancer 18:1171–1177
dos Santos LV, Souza FH, Brunetto AT et al (2012) Neurokinin-1 receptor antagonists for chemotherapy-induced nausea and vomiting: a systematic review. J Natl Cancer Inst 104:1280–1292
Takahashi T, Nakamura Y, Tsuya A et al (2011) Pharmacokinetics of aprepitant and dexamethasone after administration of chemotherapeutic agents and effects of plasma substance P concentration on chemotherapy-induced nausea and vomiting in Japanese cancer patients. Cancer Chemother Pharmacol 68:653–659
Huang JQ, Zheng GF, Chan GC et al (2004) Efficacy of current antiemetic treatments for preventing delayed chemotherapy-induced nausea and vomiting: a meta-analysis of randomized controlled trials. Clin Res Regul Aff 21:191–212
Rojas C, Stathis M, Thomas AG et al (2008) Palonosetron exhibits unique molecular interactions with the 5-HT3 receptor. Anesth Analg 107:469–478
Rojas C, Li Y, Zhang J et al (2010) The antiemetic 5-HT3 receptor antagonist palonosetron inhibits substance P-mediated responses in vitro and in vivo. J Pharmacol Exp Ther 335:362–368
Molassiotis A, Coventry PA, Stricker CT et al (2007) Validation and psychometric assessment of a short clinical scale to measure chemotherapy-induced nausea and vomiting: the MASCC antiemesis tool. Pain Symptom Manage 34:148–159
Acknowledgments
The authors thank the patients and nurses who participated in our study. We also thank David Hochman for reviewing the language of our article. Presented in part at the 12th Annual Meeting of the Japanese Society of Medical Oncology, Fukuoka, Japan, July, 2014.
Conflict of interest
All of the authors, except Yasushi Tusji, declare that they have no conflict of interest. Yasushi Tusji has received lecture fees from Ono Pharmaceutical Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kitayama, H., Tsuji, Y., Sugiyama, J. et al. Efficacy of palonosetron and 1-day dexamethasone in moderately emetogenic chemotherapy compared with fosaprepitant, granisetron, and dexamethasone: a prospective randomized crossover study. Int J Clin Oncol 20, 1051–1056 (2015). https://doi.org/10.1007/s10147-015-0823-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-015-0823-6