Abstract
Objective
This study aimed to investigate the clinical characteristics of hemifacial spasm (HFS) after Bell’s palsy and to evaluate the therapeutic efficacy of microvascular decompression (MVD).
Methods
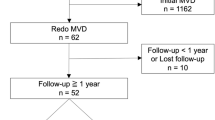
A retrospective analysis was conducted on 18 patients who underwent MVD for HFS after Bell’s palsy at our institution between January 1, 2017, and December 31, 2021. Clinical presentations, intraoperative findings, postoperative outcomes, and complications were comprehensively assessed.
Results
Neurovascular compression (NVC) was identified in all the 18 patients. The offending vessels included anterior inferior cerebellar artery (AICA) in 6 patients (33.3%), posterior inferior cerebellar artery (PICA) in 7 patients (38.9%), vertebral artery (VA) combined with AICA in 3 patients (16.7%), and VA alongside PICA in 2 patients (11.1%). Notably, marked arachnoid membrane adhesion was evident in 11 patients (61.1%). 15(83.3%) patients were cured immediately after MVD, delayed relief was found in 3 (16.7%) patients. During the follow-up period, recurrence was not documented. Surgical complications were limited to facial paralysis in 3 patients and auditory impairment in 1 patient. No additional surgical complications were recorded.
Conclusions
In patients manifesting HFS after Bell’s palsy, NVC predominantly underlies the etiology. MVD is a reliably safe and efficacious therapeutic intervention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bell’s palsy is characterized by acute, unilateral facial paralysis, typically resulting from an infection with herpes simplex virus [1]. Although the majority of Bell’s palsy patients experience partial or full recovery, complications such as synkinesis, the involuntary contraction of facial muscles during intentional facial muscle movements, can arise following recovery [2,3,4]. Hemifacial spasm (HFS) is a neurological disorder manifested by unilateral, irregular clonic or tonic contractions in the muscles innervated by the ipsilateral facial nerve. Microvascular decompression (MVD) has been widely accepted as an effective therapeutic intervention for this condition [5, 6].
Post-facial palsy synkinesis (PFPS) and HFS are two different entities with different underlying causes. The exact cause of synkinesis is still under debate. Multiple theories, such as anomalous ephaptic transmission, heightened sensitivity within the facial nucleus, and aberrant adaptive responses in the central nervous system, have been posited as potential mechanisms [7,8,9,10]. The etiology of primary HFS has been attributed to neurovascular compression (NVC) at the facial nerve root [5]. Clinical manifestations in PFPS patients, such as involuntary eye closure on the affected side while smiling or midface spasms during blinking, may bear semblance to the symptoms of HFS. Consequently, delineating between PFPS and HFS after Bell’s palsy can pose diagnostic challenges. And for HFS patients with a medical history of Bell’s palsy, it is not easy to make the right diagnosis and determine the appropriate surgical interventions.
In this study, we retrospectively analyzed the patients with HFS after Bell’s palsy in our institution from 2017 to 2021. The main objective was to investigate the clinical features and surgical efficacy of MVD.
Materials and methods
Research population
The study was approved by the Ethical Committee of Nanjing Drum Tower Hospital. A cohort of 18 patients, who underwent MVD for HFS between January 1, 2017, and December 31, 2021, were included in this retrospective analysis. All the patients had a preceding medical history of Bell’s palsy, and some time after recovering from facial paralysis, they presented with involuntary twitching of unilateral facial muscles. They all underwent high-resolution MRI, employing three-dimensional time-of-flight and fast imaging with steady-state acquisition sequences, which served to elucidate vascular structures. Every patient displayed an evoked abnormal muscle response (AMR) preoperatively, and MVD was performed under the continuous electrophysiological monitoring of AMR.
Surgery and abnormal muscle response (AMR) monitoring
Under general anesthesia, patients were placed in the lateral decubitus position. A craniotomy was performed, followed by the meticulous opening of the dura mater and arachnoids under microscopic visualization. Adequate cerebrospinal fluid (CSF) drainage was ensured. The root exit zone (REZ) of the facial nerve was comprehensively exposed, and every suspected offending vessel was isolated from the facial nerve root using shredded Teflon implants. Once assured of the absence of further compressions, the dura mater was sutured, followed by wound closure.
The intraoperative AMR was monitored using the evoked potential system (Xltek Protektor32, Natus, USA). A square-wave stimulation of 0.2ms pulse width and 1 Hz frequency was utilized. Stimulation of the marginal mandibular branch of the facial nerve allowed for AMR recordings from the orbicularis oculi and mentalis muscles. Typically, a consistent AMR could be registered with a stimulation intensity between 5 and 20 mA. AMR was evoked every 5 min prior to dura opening; After dural opening, AMR was monitored incessantly until surgical completion [11].
Statistical analysis
Follow-up was maintained either through telephonic consultations or clinic visits, and surgical outcomes, as well as complications, were meticulously documented. The average follow-up duration amounted to approximately 49.8 ± 17.92 months postoperatively.
Results
Within the cohort of 18 participants evaluated in this study, the average age at the time of surgical intervention was 56.4 ± 9.64 years. Of these, 13 (72.2%) were female, while 10 out of 18 (55.6%) presented with left-sided symptoms. Each individual in the study had a documented medical history of Bell’s palsy. The average interval from achieving complete recovery from facial paralysis to the manifestation of HFS was 4.4 ± 2.52 years (ranging from 1 to 11 years). Furthermore, the average duration of HFS prior to surgical intervention was recorded as 5.1 ± 4.48 years.
In all the patients, preoperative MRI scans either definitively identified or raised suspicions of neurovascular compression (NVC). Subsequent intraoperative microscopic evaluations substantiated these findings, confirming NVC in each case. The offending vessels were anterior inferior cerebellar artery (AICA) in 6 (33.3%), posterior inferior cerebellar artery (PICA) in 7 (38.9%), VA in association with AICA in 3 (16.7%), and VA in conjunction with PICA in 2 (11.1%). Additionally, significant thickening and adhesion of the arachnoid membrane at the cerebellopontine angle were discerned in 11 patients, accounting for 61.1% of the cohort.
Within the scope of this research, AMR was successfully evoked in each patient prior to the administration of muscle relaxants. Before the incision of the dura mater, AMR was detected in 17 participants. Following dural incision and subsequent CSF drainage, a permanent disappearance of AMR was observed in 3 individuals. Prior to commencing the decompression, AMR persisted in 14 participants. Of these, 12 patients showed a complete AMR disappearance after decompression. Though confirming adequate decompression had been achieved, AMR did not disappear in the other 2 patients.
Postoperatively, a swift resolution of symptoms was achieved in 15 patients (83.3%), with delayed relief noted in 3 (16.7%). 4 cases of postoperative complications were observed, including 3 cases of delayed facial paralysis (DFP), and 1 of auditory impairment. Of the 3 cases with DFP, one patient received lumbar puncture and the polymerase chain reaction for herpes simplex virus (HSV) was positive in the cerebrospinal fluid. Treatment with intravenous acyclovir was initiated, after which a rapid and marked improvement was observed. The other two patients were managed with oral steroids and acyclovir for 2 weeks, and one patient achieved excellent outcome at 5 weeks from the onset. A complication was deemed permanent if its presence persisted until the terminal follow-up. Within this study, barring one individual who endured permanent facial paralysis, all other participants with cranial nerve complications achieved full recovery during the follow-up duration, and recurrence remained notably absent until the study’s conclusion. More details are summarized in Table 1.
.
.
.
.
Discussion
Bell’s palsy exhibits an incidence rate ranging between 11.5 and 40.2 per 100,000, predominantly affecting individuals aged between 15 and 45 years [1]. It is reported that a majority of those afflicted with Bell’s palsy undergo spontaneous remission: approximately 84% regain near-normal facial functionality, while 71% achieve full recovery. However, a subset of these patients may experience enduring facial weakness, with a proportion of them progressing to PFPS [1, 12, 13].
Delineating between PFPS and HFS after Bell’s palsy can pose diagnostic challenges. Synkinesis is characterized by involuntary contractions in facial musculature concurrent with intentional facial movements [3, 4]. While HFS is characterized by unilateral, involuntary, synchronous, and clonic twitches of the hemifacial musculature. Typically, a diagnosis is rendered based on patient history and clinical evaluations [6, 14]. For those developing HFS after Bell’s palsy, the symptomatic presentation closely mirrors that of idiopathic HFS. Our study discerned that HFS manifestations typically emerge between 1 and 11 years following comprehensive recovery from Bell’s palsy, a temporal progression distinct from PFPS. Furthermore, AMR monitoring emerges as a pivotal tool in distinguishing between the two conditions [15, 16]. When one branch of the facial nerve is electrically stimulated, the AMR can be recorded from the muscle innervated by another branch of the facial nerve. All 18 participants in the current study exhibited prominent AMR preoperatively.
The relationship between HFS and Bell’s palsy remains ambiguously delineated in existing literature. It is uncertain whether HFS directly results from antecedent facial palsy or if the occurrence is merely coincidental. There appears to be a dearth of extensive reports specifically addressing HFS after Bell’s palsy. Wang and Jankovic examined 158 HFS patients and discerned that 9 of these had a preceding medical history of Bell’s palsy, however, there was no concentrated exploration into the etiology and therapeutic outcomes [14]. Studies conducted by Eekhof JL et al. and Oge AE et al. elucidated the electrophysiological characteristics of HFS and PFPS, yet omitted any investigation into surgical outcomes [15, 17]. A series by Li et al., encompassing 17 HFS patients post-Bell’s palsy, revealed that 15 presented with offending vessels. Moreover, pronounced thickening and arachnoid mater adhesion around the facial nerve were identified in 3 of these cases. Following microvascular decompression (MVD), 12 patients (70.5%) experienced complete spasm cessation, while 4 exhibited symptomatic improvement [16].
In the current research, NVC was a consistent finding across all 18 participants, with notable arachnoid membrane adhesion observed in 11 (61.1%) cases. Based on our intraoperative observations, we posit that NVC may serve as the principal etiological factor for HFS after Bell’s palsy. Additionally, Bell’s palsy, typically resulting from infections such as herpes zoster or other viral agents, may be instrumental in the pathogenesis of HFS. Viral infections may precipitate inflammatory reactions leading to thickening and adhesion around the facial nerve. This process may consequently anchor vessels to the facial nerve, engendering neurovascular conflicts. It is imperative to note that these suppositions are anchored in our clinical findings, necessitating further investigations to elucidate the underlying causes and pathogenesis.
16 (84.2%) patients were cured immediately after surgery, indicating that MVD is an effective treatment for these patients. However, we observed 4 instances of postoperative complications, with one individual enduring persistent facial paralysis. Relative to the outcomes for idiopathic HFS treated contemporaneously [18, 19], the complication rate for the present cohort was elevated. We hypothesize that this may be attributed to the pronounced arachnoid adhesion, which rendered facial nerve root exposure challenging. Consequently, we advocate for sharp dissection of the thickened and adherent arachnoid membrane, emphasizing the importance of minimizing cranial nerve strain during the procedure.
Nowadays, AMR monitoring is widely applied in HFS patients. Previous studies have demonstrated that AMR can be used in the diagnosis of HFS and the identification of the offending vessels [20,21,22]. Considerable reports have suggested that AMR disappearance after MVD could significantly assess good outcomes [23,24,25]. However, AMR should not be regarded as an absolute standard to predict clinical outcomes [11]. Instances of AMR disappearance have been observed at various surgical stages, including prior to decompression, during dural opening, upon CSF drainage, and amidst dissection of the arachnoid membrane [12, 25,26,27]. In the present study, we observed a permanent disappearance of AMR in 3 individuals before decompression procedure, this may be attributed to the anatomical shift of neurovascular relationship resulting from CSF drainage or dissecting the arachnoid tissue. We believe that for HFS after Bell’s palsy, AMR monitoring is necessary, but the key to successful MVD is sufficient decompression. If AMR showed a permanent disappearance before decompression, electrophysiological monitoring should be continued, and the REZ of facial nerve should be explored more carefully, thereby trying to get an adequate decompression.
Conclusion
This retrospective analysis encompassed a cohort of 18 HFS patients, all with a preceding medical history of Bell’s palsy. The findings posit that NVC might serve as the etiological underpinning for HFS after Bell’s palsy. Moreover, MVD is a safe and effective treatment.
Data availability
Not applicable.
Code availability
Not applicable.
References
Sajadi MM, Sajadi MR, Tabatabaie SM (2011) The history of facial palsy and spasm: Hippocrates to Razi. Neurology 77(2):174–178
Peitersen E (2002) Bell’s palsy: the spontaneous course of 2,500 peripheral facial nerve palsies of different etiologies. Acta Otolaryngol Suppl (549):4–30
Vakharia K, Vakharia K (2016) Bell’s Palsy. Facial Plast Surg Clin North Am 24(1):1–10
Shokri T, Patel S, Ziai K, Harounian J, Lighthall JG (2021) Facial synkinesis: a distressing sequela of facial palsy. Ear Nose Throat J. https://doi.org/10.1177/01455613211054627
Mercier P, Sindou M (2018) The conflicting vessels in hemifacial spasm: literature review and anatomical-surgical implications. Neurochirurgie 64(2):94–100
Chaudhry N, Srivastava A, Joshi L (2015) Hemifacial spasm: the past, present and future. J Neurol Sci 356(1–2):27–31
Maeyama H, Aoyagi M, Tojima H, Inamura H, Kohsyu H, Koike Y (1994) Electrophysiological study on the pathology of synkinesis after facial nerve paralysis. Acta Otolaryngol Suppl 511:161–164
Kollewe K, Mohammadi B, Dengler R, Dressler D (2010) Hemifacial spasm and reinnervation synkinesias: long-term treatment with either Botox or Dysport. J Neural Transm (Vienna) 117(6):759–763
Pinkiewicz M, Dorobisz K, Zatoński T (2022) A Comprehensive Approach to Facial Reanimation: a systematic review. J Clin Med 11(10):2890
Placheta E, Tzou CJ, Hold A, Pona I, Frey M (2014) Facial synkinesia before and after surgical reanimation of the paralyzed face. Plast Reconstr Surg 133(6):842e–851e
Jiang C, Xu W, Dai Y, Lu T, Jin W, Liang W (2017) Early permanent disappearance of abnormal muscle response during microvascular decompression for hemifacial spasm: a retrospective clinical study. Neurosurg Rev 40(3):479–484
Bacorn C, Fong NST, Lin LK (2020) Misdiagnosis of Bell’s palsy: Case series and literature review. Clin Case Rep 8(7):1185–1191
Heckmann JG, Urban PP, Pitz S, Guntinas-Lichius O, Gágyor I (2019) The diagnosis and treatment of idiopathic facial paresis (Bell’s Palsy). Dtsch Arztebl Int 116(41):692–702
Wang A, Jankovic J (1998) Hemifacial spasm: clinical findings and treatment. Muscle Nerve 21(12):1740–1747
Eekhof JL, Aramideh M, Speelman JD, Devriese PP, Ongerboer De Visser BW (2000) Blink reflexes and lateral spreading in patients with synkinesia after Bell’s palsy and in hemifacial spasm. Eur Neurol 43(3):141–146
Li X, Zheng X, Wang X, Li B, Ying T, Li Y, Li S (2013 Microvascular decompression treatment for post-Bell’s palsy hemifacial spasm. Neurol Res 35(2):187–192
Oge AE, Yayla V, Demir GA, Eraksoy M (2005) Excitability of facial nucleus and related brain-stem reflexes in hemifacial spasm, post-facial palsy synkinesis and facial myokymia. Clin Neurophysiol 116(7):1542–1554
Jiang C, Liang W, Wang J, Dai Y, Jin W, Sun X, Xu W (2020) Microvascular decompression for hemifacial spasm associated with distinct offending vessels: a retrospective clinical study. Clin Neurol Neurosurg 194:105876
Wang J, Chong Y, Jiang C, Dai Y, Liang W, Ding L (2022) Microvascular decompression for hemifacial spasm involving the vertebral artery. Acta Neurochir (Wien) 164(3):827–832
Amano Y, Asayama B, Noro S, Abe T, Okuma M, Honjyo K, Seo Y, Nakamura H (2022) Significant correlation between delayed relief after microvascular decompression and morphology of the abnormal muscle response in patients with Hemifacial Spasm. Neurol Med Chir (Tokyo) 62(11):513–520
Lee S, Park SK, Lee JA, Joo BE, Kong DS, Seo DW, Park K (2018) A new method for monitoring abnormal muscle response in hemifacial spasm: a prospective study. Clin Neurophysiol 129(7):1490–1495
Tani S, Inazuka M, Maegawa T, Takahashi Y, Kikuchi A, Yokosako S, Yoshimura C, Koseki H, Ohbuchi H, Hirota K, Hagiwara S, Hirasawa M, Sasahara A, Kasuya H (2017) Nonspastic hemifacial spasm confirmed by abnormal muscle responses. Surg Neurol Int 8:96
Son BC, Ko HC, Choi JG (2018) Intraoperative monitoring of Z-L response (ZLR) and abnormal muscle response (AMR) during microvascular decompression for hemifacial spasm. Interpreting the role of ZLR. Acta Neurochir (Wien) 160(5):963–970
Liu MX, Zhong J, Xia L, Dou NN, Sun H, Li B, Visocchi M, Li ST (2017) The significance of abnormal muscle response monitoring during Microvascular Decompression for Hemifacial Spasm. Acta Neurochir Suppl 124:297–301
Hirono S, Yamakami I, Sato M, Kado K, Fukuda K, Nakamura T, Higuchi Y, Saeki N (2014) Continuous intraoperative monitoring of abnormal muscle response in microvascular decompression for hemifacial spasm; a real-time navigator for complete relief. Neurosurg Rev 37(2):311–320
Zhong J, Xia L, Dou NN, Ying TT, Zhu J, Liu MX, Li ST (2015) Delayed relief of hemifacial spasm after microvascular decompression: can it be avoided? Acta Neurochir (Wien) 157(1):93–99
Neves DO, Lefaucheur JP, de Andrade DC, Hattou M, Ahdab R, Ayache SS, Le Guerinel C, Keravel Y (2009) A reappraisal of the value of lateral spread response monitoring in the treatment of hemifacial spasm by microvascular decompression. J Neurol Neurosurg Psychiatry 80(12):1375–1380
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Contributions
Chengrong Jiang: original draft, data curation, formal analysis. Jing Wang: review and editing, formal analysis. Yulong Chong: data curation, visualization, methodology. Wu Xu : conceptualization, resources, project administration, supervision, review and editing. Weibang Liang: conceptualization, resources, project administration, supervision, review and editing.
Corresponding authors
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Consent to participate
All the patients mentioned in this article gave their consent for inclusion.
Conflict of interest
The authors declare no competing interests.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, C., Wang, J., Chong, Y. et al. Microvascular decompression for hemifacial spasm after Bell’s palsy: a retrospective clinical study. Neurosurg Rev 47, 92 (2024). https://doi.org/10.1007/s10143-024-02328-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-024-02328-w