Abstract
Although choroid plexus cysts are a frequent incidental neuroimaging finding, symptomatic ones are rare—a series of more than five cases are hard to find. In the absence of high-volume studies, there are no generally accepted algorithms for diagnosis and treatment for this pathology. Proposed surgical techniques include microsurgical excision or fenestration and endoscopic excision or fenestration with or without additional shunting. No definitive conclusions exist about the superiority of a certain technique. Here, we introduce an illustrative case of a patient with a symptomatic choroid plexus cyst in the trigone of the lateral ventricle and a systematic review of 65 additional published cases with the aim of identifying epidemiological features, variants of localization of the cysts, their symptoms, persistence of concomitant obstructive hydrocephalus, and treatment modalities. A PRISMA-based literature search was performed on the PubMed, MEDLINE, Scopus, and Web of Knowledge databases. We include in the review case reports and case series of symptomatic choroid plexus cysts with full texts or valuable abstracts available online in English and published by April 2023. All abstracts of retrieved studies were assessed by two independent researchers to avoid bias. Only descriptive statistics were used for the presentation of the results. A total of 48 studies (39 case reports and 9 case series) with 65 depicted cases met the eligibility criteria. The review showed a slight predominance of choroid plexus cysts in men. The most common localizations of cysts were the trigone and the body of the lateral ventricle. Obstructive hydrocephalus is often present in patients with choroid plexus cysts. The most common symptoms of cysts were signs of increased ICP: headaches and vomiting. The main treatment approaches for symptomatic choroid plexus cysts were microsurgical excision, microsurgical fenestration, endoscopic fenestration, and total endoscopic excision. The tendency has been noted to shift from microsurgical to endoscopic procedures over the past two decades. Some data on the classification of cysts of the central nervous system and the underlying mechanisms of the pathogenesis of choroid plexus cysts are also presented.
Although symptomatic cases of choroid plexus cysts are rare, by summarizing currently available data, one could clarify their common features and identify a preferable treatment modality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Choroid plexus cysts (CPCs) are a frequent incidental neuroimaging finding and completely asymptomatic in the vast majority of cases [1]. However, symptomatic CPCs could cause severe disability in either infants [2] or adults [3, 4]. Some researchers describe difficulties with the diagnosis of CPCs when applying standard protocols for CT and MRI [5]. Given the vast spectrum of applicable surgical interventions (from cystoperitoneal shunting or stereotactic aspiration to complete microsurgical excision), optimal treatment modalities are also a matter of debate [6]. Taking into account the rarity of symptomatic CPCs and the abovementioned difficulties with their proper diagnosis and treatment, we decided to perform a systematic review based on case reports and case series [7, 8] in order to determine epidemiological features, localization, symptoms, persistence of concomitant obstructive hydrocephalus, and treatment modalities for symptomatic CPCs.
Materials and methods
Literature search
The literature review was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist [9] with the aim of identifying epidemiological features, variants of localization of the cysts, their symptoms, persistence of concomitant obstructive hydrocephalus, and treatment modalities.
Search strategy
PubMed, Scopus, and Web of Knowledge were searched for available online English-language studies published by April 2023 reporting cases of symptomatic choroid plexus cysts by MeSH terms “choroid plexus”, “cyst”, and “symptomatic”. Reference lists of included studies were consulted as an additional source for literature. No restrictions were included at this stage.
Study selection and inclusion criteria
Abstracts of all identified studies were thoroughly assessed by two independent researchers (A.S. and G.G.), papers that met eligibility criteria were retained, and duplicates were removed. Then the full texts of selected papers were reviewed by authors to apply inclusion criteria. Only published articles with full text or an abstract (containing information of interest) available online in English were included. Data from book chapters and conference abstracts were not included. Exclusion criteria included reviews, letters, and studies not reporting sufficient data. In addition, cases of small asymptomatic CPCs or intraparenchymal cysts due to ectopic choroid plexus [10,11,12] were not included.
Qualitative assessment
Considering that we performed the review based on case reports and case series, we possessed all case series and case reports containing sufficient data and published in journals indexed in internationally recognized databases as being of “good” quality. Case reports missing one investigated parameter were considered “fair”, and those with missing two or more parameters and data extracted from abstracts were considered “poor”.
Data collection
The following data were extracted by two authors (A.S. and M.K.) and critically reviewed by two authors (A.S. and D.S.): number of cases per paper, age and sex of participants, localization of CPCs, clinical presentation on admission, persistence of concomitant obstructive hydrocephalus, treatment method, histological verification, outcome, and follow-up. The presence of obstructive hydrocephalus was assessed by announcement through the text or by reviewing the attached CT and MRI images.
The strategy of literature search is presented in Fig. 1.
Results
Search results
Initial searches revealed 301 articles. After excluding 184 duplicates, the titles and abstracts of the remaining 117 papers were screened. A total of 64 Articles were found to be ineligible, and 53 studies met the eligibility criteria. Full text was available for 45 studies. Three valuable abstracts were also presented. Data collected from the literature about published cases of symptomatic CPCs are summarized in Table 1.
Quality assessment
The quality assessment rated 44 studies as “good”, one as “fair”, and three as “poor”.
Demographics, clinics, and surgical data
Overall, 65 cases of symptomatic CPCs were reviewed. The age ranged from newborn to 79 years old: 1 year old and younger—15 (23%); from 1 to 18 years old—18 (28%); and older than 18 years—32 (49%). Among them, 42 (64.6%) were male.
In 27 cases, CPCs were located in the left lateral ventricles, in 22 cases, they were located in the right. The most common localizations among CPCs of the lateral ventricles were trigone (26 cases, 40%) and the body of the lateral ventricle (14 cases, 21.5%). CPCs were located in the frontal and temporal horns of the lateral ventricles in 7 and 1 cases, respectively. Localization in the third ventricle was observed in 14 cases. Two cases of CPCs of the fourth ventricle were also described.
CPCs were the cause of obstructive hydrocephalus in 34 cases. It seems to be associated with the localization of CPCs. Thus, all 7 CPCs located in the frontal horns and 12/14 CPCs in the third ventricle caused CSF pathway obstruction, while only 14/40 (35%) of CPCs in the trigone or body of the lateral ventricle were associated with hydrocephalus.
The most common symptoms of CPCs were signs of increased ICP: headaches and vomiting (32 and 18 cases, respectively). Drowsiness was observed in 4 cases and loss of consciousness in 9 cases. In five cases, CPCs caused seizures. The most common symptom of CPCs in children was increased head circumference, which was noted in 10 cases. Among patients older than 50 years old, gait disturbance was a common symptom (4/10). Short-term memory loss or episodes of global amnesia were presented in 4 cases. Hemiparesis or monoparesis was noted in four cases. Other signs and symptoms such as hallucinations (1), blurred vision (1), myoclonic jerks of the extremities (1), disconjugate or restricted gaze (1), and CN VI palsy (1) were considered non-specific.
The main treatment approaches for symptomatic CPCs were microsurgical excision (24, 37%), microsurgical fenestration (11, 17%), endoscopic fenestration (13, 20%), and total endoscopic excision (7, 11%). In three cases, ventriculoperitoneal or cystoperitoneal shunting was performed as a first-line procedure. In most cases, microsurgical excision was curative, while in one case, it was supplemented by ventriculoperitoneal shunting. Microsurgical fenestration tends to be more often supplemented by cysto-ventriculoperitoneal shunting, which was performed in 4/10 cases. In one case, microsurgical fenestration was unsuccessful, and further microsurgical excision of the cyst was to be performed. On the contrary, there were no cases of endoscopic excision or fenestration that demanded conversion to microsurgical procedures. In one case, endoscopic fenestration was supplemented by cysto-ventriculoperitoneal shunting. Two cases of cystoperitoneal shunting performed initially as a curative procedure demanded further microsurgical excision of the CPCs. One case of stereotactic puncture of the CPC was described. In 10 cases, observation was chosen with no mention of further treatment.
In 44 (80%) cases, patients improved after surgery. There was no evidence of serious complications. In two cases, patients had persistent headaches, in one—memory impairment and hemianopsia, and in one other case—transient hemiparesis. One patient died from other pathology unrelated to the treatment and surgery. In one case, endoscopic excision of the cyst’s wall was followed by intraventricular hemorrhage [40], which resolved within a hospital stay. No recurrent CPCs were observed. The mean follow-up was 10 months, ranging from assessment during in-hospital period to 10 years.
Illustrative case
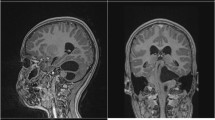
A previously healthy 32-year-old male was presented to our neurosurgical department with a 2-month history of postural headaches (from 4 to 6 points on the visual analog scale on admission) and dizziness. He had no history of trauma or illness. The general physical and neurological examination on admission was unremarkable, with the exception of a slight unsteadiness during the Romberg test without lateralization. MRI scans revealed a large cystic lesion in the trigone of the left lateral ventricle with signs of an isolated occipital horn (Fig. 2A). Other parts of the lateral ventricles, the third and fourth ventricles, were not dilated. Fundoscopy revealed no signs of papilledema. A lumbar puncture was performed. The CSF opening pressure was 20 cm H2O.
Endoscopic fenestration of the CPC through the standard occipital access route was performed, and the cyst was communicated with the left lateral ventricle (Fig. 3).
A The large CPC seen immediately after the introduction of the endoscope into the occipital horn of the left lateral ventricle. B After the coagulation of the cyst wall, the choroid plexus was observed. C Smaller daughter cyst. D Fenestration of the cyst wall was performed after additional coagulation
An endoscopic septostomy was also performed. Control CT scans revealed a remarkable decrease in the cyst’s volume (Fig. 2B). The postoperative course was uneventful, and symptoms resolved. The patient was discharged on postoperative day 3 and was symptom-free on one-month follow-up.
Discussion
CPCs are usually detected prenatally, with an incidence of 1.2% in fetuses [50]. However, cases of de-novo formation of CPCs in adults, presumably after VP-shunting, are also described [51]. The vast majority of CPCs are asymptomatic and are incidental findings during neuroimaging studies or autopsies.
Instead of incidentally discovered CPCs, symptomatic ones are rare. Walter Dandy stated: “I know of no instance when a cyst of the choroid plexus has caused symptoms…” [52]. The first symptomatic case of supposed CPC was described in 1876 by Brown [53]. Although the lesion was distinctly described as a thin-walled cyst arising from the choroid plexus and filled with clear serous fluid, no histopathological description was provided. For this reason, some authors [15] refuse to recognize the described case as the first evidence of a symptomatic choroid plexus cyst. The first histologically verified case of symptomatic CPC was presented by Baker and Gottlieb in 1956 [13]. The cyst was removed by microsurgery with complete resolution of symptoms. Since then, the vast majority of publications on the symptomatic CPCs are case reports with rare small case series.
Classification and probable pathogenesis
By their nature, choroid plexus cysts are intraventricular, non-colloid neuroepithelial cysts [29]. According to Czervionke et al., CPCs are the most common intraventricular neuroepithelial cysts (Fig. 4).
Classification of the neuroepithelial cysts by Czervionke et al. [3]
Yeates et al. postulated [4] that intraventricular arachnoid cysts originate from the ependymal layer of the tela choroidea covering the vascular mesenchyma, which later projects into the lateral ventricle through the choroid fissure as the choroid plexus. The blood vessels traverse the thin tela choroidea, which consists of an invagination of pia mater enclosing the arachnoid stroma, on their way to the choroid plexus [11].
Martinez-Lage et al. consider CPCs intraventricular arachnoid cysts and propose their origin to be secondary to the displacement of arachnoid cells by the vascular mesenchyma, through the choroid fissure, during the process of choroid plexus development [11].
According to Parizek et al. [37], the accumulation of cerebrospinal fluid secreted by the choroid plexus is responsible for the increase in the cyst volume.
Contrary to ependymal cysts, the walls of CPCs could easily be peeled off the ventricular ependyma but were firmly attached to the choroid plexus, usually at the point of the temporal choroidal fissure [1, 23]. In one case described by Martinez-Lage et al., the cyst’s wall was firmly adhered to the ventricular wall, although histological examination confirmed an arachnoid cyst [11].
Histologically, CPCs are characterized by the presence of cuboid choroid plexus cells. This type of cell was found in specimens and described in 47 reports included in this review.
Symptoms
The choroid plexus is a mobile structure; thus, if firmly attached to the plexus, CPCs could migrate within the lateral ventricle and even protrude to the third ventricle, causing dynamic occlusion of the foramen of Monro. This mechanism is more common for CPCs located within the frontal horn of the lateral ventricle or in the third ventricle [34, 37, 39, 48]. Although a few authors described patients suffering from postural headaches who had had CPCs in trigone [4; 35], Baalen et al. described the protrusion of a choroid plexus cyst from the third ventricle to the lateral ventricle through the foramen of Monro while an infant was crying [41].
Numaguchi et al. presented two cases of big CPCs of the lateral ventricles that somehow were asymptomatic [29]. In two additional cases, although not clinically manifested, occlusive hydrocephalus was obvious [Czrevionke, 1987; Becker, 2002]. Baka et al. described a relatively small CPS that manifested as mild confusion and loss of balance after hemorrhage inside the cyst [35].
Diagnosis
Before the widespread introduction of CT and MRI techniques, the most common method for visualization of CPCs was pneumoventriculography. This approach—to see isolated cysts within the ventricle by contrasting the outside ventricular cave—is still up-to-date. Routine CT and MRI are unable to detect the cyst’s wall, misdiagnosing actual CPC as monoventricular hydrocephalus [5]. The application of positive ventriculography by lumbar injection of contrast substantially increases the diagnostic value of CT in cases of CPCs. Usually, 1,5T MRI with multiplanar CISS, FIESTA, or DRIVE protocols could clearly show cyst walls, evaluate ventricular anatomy, and plan surgical intervention [2, 42, 45].
Treatment options
Complete microsurgical excision of the cyst’s walls was the main surgical strategy until 1986, when Nakasu et al. showed comparable effects of fenestration of the cyst [25]. Depending on the localization of the cyst within the ventricular system, transcallosal and transcortical (through the middle temporal gyrus) approaches were most applicable. In some cases, microsurgical excision was the final treatment option after an unsuccessful shunting [20, 31]. On the other hand, total excision had never required adjuvant treatment, with the exception of one case of additional cysto-ventriculoperitoneal shunting [32].
The first use of a ventriculoscope for cyst wall fenestration and cysto-ventriculoperitoneal shunt placement was reported by Martinez-Lage et al. in 1992 [11]. Although the current development of minimally invasive techniques has made microsurgical procedures to a large extent safe and effective, the tendency has been noted to shift from microsurgical to endoscopic procedures over the past two decades (Fig. 5).
Despite one case of minor intraventricular hemorrhage after endoscopic excision of the cyst walls, no serious complications were reported, neither after microsurgical nor endoscopic procedures. There is no data available confirming the advantage of total excision of the cyst walls on fenestration, either microsurgically or endoscopically.
Obviously, every surgeon prefers to choose treatment options from his personal field of expertise, but in the case of equally available microsurgical and endoscopic approaches (which are necessarily in high-volume neurosurgical departments), the last one seems to be preferable in cases of symptomatic CPCs.
Cystoperitoneal or cysto-ventriculoperitoneal shunting is more likely to be an adjuvant procedure than a curative one for patients with CPCs. This statement is supported by the fact that only one successful VP-shunting case of CPC has been described in the literature. Stereotactic puncture and aspiration of the cyst’s contents, as described by Pelletier et al. [33], are not widespread treatment options for CPCs.
Some previously symptomatic CPCs could resolve spontaneously [Lin, 2021], so observation could also be a valuable option in selected cases.
Proposed diagnostic algorithm and treatment strategy for CPC
Based on the reviewed literature, we recommend supplementing routine MRI studies with CISS, FIESTA, or the DRIVE protocol in all patients with suspected CPC or monoventricular hydrocephalus. Positive CT-ventriculography may be considered as an alternative or adjunct to the MRI. Symptomatic cases of CPC with obvious signs of cerebral compression or obstructive hydrocephalus should be considered as candidates for surgical treatment. We suggest endoscopic fenestration of the cyst’s walls as the first-line option that could be converted to microsurgical fenestration if required. A complete excision of the cyst walls is not necessary. Considering the possibility of life-long complications, shunting procedures should be avoided. Observation as a treatment strategy can be used in carefully selected cases due to contraindications for surgery.
Conclusion
The rarity of symptomatic cases of CPCs makes an evidence-based approach to diagnosis and treatment difficult. The collection of new well-described series in the future will allow for the discovery of optimal management algorithms through performing meta-analyses. Nevertheless, we hope that our systematic review will help to solve some difficulties in decision-making for specialists who are to treat the patient with CPC for the first time or to perform proper high-volume research. Based on the current literature and personal experience, we are advised to use endoscopic procedures as the primary approach for symptomatic cases of CPCs because they are most effective and complication-free.
Data availability
All data are included in the main text.
References
Basilotta Marquez Y, Gromadzyn G, Tcherbbis Testa V, Rugilo C, Argañaraz R, Mantese B (2022) Choroid plexus cyst causing acute hydrocephalus and transtentorial herniation: report of a rare case and its successful neuroendoscopic treatment. Childs Nerv Syst 38(2):435–439. https://doi.org/10.1007/s00381-021-05184-x Epub 2021 May 4
Spennato P, Chiaramonte C, Cicala D, Donofrio V, Barbarisi M, Nastro A, Mirone G, Trischitta V, Cinalli G (2016) Acute triventricular hydrocephalus caused by choroid plexus cysts: a diagnostic and neurosurgical challenge. Neurosurg Focus 41(5):E9. https://doi.org/10.3171/2016.8.FOCUS16269
Czervionke LF, Daniels DL, Meyer GA, Pojunas KW, Williams AL, Haughton VM (1987) Neuroepithelial cysts of the lateral ventricles: MR appearance. AJNR Am J Neuroradiol 8(4):609–613 PMID: 3113198; PMCID: PMC8333684
Yeates A, Enzmann D (1979) An intraventricular arachnoid cyst. J Comput Assist Tomogr 3(5):697–700. https://doi.org/10.1097/00004728-197910000-00025
Kariyattil R, Panikar D (2008) Choroid plexus cyst of the third ventricle presenting as acute triventriculomegaly. Childs Nerv Syst 24(7):875–877. https://doi.org/10.1007/s00381-008-0622-8 Epub 2008 Apr 18
Draghi R, Mongardi L, Panzacchi R, Godano U, Barni I, Calbucci F, Borghesi I (2020) Choroid plexus cyst of the fourth ventricle associated with intermittent obstructive hydrocephalus. World Neurosurg 143:152–157. https://doi.org/10.1016/j.wneu.2020.07.164 Epub 2020 Aug 1
Nambiema A, Sembajwe G, Lam J, Woodruff T, Mandrioli D, Chartres N, Fadel M, Le Guillou A, Valter R, Deguigne M, Legeay M, Bruneau C, Le Roux G, Descatha A (2021) A Protocol for the use of case reports/studies and case series in systematic reviews for clinical toxicology. Front Med (Lausanne) 6(8):708380. https://doi.org/10.3389/fmed.2021.708380 PMID: 34552944; PMCID: PMC8450367
Sampayo-Cordero M, Miguel-Huguet B, Malfettone A, Pérez-García JM, Llombart-Cussac A, Cortés J, Pardo A, Pérez-López J (2020) The value of case reports in systematic reviews from rare diseases. The example of enzyme replacement therapy (ERT) in patients with mucopolysaccharidosis type II (MPS-II). Int J Environ Res Public Health 17(18):6590. https://doi.org/10.3390/ijerph17186590 PMID: 32927819; PMCID: PMC7558586
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Seike M, Kurisaka M, Mori K (1987) Intraventricular arachnoid cyst. Case report. Neurol Med Chir (Tokyo) 27(2):154–158. Japanese. https://doi.org/10.2176/nmc.27.154
Martinez-Lage JF, Poza M, Sola J, Puche A (1992) Congenital arachnoid cyst of the lateral ventricles in children. Childs Nerv Syst 8(4):203–206. https://doi.org/10.1007/BF00262846
Iglesias JR, Sanjuanbenito L, Martinez-Cubells J, Lousa M, Redondo C (1981) Intracerebral arachnoidal cyst containing choroid plexus. Case report. Acta Neurochir (Wien) 58(3-4):229–234. https://doi.org/10.1007/BF01407129
Baker GS, Gottlieb CM (1956) Cyst of the choroid plexus of the lateral ventricle causing disabling headaches and unconsciousness-report of case. Proc Staff Meet Mayo Clin 31:95–97
de la Torre E, Alexander E Jr, Davis CH Jr, Crandell DL (1963) Tumors of the lateral ventricles of the brain. Report of eight cases, with suggestions for clinical management. J Neurosurg 20:461–470. https://doi.org/10.3171/jns.1963.20.6.0461
Neblett CR, Robertson JW (1971) Symptomatic cysts of the telencephalic choroid plexus. J Neurol Neurosurg Psychiatry 34(3):324–328. https://doi.org/10.1136/jnnp.34.3.324 PMID: 5571320; PMCID: PMC1083471
Andreussi L, Cama A, Cozzutto C, Gianbartolomei G, Grossi G (1979) Cyst of the choroid plexus of the left lateral ventricle. Surg Neurol 12(1):53–57
Giorgi C (1979) Symptomatic cyst of the choroid plexus of the lateral ventricle. Neurosurgery 5(1 Pt 1):53–56. https://doi.org/10.1227/00006123-197907010-00009
Vaquero J, Cabezudo JM, Leunda G (1980) Symptomatic cyst of choroid plexus of the lateral ventricle. J Neurol Neurosurg Psychiatry 43(6):555. https://doi.org/10.1136/jnnp.43.6.555 PMID: 6782197; PMCID: PMC490601
Dempsey RJ, Chandler WF (1981) Choroid plexus cyst in the lateral ventricle causing obstructive symptoms in an adult. Surg Neurol 15(2):116–119. https://doi.org/10.1016/0090-3019(81)90026-4
New PF, Davis KR (1981) Intraventricular noncolloid neuroepithelial cysts. AJNR Am J Neuroradiol 2(6):569–576 PMID: 6797281; PMCID: PMC8335256
Hatashita S, Takagi S, Sakakibara T (1984) Choroid plexus cyst of the lateral ventricle in an elderly man. Case report. J Neurosurg 60(2):435–437. https://doi.org/10.3171/jns.1984.60.2.0435
Yoshida T, Ikeda T, Maeda Y, Ushio Y, Koh S, Mogami H (1984 Jul) An arachnoid cyst in the left lateral ventricle. No Shinkei Geka 12(8):969–973 Japanese
Inoue T, Kuromatsu C, Iwata Y, Matsushima T (1985) Symptomatic choroidal epithelial cyst in the fourth ventricle. Surg Neurol 24(1):57–62. https://doi.org/10.1016/0090-3019(85)90065-5
Blom R, Witt N, Johnson ES (1986) Demonstration of a symptomatic intraventricular cyst using direct intraventricular metrizamide instillation. AJNR Am J Neuroradiol 7(6):1093–1095 PMID: 3098074; PMCID: PMC8334066
Nakasu Y, Handa J, Watanabe K (1986) Progressive neurological deficits with benign intracerebral cysts. Report of two cases. J Neurosurg 65(5):706–709. https://doi.org/10.3171/jns.1986.65.5.0706
Nakase H, Hisanaga M, Hashimoto S, Imanishi M, Utsumi S (1988) Intraventricular arachnoid cyst. Report of two cases. J Neurosurg 68(3):482–486. https://doi.org/10.3171/jns.1988.68.3.0482
Numaguchi Y, Kumra A, Schmidt RD, Martino C (1988) Noncolloid neuroepithelial cysts in the lateral ventricle: magnetic resonance features. J Comput Tomogr 12(3):174–181. https://doi.org/10.1016/0149-936x(88)90002-1
Lee K, Bae H, Yun I (1989) Intraventricular arachnoid cyst. J Neurosurg 70(1) Retrieved May 25, 2023, from. https://doi.org/10.3171/jns.1989.70.1.0154
Numaguchi Y, Foster RW, Gum GK (1989) Large asymptomatic noncolloid neuroepithelial cysts in the lateral ventricle: CT and MR features. Neuroradiology. 31(1):98–101. https://doi.org/10.1007/BF00342042
Goda K, Tsunoda S, Sakaki T, Nakase H, Yoshimura Y, Sato N, Nakagawa H, Iwasaki S (1990 Aug) Intraventricular arachnoid cyst appearing with attacks of orbital pain: case report and review of the literature. No Shinkei Geka 18(8):757–760 Japanese
Kurokawa Y, Sohma T, Tsuchita H et al (1990) A case of intraventricular arachnoid cyst. Childs Nerv Syst 6:365–367. https://doi.org/10.1007/BF00298286
Odake G, Tenjin H, Murakami N (1990) Cyst of the choroid plexus in the lateral ventricle: case report and review of the literature. Neurosurgery. 27(3):470–476. https://doi.org/10.1097/00006123-199009000-00024
Pelletier J, Milandre L, Péragut JC, Cronqvist S (1990) Intraventricular choroid plexus “arachnoid” cyst. MRI findings. Neuroradiology 32(6):523–525. https://doi.org/10.1007/BF02426471
Lam AH, Villanueva AC (1992) Symptomatic third ventricular choroid plexus cysts. Pediatr Radiol 22(6):413–416. https://doi.org/10.1007/BF02013499
Baka JJ, Sanders WP (1993) MRI of hemorrhagic choroid plexus cyst. Neuroradiology. 35(6):428–430. https://doi.org/10.1007/BF00602822
Okamura K, Watanabe M, Inoue N, Kanoh M, Ohno T, Mitsui Y, Wakabayashi K (1996Nov) Intraventricular arachnoid cyst–on the origin of intraventricular arachnoid cysts. No To Shinkei. 48(11):1015–1021. Japanese
Parizek J, Jakubec J, Hobza V, Nemecková J, Cernoch Z, Sercl M, Zizka J, Spacek J, Nemecek S, Suba P (1998) Choroid plexus cyst of the left lateral ventricle with intermittent blockage of the foramen of Monro, and initial invagination into the III ventricle in a child. Childs Nerv Syst 14(12):700–708. https://doi.org/10.1007/s003810050301
Hanbali F, Fuller GN, Leeds NE, Sawaya R (2001) Choroid plexus cyst and chordoid glioma. Report of two cases. Neurosurg Focus 10(6):E5. https://doi.org/10.3171/foc.2001.10.6.6
Radaideh MM, Leeds NE, Kumar AJ, Bruner JM, Sawaya R (2002) Unusual small choroid plexus cyst obstructing the foramen of monroe: case report. AJNR Am J Neuroradiol 23(5):841–843 PMID: 12006289; PMCID: PMC7974717
Jeon JH, Lee SW, Ko JK, Choi BG, Cha SH, Song GS, Choi CH (2005) Neuroendoscopic removal of large choroid plexus cyst: a case report. J Korean Med Sci 20(2):335–339. https://doi.org/10.3346/jkms.2005.20.2.335\ PMID: 15832013; PMCID: PMC2808618
van Baalen A, Stephani U (2007) Flexible and floating choroid plexus cyst of the third ventricle: an ultrasonographic video documentation. Childs Nerv Syst 23(2):259–261. https://doi.org/10.1007/s00381-006-0254-9 Epub 2006 Nov 15
Nahed BV, Darbar A, Doiron R, Saad A, Robson CD, Smith ER (2007) Acute hydrocephalus secondary to obstruction of the foramen of Monro and cerebral aqueduct caused by a choroid plexus cyst in the lateral ventricle. Case report. J Neurosurg 107(3 Suppl):236–239. https://doi.org/10.3171/PED-07/09/236
Bozić B, Rotim K, Houra K (2008) Giant choroid plexus cyst as an accidental finding in an older man. Coll Antropol 32(Suppl 1):195–197
Chamczuk AJ, Grand W (2010) Endoscopic cauterization of a symptomatic choroid plexus cyst at the foramen of Monro: case report. Neurosurgery. 66(6 Suppl Operative):376–377; discussion 377. https://doi.org/10.1227/01.NEU.0000369186.99306.E0
Filardi TZ, Finn L, Gabikian P, Giussani C, Ebenezer S, Avellino AM (2009) Treatment of intermittent obstructive hydrocephalus secondary to a choroid plexus cyst. J Neurosurg Pediatr 4(6):571–574. https://doi.org/10.3171/2009.7.PEDS08247
Eboli P, Danielpour M (2011) Acute obstructive hydrocephalus due to a large posterior third ventricle choroid plexus cyst. Pediatr Neurosurg 47(4):292–294. https://doi.org/10.1159/000336046 Epub 2012 Feb 22
de Lara D, Ditzel Filho LF, Muto J, Prevedello DM (2013) Endoscopic treatment of a third ventricle choroid plexus cyst. Neurosurg Focus 34(1 Suppl):Video 9. https://doi.org/10.3171/2013.V1.FOCUS12332
Azab WA, Mijalcic RM, Aboalhasan AA, Khan TA, Abdelnabi EA (2015) Endoscopic management of a choroid plexus cyst of the third ventricle: case report and documentation of dynamic behavior. Childs Nerv Syst 31(5):815–819. https://doi.org/10.1007/s00381-015-2649-y Epub 2015 Feb 26
Tamai S, Hayashi Y, Sasagawa Y, Oishi M, Nakada M (2018) A case of a mobile choroid plexus cyst presenting with different types of obstructive hydrocephalus. Surg Neurol Int 23(9):47. https://doi.org/10.4103/sni.sni_377_17\ PMID: 29541488; PMCID: PMC5843973
Yang R, Yan H, Dewan MC, Tailor JK, Santisukwongchote S, Hawkins C, Ibrahim GM (2021) Giant choroid plexus cysts with calvarial erosion: a case report and literature review. Childs Nerv Syst 37(7):2381–2385. https://doi.org/10.1007/s00381-020-04930-x Epub 2020 Oct 15
Binning MJ, Couldwell WT (2008) Choroid plexus cyst development and growth following ventricular shunting. J Clin Neurosci 15(1):79–81. https://doi.org/10.1016/j.jocn.2006.05.021 Epub 2007 Nov 26
Dandy WE (1934) Benign encapsulated tumors in the lateral ventricle of the brain: diagnosis and treatment. Williams and Wilkins, Baltimore, p 23
Brown G (1876) Cyst of the choroid plexus of large size in an infant. Trans Path Soc Lond 27:25–26
Author information
Authors and Affiliations
Contributions
Artem Stanishevskiy contributed as the first author to the paper and wrote the main manuscript text. Dmitriy Svistov and Vladislav Cherebillo have made substantial contributions to the conception and design of the manuscript and Artem Stanishevskiy and Mariia Kurnukhina to the acquisition, analysis, and interpretation of the data. Artem Stanishevskiy and Gaspar Gavrilov participated in drafting the manuscript, and Dmitriy Svistov and Vladislav Cherebillo revised it critically. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. This is an observational study, and no ethical approval is required. Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stanishevskiy, A., Gavrilov, G., Svistov, D. et al. Symptomatic intraventricular choroid plexus cysts. Illustrative case and systematic review. Neurosurg Rev 46, 264 (2023). https://doi.org/10.1007/s10143-023-02176-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-023-02176-0