Abstract
Controversies persist regarding the benefits of surgery in elderly patients with meningiomas. The objective of this study was to develop decision-making scale to clarify the necessity for surgical intervention and provide clinical consultation for this special population. This retrospective cohort study was conducted at a single center and included 478 elderly patients (≥ 65 years) who underwent meningioma resection. Follow-up was recorded to determine recurrence and mortality rates. Univariate and multivariate analyses were performed to identify significantly preoperative factors, and prognostic prediction models were developed with determined cutoff values for the prognostic index (PI). Model discrimination was evaluated using Kaplan-Meier curves based on the PI stratification, which categorized patients into low- and high-risk groups. A decision-making tree was then established based on the risk stratification from both models. Among all patients analyzed (n = 478), 62 (13.0%) experience recurrence and 47 (10.0%) died during the follow-up period. Significantly preoperative parameters from both models included advanced age, aCCI, recurrent tumor, motor cortex involvement, male sex, peritumoral edema, and tumor located in skull base (all P < 0.05). According to the classification of PI from the two models, the decision-making tree provided four recommendations that can be used for clinical consultation. Surgery is not recommended for patients assigned to the high-risk group in both models. Patients who meet the low-risk criteria in any model may undergo surgical intervention, but the final decision should depend on the surgeon’s expertise.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meningiomas, accounting for about one-third of the CNS tumors, have become the most common intracranial lesions [1, 2]. Epidemiological studies indicated that the highest incidence rates are observed in individuals ranging from 55 to 89 years old [3,4,5,6]. Around 20% of meningiomas exhibit aggressive behavior, and the recurrence rate ranges from 7% to 94% [7,8,9,10,11,12]. Surgical treatment remains the leading therapeutic approach, significantly improving life expectancy. With development of microsurgical techniques, the benefits from meningioma resection are significantly increased [13]. However, the benefits of surgery for elderly patients vary due to age-related health declines in health and reduced physiological reserves that lead to poor outcomes under surgical stress. Currently, there is no consensus on surgical treatment decision for this vulnerable population [14]. Likewise, while radiotherapy can be used as a stand-alone approach or as an adjuvant for more aggressive lesions, its potential adverse effects are still under discussion [11, 15]. Although previous studies have reported prognosis-related predictors for the elderly meningioma patients [16,17,18], there is currently no consensus on the risk factors affecting overall survival (OS) and recurrence-free survival (RFS) after surgery. Furthermore, little is known about which elderly meningioma patients would benefit from surgical intervention.
Therefore, this study aims to provide a comprehensive analysis of the prognosis of elderly patients who undergo meningioma resection using one of the largest cohorts to date. Subsequently, a decision-making tree for surgical intervention in this susceptible group is established.
Methods
Patient cohorts
This was a single-center retrospective cohort study that was conducted in the Department of Neurosurgery at Beijing Tiantan Hospital, Capital Medical University, Beijing, China, from January 2008 to December 2018. Ethical approval was obtained from the Ethics Committee of Beijing Tiantan Hospital, and this study was conducted according to the declaration of Helsinki. Elderly patients who were aged 65 years or older, pathologically diagnosed with meningiomas and underwent surgical resection were included. The exclusion criteria were as follows: (i) patients with other brain or spine lesions; (ii) patients with neurofibromatosis type 1 or 2; (iii) concurrence with other malignancies or death from other lethal diseases in hospital or after discharge; (iv)multiple lesions; (v) loss of follow-up data; and (vi) other (e.g. incomplete clinical data). Supplementary Figure S1 shows the study flowchart.
Clinical data collection
Demographic data were collected, including age, gender, neurological symptoms, comorbidities (hypertension, diabetes mellitus, cerebrovascular accident, heart disease, hydrocephalus, pulmonary disease), age-related Charlson Comorbidity Index (aCCI) [19], history of other surgery, recurrent meningioma, smoking, drinking, and preoperative KPS. Imaging features were analyzed through the review of magnetic resonance imaging (MRI) scans and reports documented by 2 experienced radiologists. All individuals underwent MRI tests and the radiology characteristics contained tumor location (non-skull base and skull base/non-posterior fossa and posterior fossa), tumor maximal diameter, involvement of nerves and vessels, involvement of motor cortex, venous sinus invasion, peritumoral edema, and edema index (EI). The tumor maximum diameter was calculated in coronal, sagittal, and axial images on account of the preoperative contrast-enhanced MRI and the cutoff value was based on the median (i.e., ≤ 6 or > 6 cm). The peritumoral edema was defined as hyperintensity on axial T2-weighted or fluid-attenuated inversion recovery (FLAIR) MRI. Clinicopathological parameters were recorded by 2 neuropathologists and meningiomas were graded in accordance with 2021 WHO classification scheme [20]. The extent of resection (EOR) according to Simpson grade was extracted from the surgical reports. The EOR was classified into two categories: gross total resection (GTR; Simpson grades I–III) or subtotal resection (STR; Simpson grades IV and V). Radiologically, GTR was evaluated when patients underwent routine MRI 3 months after surgery to rule out early unspecific postoperative contrast enhancement and other possible confounding effects. Surgical/medical complications included intracranial hemorrhage, hydrocephalus, pneumonia, deep venous thrombosis, central nervous system infection, and wound infection. We also included Therapy-Disability-Neurology (TDN) grade [21] to predict adverse events after surgery. The postoperative KPS was obtained from the discharge records.
Follow-up
All cases treated at our institution underwent regular follow-ups until the patient experienced a recurrence or died. Specifically, (1) follow-up of WHO grade 1 meningiomas was done annually, then every 2 years after 5 years; (2) follow-up of WHO grade 2 meningiomas was done every 6 months, then annually after 5 years; (3) follow-up of WHO grade 3 meningiomas was done every 3–6 months indefinitely [22]. The surviving patients were reviewed at the final follow-up (Jan. 2023). MRI scan was scheduled in each follow-up. Patients were encouraged to have regular face-to-face clinic visits. However, for those who are unwilling or unable to travel to follow-up appointments, we recommend that they get an MRI scan at a local medical institute. Information regarding their scheduled follow-up is available through network communication, which allows for the sharing of messages, video-calls, videos, photographs, or electronic mail attachments of the MRI films. Radiologically, we defined tumor recurrence as the detectable appearance of a new enhanced lesion on a serial postoperative MR image that required therapeutic intervention such as secondary surgery, or an increase of more than 25% in a residual tumor on an MR image [23]. Postoperative radiotherapy was documented at follow-up. The median (interquartile range [IQR]) follow-up time was 65 (51–80) months.
Statistical analysis
Demographic and clinical data of all participants were generalized by mean ± SD or median (IQR) for continuous variables and counts with proportions for categorical features. The differences in recurrence and death cohorts were compared using the chi-squared test, Student’s t test, Fisher’s exact or Mann-Whitney U test as necessary. To reduce type I errors, Benjamini-Hochberg procedure was used to conduct the false discovery rate (FDR) multiple comparison correction analysis on comparison tests. The RFS and OS cumulative rates were plotted by Kaplan-Meier curves with log-rank tests, respectively. Univariate and multivariate time-to-death analyses were performed using Cox proportional hazard regression models. The potential risk estimators were identified by conducting univariate Cox regression analysis with a p < 0.05. In addition, variables that were deemed clinically important and predictors previously identified in published articles were also considered [16, 17, 24, 25]. Next, the potential risk estimators were subjected to multivariate analysis using the Enter approach.
To make a decision-making tree, models incorporated significant preoperative covariates from the multivariate analyses were developed. Prognostic index (PI) was calculated by β coefficients. Model performance was assessed by receiver operating characteristic (ROC) curve and cutoff values of PI were determined. An area under the curve (AUC) of 0.5 indicates no discrimination, while an AUC of 1 reflects perfect discrimination. A scoring system with an AUC ≥ 0.7 is considered to have acceptable clinical relevance for discrimination capacity [26]. Model discrimination was further checked by the Kaplan-Meier curve of PI, which separated patients into low-risk and high-risk groups. Moreover, the calibration of the model was evaluated using the Hosmer-Lemeshow goodness-of-fit test. Statistical calculations were performed with SPSS v26.0. All tests were two-sided, and p < 0.05 was considered as statistical significance.
Results
Baseline features
The clinical histories of 563 elderly individuals admitted to our institution were reviewed. A total of 478 patients met the inclusion criteria. The descriptive characteristics were listed in Supplementary Table S1. There were 139 males and 339 females (ratio 1:2.4). The mean age at surgery was 68.75 ± 3.42 years, and the patients with recurrent tumors accounted for 44 (9.3%). The tumors were more likely to be located in the non-skull base (60.3%) and non-posterior fossa (77.0%).
Survival
Of 478 elderly participants, 47 (10.0%) died during the follow-up. The cumulative rates of OS at 3, 5, and 10 years were 94.1% (95% CI 93.1–95.1%), 90.7% (95% CI 89.4–92.0%), and 87.5% (95% CI 85.8–89.2%), respectively (Fig. 1A). The Kaplan-Meier curves showed the variables including advanced age (P < 0.001), male sex (P < 0.001), STR (P < 0.001), and either WHO 2 or 3 grade (P < 0.001) significantly associated with lower life expectancy (Fig. 1B–E). In univariate analysis, the proportions of patients with advanced age (χ2 = 15.31, P [FDR-corrected] < 0.001), male gender (χ2 = 17.40, P < 0.001), recurrent meningioma (χ2 = 32.17, P < 0.001), maximal diameter of tumor > 6 cm (χ2 = 17.91, P < 0.001), peritumoral edema (χ2 = 16.40, P < 0.001), WHO 2–3 grade (χ2 = 46.10, P < 0.001), STR (χ2 = 11.63, P = 0.001), perioperative medical/surgical complications (χ2 = 12.12, P = 0.001), lower preoperative and postoperative KPS (P = 0.004; P < 0.001), 3–5 TDN grade (χ2 = 34.16, P < 0.001), and recurrence during follow-up (χ2 = 16.57, P < 0.001) were higher in the mortality cohorts (Table 1). We also found that there was positive association between aCCI and mortality in elderly meningioma patients (t = −1.92 , P = 0.055). These results were almost consistent with univariate Cox regression analysis (Table 2).
Cumulative OS or RFS rates stratified by different variables. A OS possibility for the elderly with meningiomas after resection. B Comparison of survival rate between different age-stratified for the elderly with meningiomas after resection. C Comparison of survival rate between males and females for the elderly with meningiomas after resection. D Comparison of survival rate between different WHO grades for the elderly with meningiomas after resection. E Comparison of survival rate between different extent of resection for the elderly with meningiomas after resection. F RFS possibility for the elderly with meningiomas after resection. G Comparison of recurrence rate between different age-stratified for the elderly with meningiomas after resection. H Comparison of recurrence rate between males and females for the elderly with meningiomas after resection. I Comparison of recurrence rate between different WHO grades for the elderly with meningiomas after resection. J Comparison of recurrence rate between different extent of resection for the elderly with meningiomas after resection
All parameters fitted in the multivariable analysis were further estimated. The results showed male gender (HR 1.98, 95% CI 1.06–3.71, P = 0.032), age at surgery (HR 1.15, 95% CI 1.06-1.25, P = 0.001), recurrent meningioma (HR 3.50, 95% CI 1.64–7.46, P = 0.001), tumor located in skull base (HR 1.84, 95% CI 1.00–3.41, P = 0.049), peritumoral edema (HR 2.07, 95% CI 1.08–4.00, P = 0.029), WHO 2–3 grade (HR 4.31, 95% CI 2.21–8.42, P < 0.001), medical/surgical complications (HR 3.63, 95% CI 1.82–7.23, P < 0.001), and postoperative KPS (HR 0.97, 95% CI 0.96–0.99, P = 0.006) were independently and significantly associated with mortality (Table 2).
Recurrence
Sixty-two (13.0%) elderly patients experienced recurrence during follow-up. The cumulative rates of RFS at 3, 5, and 10 years were 93.1% (95% CI 90.6–95.4%), 89.4% (95% CI 86.5–92.3%), and 79.1% (95% CI 72.0–86.2%), respectively (Fig. 1F). The Kaplan-Meier curves revealed that age ≥ 75 years (P = 0.005), STR (P < 0.001), and WHO grade 2–3 (P < 0.001) significantly associated with the increased recurrence rate (Fig. 1G, I, and J). Additionally, male gender was more likely to have an association with recurrence but the statistical significance was not reached (P = 0.062) (Fig. 1H). In the univariate analysis, the proportions of elderly patients with advanced age (χ2 = 12.17, P = 0.002), recurrent meningioma (χ2 = 28.28, P < 0.001), involvement of motor cortex (χ2 = 4.29, P = 0.038), peritumoral edema (χ2 = 7.07, P = 0.008), WHO 2–3 grade (χ2 = 38.68, P < 0.001), subtotal resection (χ2 = 24.92, P < 0.001), and lower preoperative KPS (P = 0.001) were significantly higher in the recurrence group (Table 1). Besides, maximal diameter of tumor > 6 cm was marginally significantly associated with recurrence. These results were almost consistent with the univariate Cox regression analysis (Table 2). Furthermore, in multivariate Cox analysis, we found that older age (HR 1.08, 95% CI 1.01–1.16, P = 0.037), higher aCCI (HR 1.33, 95% CI 1.08–1.64, P = 0.008), recurrent meningioma (HR 1.84, 95% CI 1.01–3.48, P = 0.049), involvement of motor cortex (HR 1.99, 95% CI 1.02–4.06, P = 0.047), WHO 2–3 grade (HR 3.84, 95% CI 2.15–6.86, P < 0.001), and STR (HR 2.61, 95% CI 1.46–4.65, P = 0.001) became independent risk predictors for tumor recurrence (Table 2).
Development of prognostic assessment models
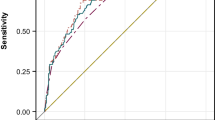
To identify elderly individuals with meningiomas who may benefit from tumor resection before surgery, the first step is to establish prognostic assessment models based on significant variables available preoperatively. Specifically, the aforementioned multivariate analysis revealed that the preoperative variables were independently associated with recurrence included advanced age, aCCI, recurrent meningioma, and involvement of motor cortex. Therefore, a predictive model (recurrence) was developed (Supplementary Table S2). Each variable corresponded to a β coefficient and PI = (0.08 * I [age]) + ( 0.28 * I [aCCI]) + ( 0.61 * I [recurrent tumor]) + (0.69 * I [motor cortex involved]), where I denotes the indicator function equal to 1 if the condition in parenthesis is met and 0 otherwise. Furthermore, a predictive model (mortality) was developed to predict death by incorporating significant preoperative variables from the multivariate analysis (Supplementary Table S3). The weighted risk factors were used to calculate PI: (0.12 * I [age] + (1.41 * I [recurrent tumor]) + (0.92 * I [male sex]) + (1.13 * I [peritumoral edema]) + (0.63 * I [skull base]). The discrimination of both models with AUC was 0.70 (95% CI 0.66–0.74) and 0.83 (95% CI 0.79–0.86) (Fig. 2A–B). Furthermore, low- and high-risk groups for both models were stratified according to the cutoff value of PI. Kaplan-Meier curves revealed each group was well separated (Fig. 2C–D). In addition, Hosmer-Lemeshow goodness-of-fit tests yielded chi-squares of 7.86 (P = 0.447) and 14.46 (P = 0.064) for recurrence model and mortality model, respectively, suggesting good calibration and no significant deviation between observed and predicted events in both datasets.
Validation of model performance and risk stratification according to PI. A–B ROC curves showing model (recurrence) with AUC 0.700 and model (mortality) with AUC 0.826. C–D Kaplan-Meier curves demonstrating recurrence and mortality in patients after resection for meningiomas according to cutoff values of PI
Development of decision-making tree
The next step is to develop decision-making tree by combining both prognostic assessment models for clinical consultation. Based on the Youden index, the cutoff values of PI for two aforementioned models were 7.01 (recurrence model) and 9.24 (mortality model), respectively. Using the risk stratification from the two models, decision-making tree was developed (details in Fig. 3).
Surgical decision-making tree for the elderly patients with meningiomas. According to NCCN guidelines, for asymptomatic meningioma with a tumor maximum diameter ≤ 3 cm, the “waiting and watching” approach is recommended. According to the cutoff value of PI, patients who are assigned to the low-risk group in both models should be advised to undergo surgical procedures. Conversely, surgery may not be recommended if a patient is assigned to the high-risk group in both models. Furthermore, surgical resection should be considered when a patient is assigned to the low-risk group in model (mortality) and to the high-risk group in model (recurrence). In other cases, the choice of surgery depends on the clinical assessment of neurosurgeons and patients’ wishes. Blue and purple solid boxes represent risk stratification from model (recurrence) and model (mortality), respectively. Contents in solid colored boxes are our proposed treatment patterns for meningiomas in the elderly during pre-operative consultations
Discussion
The incidence of meningiomas continues to increase with the aging of the population [22]. However, there is a lack of comprehensive studies evaluating the specific characteristics and risk factors that impact recurrence and survival in elderly patients with meningiomas resection. Additionally, there is no consensus on the optimal therapeutic strategies for elderly patients with meningiomas, particularly regarding surgical intervention. In this study, we aimed to address these gaps by examining the clinical, histological, and radiological characteristics associated with recurrence and death after resection. Furthermore, we developed decision-making tree based on recurrence and mortality models to identify those who can benefit from surgery.
Previous studies have suggested that advanced age is a significant independent predictor that influences the recurrence and mortality rate following resection [27,28,29,30]. While Hanna et al. reported a decrease in OS but an increase in PFS with advanced age [31], our study found that both OS and RFS rates decreased with advanced age. Our study also demonstrated the significant impact of preoperative comorbidities and higher aCCI on poor prognosis in those special population. Several previous studies have reported a poor prognosis following meningioma excision in elderly patients. This has been attributed to various factors, including advanced age, male gender, larger tumor volume, peritumoral edema, lower KPS scores, tumors located in the skull base, WHO grade II–III, STR, and the occurrence of postoperative complications [16, 27,28,29,30, 32,33,34,35,36]. These findings are consistent with the results in our study. Interestingly, gender has bidirectional aspects regarding the incidence and prognosis of meningioma. On the one hand, females predominated the incidence due to the frequent distribution of estrogen and progesterone receptors in meningioma [37, 38]. On the other hand, we identified male sex as a poor prognostic factor, which is consistent with reports by Sacko et al. and Caroli et al. [17, 32]. However, the association of male sex with a worse prognosis at the molecular or hormonal level remains unclear. Furthermore, Cohen-Inbar et al. [30] suggested that tumor size played a pivotal role in the necessity of surgical intervention for the elderly with meningioma. In our study, we found that tumor size was significantly associated with prognosis in the univariate analysis, but not in the multivariable analysis. Therefore, further studies with larger sample sizes are required to validate these findings. Another study by Ehresman et al. [39] reported that cerebellopontine angle tumors are independently associated with postoperative deficits after resection and that impaired functional outcomes after surgery are linked to a poor prognosis. In our study, we found that the tumor located in the skull base was an independent risk factor for mortality after surgery. Additionally, several reports have indicated that the extent of peritumoral edema is significantly correlated with a poor prognosis [30, 33], which is also consistent with our study. Moreover, other factors such as involvement of motor cortex and STR were also associated with an increased susceptibility to relapse, possibly due to the preservation of maximal neurological function. Our study also highlighted a similar conclusion.
Previously, prognostic models for meningioma have primarily focused on the general population, with limited emphasis on the elderly patients. However, given the higher incidence, recurrence, and mortality rates of meningioma in elderly patients, it is crucial to develop prognostic models specially for elderly patients. Previous studies have constructed predictive models based on small sample sizes [17, 30, 32, 33], which limits their clinical applicability. Our study presented one of the largest single-center cohort of elderly patients with meningiomas, allowing for a more comprehensive exploration of prognosis and reducing potential biases associated with inadequate sample size. Furthermore, existing models have primarily focused on the risk of postoperative mortality rather than recurrence [17, 32], and they have not been integrated to guide surgical decision-making. Given the advanced age and reduced surgical resilience of elderly patients, as well as the slow growth of some meningiomas that may not significantly affect a patient’s expected survival, the decision to undergo surgery becomes particularly crucial for this population and requires careful consideration. Therefore, we developed a comprehensive surgical decision-making tree specifically for elderly patients with meningiomas. This tree combines two models for mortality and recurrence risk stratification based on cutoff values of PI to facilitate the decision-making process in clinical consultation condition. This could potentially be the first comprehensive decision tree analysis for elderly patients undergoing meningioma surgery. The advantage of this decision-making tree lies in the simplicity of its variables and its ability to identify high-risk individuals through basic mathematical calculations.
In our study, both models demonstrated the impact of age on surgical decision is very crucial regardless of other risk factors considered in the PI formula. Previous studies have identified the risk of postoperative mortality commonly as the primary factor that contraindicates intracranial surgery in patients who are over 70 years of age [17]. However, Oumar et al. suggested that craniotomy may still be a viable option for meningioma patients over 80 years old [17]. Applying the PI formula, we observed that patients aged over 80 years without any additional risk factors should be assigned to the high-risk group in mortality model. Consequently, surgical intervention may not be the first choice for these patients. Additionally, the surgical decision-making tree incorporated two prognostic models, which can be used in clinical practice to guide more optimized therapeutic alternatives for the elderly patients with meningiomas.
According to the decision-making tree, the “waiting and watching” approach is recommended for asymptomatic meningiomas with maximal diameter ≤ 3 cm, in accordance with NCCN guidelines. However, if the tumor does not meet the aforementioned criteria, surgical intervention may be recommended if the patient is assessed as low-risk in both models. Conversely, surgery may not be the optimized choice if a patient is assessed as high-risk in both models. For cases assessed as the low-risk in mortality model and high-risk in recurrence model, we suggest that the surgical intervention may be considered. In other cases, the decision regarding surgery depends on the clinical judgment of the neurosurgeons and patients’ wishes (Fig. 3).
Our study has some limitations. Firstly, the age range of enrolled elderly patients was relative narrow, and we did not include an adequate number of individuals over 80 years old. Secondly, this is a retrospective study, inherent biases may exist in certain aspects. For example, the lack of information on the nature history in healthy controls could result in overestimation of meningioma-related mortality rates. Furthermore, we were unable to examine some molecular information, such as TERT promoter mutation and CDKN2A/B homozygous deletion, due to unavailability of genetic test data. Thirdly, we were unable to incorporate certain important prognosis-related predictors, such as WHO classification and postoperative complications into our surgical decision diagram, as these factors were undetectable prior to surgery. Fourthly, due to lack of the data on the outcomes of non-surgical treatment of meningiomas, this decision-making tree only can provide recommendations for those who are suitable for surgical intervention. Finally, extensive external validation is required to further refine and validate our surgical decision-making tree.
Conclusion
In one of the largest elderly patient cohorts to date, this study identified several key predictors that significantly affect postoperative RFS and OS. These findings have led to the development of a comprehensive surgical decision-making tree specifically tailored for elderly patients with meningiomas. By utilizing risk stratification from two prognostic models, this decision-making tree offers four recommendations that can be used during clinical consultation for these special population. It is important to note that further validation of our surgical making-tree is warranted. However, this innovative approach holds the potential to provide valuable guidance to both neurosurgeons and patients in the future.
Data availability
Not applicable.
Code availability
Not applicable.
References
Baldi I, Engelhardt J, Bonnet C, Bauchet L, Berteaud E, Grüber A, Loiseau H (2018) Epidemiology of meningiomas. Neuro-Chirurgie 64:5–14. https://doi.org/10.1016/j.neuchi.2014.05.006
Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS (2013) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol 15(Suppl 2):ii1–ii56. https://doi.org/10.1093/neuonc/not151
Davis FG, Kupelian V, Freels S, McCarthy B, Surawicz T (2001) Prevalence estimates for primary brain tumors in the United States by behavior and major histology groups. Neuro Oncol 3:152–158. https://doi.org/10.1093/neuonc/3.3.152
McCarthy BJ, Davis FG, Freels S, Surawicz TS, Damek DM, Grutsch J, Menck HR, Laws ER Jr (1998) Factors associated with survival in patients with meningioma. J Neurosurg 88:831–839. https://doi.org/10.3171/jns.1998.88.5.0831
Porter KR, McCarthy BJ, Freels S, Kim Y, Davis FG (2010) Prevalence estimates for primary brain tumors in the United States by age, gender, behavior, and histology. Neuro Oncol 12:520–527. https://doi.org/10.1093/neuonc/nop066
Radhakrishnan K, Mokri B, Parisi JE, O'Fallon WM, Sunku J, Kurland LT (1995) The trends in incidence of primary brain tumors in the population of Rochester, Minnesota. Ann Neurol 37:67–73. https://doi.org/10.1002/ana.410370113
Bailo M, Gagliardi F, Boari N, Castellano A, Spina A, Mortini P (2019) The role of surgery in meningiomas. Curr Treat Options Neurol 21:51. https://doi.org/10.1007/s11940-019-0587-9
Cea-Soriano L, Wallander MA, García Rodríguez LA (2012) Epidemiology of meningioma in the United Kingdom. Neuroepidemiology 39:27–34. https://doi.org/10.1159/000338081
Christensen HC, Kosteljanetz M, Johansen C (2003) Incidences of gliomas and meningiomas in Denmark, 1943 to 1997. Neurosurgery 52:1327–1333. https://doi.org/10.1227/01.neu.0000064802.46759.53
Jukich PJ, McCarthy BJ, Surawicz TS, Freels S, Davis FG (2001) Trends in incidence of primary brain tumors in the United States, 1985-1994. Neuro Oncol 3:141–151. https://doi.org/10.1093/neuonc/3.3.141
Kim KH, Kang SJ, Choi JW, Kong DS, Seol HJ, Nam DH, Lee JI (2018) Clinical and radiological outcomes of proactive Gamma Knife surgery for asymptomatic meningiomas compared with the natural course without intervention. J Neurosurg:1–10. https://doi.org/10.3171/2017.12.Jns171943
Nakamura H, Makino K, Yano S, Kuratsu J (2011) Epidemiological study of primary intracranial tumors: a regional survey in Kumamoto prefecture in southern Japan--20-year study. Int J Clin Oncol 16:314–321. https://doi.org/10.1007/s10147-010-0178-y
Hoffman S, Propp JM, McCarthy BJ (2006) Temporal trends in incidence of primary brain tumors in the United States, 1985-1999. Neuro-oncology 8:27–37. https://doi.org/10.1215/s1522851705000323
Nabors LB, Portnow J, Ahluwalia M, Baehring J, Brem H, Brem S, Butowski N, Campian JL, Clark SW, Fabiano AJ, Forsyth P, Hattangadi-Gluth J, Holdhoff M, Horbinski C, Junck L, Kaley T, Kumthekar P, Loeffler JS, Mrugala MM et al (2020) Central nervous system cancers, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 18:1537–1570. https://doi.org/10.6004/jnccn.2020.0052
de Boer AW, Drewes YM, de Mutsert R, Numans ME, den Heijer M, Dekkers OM, de Roos A, Lamb HJ, Blom JW, Reis R (2018) Incidental findings in research: a focus group study about the perspective of the research participant. J Magn Reson Imaging 47:230–237. https://doi.org/10.1002/jmri.25739
Patil CG, Veeravagu A, Lad SP, Boakye M (2010) Craniotomy for resection of meningioma in the elderly: a multicentre, prospective analysis from the National Surgical Quality Improvement Program. J Neurol Neurosurg Psychiatry 81:502–505. https://doi.org/10.1136/jnnp.2009.185074
Sacko O, Sesay M, Roux FE, Riem T, Grenier B, Liguoro D, Loiseau H (2007) Intracranial meningioma surgery in the ninth decade of life. Neurosurgery 61:950–954. https://doi.org/10.1227/01.neu.0000303190.80049.7d
Yamamoto J, Takahashi M, Idei M, Nakano Y, Soejima Y, Akiba D, Kitagawa T, Ueta K, Miyaoka R, Nishizawa S (2017) Clinical features and surgical management of intracranial meningiomas in the elderly. Oncol Lett 14:909–917. https://doi.org/10.3892/ol.2017.6174
Koppie TM, Serio AM, Vickers AJ, Vora K, Dalbagni G, Donat SM, Herr HW, Bochner BH (2008) Age-adjusted Charlson comorbidity score is associated with treatment decisions and clinical outcomes for patients undergoing radical cystectomy for bladder cancer. Cancer 112:2384–2392. https://doi.org/10.1002/cncr.23462
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW (2021) The 2021 WHO Classification of tumors of the central nervous system: a summary. Neuro Oncol 23:1231–1251. https://doi.org/10.1093/neuonc/noab106
Terrapon APR, Zattra CM, Voglis S, Velz J, Vasella F, Akeret K, Held U, Schiavolin S, Bozinov O, Ferroli P, Broggi M, Sarnthein J, Regli L, Neidert MC (2021) Adverse events in neurosurgery: the novel therapy-disability-neurology grade. Neurosurgery 89:236–245. https://doi.org/10.1093/neuros/nyab121
Goldbrunner R, Minniti G, Preusser M, Jenkinson MD, Sallabanda K, Houdart E, von Deimling A, Stavrinou P, Lefranc F, Lund-Johansen M, Moyal EC, Brandsma D, Henriksson R, Soffietti R, Weller M (2016) EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol 17:e383–e391. https://doi.org/10.1016/S1470-2045(16)30321-7
Spille DC, Adeli A, Sporns PB, Heß K, Streckert EMS, Brokinkel C, Mawrin C, Paulus W, Stummer W, Brokinkel B (2021) Predicting the risk of postoperative recurrence and high-grade histology in patients with intracranial meningiomas using routine preoperative MRI. Neurosurg Rev 44:1109–1117. https://doi.org/10.1007/s10143-020-01301-7
Czernicki T (2020) Surgical management of intracranial meningiomas in the elderly: early and long-term outcomes. Clin Interv Aging 15:2439–2451. https://doi.org/10.2147/CIA.S283678
Monden D, Raimann FJ, Neef V, Dubinski D, Gessler F, Keil F, Forster MT, Ronellenfitsch MW, Harter PN, Freiman TM, Hattingen E, Seifert V, Senft C, Baumgarten P (2021) Meningioma surgery in patients >/=70 years of age: clinical outcome and validation of the SKALE score. J Clin Med 10. https://doi.org/10.3390/jcm10091820
Hajian-Tilaki K (2013) Receiver Operating Characteristic (ROC) Curve analysis for medical diagnostic test evaluation. Caspian J Intern Med 4:627–635
Achey RL, Gittleman H, Schroer J, Khanna V, Kruchko C, Barnholtz-Sloan JS (2019) Nonmalignant and malignant meningioma incidence and survival in the elderly, 2005-2015, using the Central Brain Tumor Registry of the United States. Neuro-oncology 21:380–391. https://doi.org/10.1093/neuonc/noy162
Bateman BT, Pile-Spellman J, Gutin PH, Berman MF (2005) Meningioma resection in the elderly: nationwide inpatient sample, 1998-2002. Neurosurgery 57:866–872. https://doi.org/10.1227/01.neu.0000179923.66729.87
Brokinkel B, Holling M, Spille DC, Heß K, Sauerland C, Bleimüller C, Paulus W, Wölfer J, Stummer W (2017) Surgery for meningioma in the elderly and long-term survival: comparison with an age- and sex-matched general population and with younger patients. J Neurosurg 126:1201–1211. https://doi.org/10.3171/2016.2.Jns152611
Cohen-Inbar O, Soustiel JF, Zaaroor M (2010) Meningiomas in the elderly, the surgical benefit and a new scoring system. Acta Neurochir 152:87–97. https://doi.org/10.1007/s00701-009-0552-6
van Alkemade H, de Leau M, Dieleman EM, Kardaun JW, van Os R, Vandertop WP, van Furth WR, Stalpers LJ (2012) Impaired survival and long-term neurological problems in benign meningioma. Neuro-oncology 14:658–666. https://doi.org/10.1093/neuonc/nos013
Caroli M, Locatelli M, Prada F, Beretta F, Martinelli-Boneschi F, Campanella R, Arienta C (2005) Surgery for intracranial meningiomas in the elderly: a clinical-radiological grading system as a predictor of outcome. J Neurosurg 102:290–294. https://doi.org/10.3171/jns.2005.102.2.0290
Chen ZY, Zheng CH, Tang L, Su XY, Lu GH, Zhang CY, Xiao SW, Tan YF (2015) Intracranial meningioma surgery in the elderly (over 65 years): prognostic factors and outcome. Acta Neurochir 157:1549–1557. https://doi.org/10.1007/s00701-015-2502-9
Lieu AS, Howng SL (1998) Surgical treatment of intracranial meningiomas in geriatric patients. Kaohsiung J Med Sci 14:498–503
Poon MT, Fung LH, Pu JK, Leung GK (2013) Outcome comparison between younger and older patients undergoing intracranial meningioma resections. J Neurooncol 114:219–227. https://doi.org/10.1007/s11060-013-1173-8
Poon MT, Fung LH, Pu JK, Leung GK (2014) Outcome of elderly patients undergoing intracranial meningioma resection--a systematic review and meta-analysis. Br J Neurosurg 28:303–309. https://doi.org/10.3109/02688697.2013.841857
Cahill DW, Bashirelahi N, Solomon LW, Dalton T, Salcman M, Ducker TB (1984) Estrogen and progesterone receptors in meningiomas. J Neurosurg 60:985–993. https://doi.org/10.3171/jns.1984.60.5.0985
Tilzer LL, Plapp FV, Evans JP, Stone D, Alward K (1982) Steroid receptor proteins in human meningiomas. Cancer 49:633–636. https://doi.org/10.1002/1097-0142(19820215)49:4<633::aid-cncr2820490404>3.0.co;2-4
Ehresman JS, Garzon-Muvdi T, Rogers D, Lim M, Gallia GL, Weingart J, Brem H, Bettegowda C, Chaichana KL (2019) Risk of developing postoperative deficits based on tumor location after surgical resection of an intracranial meningioma. J Neurol Surg B Skull Base 80:59–66. https://doi.org/10.1055/s-0038-1667066
Funding
Dr Huawei Huang supported this work, granted by the National Natural Science Foundation of China (81801042), Beijing Hospital Authority Youth Programme (20190504) and Beijing Municipal Administration of Hospitals Incubating Program (PX2023021). Dr Guobin Zhang also supported this work, granted by Beijing Municipal Administration of Hospitals Incubating Program (PX2023018).
Author information
Authors and Affiliations
Contributions
Conception and design: GZ and HL; data collection and assembly: HL, DZ, YY, ZJ, YW, SL, and DS; analysis and interpretation of data: HL, GZ, and HH; drafting the article: HL, GZ, and DZ; and critically revising the article: GZ, HH, and ZJ. All authors provided critical revisions to the manuscript for important intellectual content and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This study was approval by Ethics Committee of Beijing Tiantan Hospital and was conducted in accordance with the principles of the declaration of Helsinki.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 48.8 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, H., Zheng, D., Wang, Y. et al. Decision-making tree for surgical treatment in meningioma: a geriatric cohort study. Neurosurg Rev 46, 196 (2023). https://doi.org/10.1007/s10143-023-02103-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-023-02103-3