Abstract
To investigate variations regarding the formation and course of the sural nerve (SN). We dissected 60 formalin-fixed Brazilian fetuses (n = 120 lower limbs) aged from the 16th to 34th weeks of gestational age. Three incisions were made in the leg to expose the SN, and the gastrocnemius muscle was retracted to investigate the SN course. Statistical analyses regarding laterality and sex were performed using the Chi-square test. Eight SN formation patterns were classified after analysis. Type 4 (in which the SN is formed by the union of the MSCN with the LSCN) was the most common SN formation pattern. Although there was no statistical association between the formation patterns and the lower limb laterality (p = 0.9725), there was as to sex (p = 0.03973), indicating an association between anatomical variation and sex. The site of branch joining was in the distal leg most time (53.75%). In all lower limbs, the SN or its branches crossed from the medial aspect of the leg to the lateral margin of the calcaneal tendon (CT). Most often, the SN is formed by joining the MSCN and the LSCN in the distal leg. The SN or its branches ran close to the saphenous vein, crossed the CT from medial to lateral, and distributed around the lateral malleolus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The sensory innervation of the posterolateral part of the leg is complex because of the arrangement and distribution of several nerves, which may be joined or independent. This anatomical complexity requires a complete anatomical understanding of the origin, formation, and course of these nerve branches in the lower limb [1].
The sural nerve (SN) is one of the main sensory nerves of the lower extremity [2]. It supplies the sensory innervation of the posterolateral aspect of the distal leg as well as the lateral calcaneal region and dorsum of the foot [3, 4]. However, although it is traditionally described as a sensory nerve, some studies have emphasized the occasional presence of motor fibers in the SN for muscles that influence the adduction/abduction of the foot [5,6,7,8].
Three main nerve branches typically form the SN: the medial sural cutaneous nerve (MSCN) (which arises from the tibial nerve [TN]); the lateral sural cutaneous nerve (LSCN) (which arises from the common fibular nerve [CFN]); and the fibular communicating branch (FCB) (which may arise from either the LSCN or the CFN) [9]. The site of SN formation is between the popliteal fossa and the level of the ankle joint [10], but the nerves may course down the leg independently [3].
The SN passes between the heads of the gastrocnemius muscle, near the small saphenous vein (SVS) and the lateral border of the Achilles tendon (TC) [11, 12]. The SN runs posterior-inferior to the lateral malleolus (LM) and innervates the dorsolateral aspect of the foot [2]. However, the SN can have several variations along its course. The site of origin and pattern of formation of the SN can vary, with four or more patterns of anatomical classification [7, 13].
A thorough anatomical knowledge regarding SN anatomy may avoid inaccurate neuropathy diagnoses and iatrogenic injuries [3]. Sural nerve variations are relevant for nerve fiber protection during operative and clinical approaches, such as nerve grafting, nerve biopsies, nerve conduction, and local anesthetic techniques [14].
Surgical procedures for LM fractures and CT repair are associated with approximately 60% of SN injuries due to nerve variations [14]. Sural nerve variations can lead to inadequate stimulation of the nerve complex and misinterpretation of diagnoses in nerve conduction studies and biopsies for the diagnosis of focal or generalized lower limb neuropathies [3, 15]. The SN is the main donor for nerve transplantation in facial paralysis reconstruction, post-traumatic injuries, and post-obstetric brachial plexus paralysis [3, 16].
Sural nerve surgery requires a thorough preoperative anatomical understanding, as iatrogenic SN injury may cause sensory and motor deficits or inaccurate diagnosis [7]; therefore, the current study aims to investigate the anatomical variations in the formation and course of the SN in fetuses.
Material and methods
Sample characteristics
For the present study, an analysis of 120 adult lower limbs was performed to investigate the prevalence of anatomical variations of the SN. The determination of the sample size was based on statistical calculations, considering an acceptable margin of error of 5%, a confidence level of 95%, and estimated variability from previous studies in this field. Thus, the final sample of 120 lower limbs was considered sufficient to obtain significant and representative results for the variations studied.
We included 60 fetal cadaver specimens (30 male and 30 female) (n = 120 legs) ranging in gestational age from 16 to 34 weeks. Gestational age was calculated based on the measurement of right foot length [17]. Only fetuses without malformations of the SN anatomy or leg anatomy were included. The fetuses were obtained from cadaver donation programs for anatomical research at the Federal University of Sergipe.
Ethical considerations
We declare that every effort was made to follow all local and international ethical guidelines and laws concerning using human cadaveric donors in anatomical research, as recommended by Iwanaga et al. [18]. We have included the approval of the Human Research Ethics Committee of the Federal University of Sergipe at the end of the manuscript. Data collection began after ethical approval.
Dissection method
A number 03 scalpel handle and number 15 blades were used to expose the formation and course of the SN. The fetus was placed in the prone position, and three incisions were made: one vertical incision in the midline of the leg from the popliteal fossa to the lateral border of the CT, one in the upper half of the popliteal fossa, and one at the level of the intermalleolar line. We used Metzembaum straight scissors 13. The superficial and deep fascia were removed with curved Iris scissors (10.5 cm), straight anatomical forceps (13 cm), and rat tooth dissecting forceps (11.5 cm). The two heads of the gastrocnemius muscle were retracted [3].
Variables analyzed
We collect data on the pattern of SN formation, the sites of SN formation, the course of the SN concerning the SSV, CT, and LM, and the bilateral symmetry of SN formation.
Classification of anatomical variations
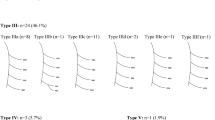
We have adapted Popieluszko’s classification of SN formation patterns [7]: Type 1, the SN is formed by the joining of the MSCN (arising from the TN) and the FCB (arising from the LSCN); Type 2, the SN is formed by the joining of the MSCN with the FCB (arising from the NCSL), which branches into 2 or 3 more branches in the distal leg; Type 3, there is the independent coexistence of the MSCN and the FCB, without forming the SN; Type 4, the SN is formed by the joining of the MSCN with the LSCN (arising from the FCN); Type 5, there is the independent coexistence of the MSCN and the LSCN, without forming the SN; Type 6, the SN is a continuation of the MSCN; Type 7, the SN is a continuation of the MSCN, with the presence of the LSCN, although it does not contribute to the formation of the SN; Type 8, the SN is a continuation of the LSCN and receives one or two communicating branches of the MSCN (Fig. 1).
The eight SN formation patterns. SCN, sciatic nerve; TN, tibial nerve; CFN, common fibular nerve; FCB, fibular communicating branch; LSCN, lateral cutaneous sural nerve; LSCNB, lateral cutaneous sural nerve branches; MSCN, medial cutaneous sural nerve; MSCNB, medial cutaneous sural nerve branch; SN, sural nerve
Statistical analysis
Data analysis was performed by the χ2 test (chi-square) using R statistical software (version 4.0.5). The significance level was set at 5% (p < 0.05).
Results
Type 4 anatomy was the most common (45%), followed by type 1 (17.5%). The third most common variant was Type 6, in which the SN is a continuation of the MSCN (14.17%). Type 7 was seen in 8.33% of cases, Type 5 in 6.67%, Type 3 in 5%, and Type 2 in 2.5%. The least common pattern was Type 8, with only one right lower extremity (0.83%) (Fig. 1).
Type 2 has not been reported in any identified study. In the present study, this anatomical variation was present in 3 of the 120 limbs analyzed (2.5%) (Fig. 2).
SN formation patterns did not show a statistically significant association with lower limb laterality (p = 0.9725) but did show a significant association with gender (p = 0.03973).
Type 1 was more common in males (13 cases [21.67%]) than in females (8 cases). Type 5 occurred in 6 legs in males out of a total of 6.67% (8 cases). Type 2 (2.5%) and type 8 (0.83%) occurred only in males. On the other hand, Types 6 and 7 had a higher incidence in females (21.67% and 11.67%, respectively). Types 3 and 4 showed an equal distribution between the sexes. The results are presented in Table 1.
The bilateral symmetry of the SN formation pattern was analyzed. The symmetrical group with the same type of nerve formation between the legs was present in 70% of the 60 fetal cadavers presented. An asymmetrical distribution was found in 30% of the fetuses.
The communication between the branches forming the SN is highly variable, and anastomosis sites may occur between the popliteal fossa and the ankle joint. Of the 80 legs in which nerve branches joined to form the SN (Types 1, 2, and 4), the most common site of union was the distal third of the leg (53.75%), followed by the middle third (40%). Nerve fusion occurred at the level of the ankle joint in 3.75% of cases. The least common locations were the popliteal fossa and the upper third of the leg, with one case each (1.25%). There was a statistically significant association (p < 0.001) between the location of the bifurcation (Fig. 3).
The course of the SN and its component nerves were observed. Both the SN and its component nerves that descended independently without forming the SN, mainly the MSCN, crossed from the lateral side of the leg to the lateral margin of the CT, traversing posteroinferior to the LM in 100% of cases.
The SSV ran close to the SN. Of 40 legs (in which the SSV was visible or not ruptured), 50% had the SSV running medial to the SN, whereas 30% had the SSV running lateral to the SN. In 20%, the SSV crossed from medial to lateral, passing anterior to the SN in the distal third of the leg. The course of the vein relative to the nerve showed no statistically significant association (p = 0.06081).
Shield et al. [19], while studying the gestational development of the sural nerve, reported that the only difference from the 15th week of gestation was the onset of myelination of the nerve starting at 21 weeks, with only morphometric variations found. Thus, given the morphological stability of the sural nerve since the consolidation of the peripheral nervous system, we did not perform subgroup analyses by gestational age.
Discussion
The SN has a large individual anatomical variability [20]. The pattern of SN formation and the location of SN may vary. Therefore, the possibility of anatomical variation should be considered in both clinical and surgical settings [6].
We found Type 4 in most of the lower limbs examined. This is supported by previous studies in fetuses, such as those by Büyükmumcu et al. and Ulcay et al. [9, 16], which show a high prevalence of this anatomical pattern. Studies in adults have confirmed this information and established Type 4 as the classic pattern [2, 4, 10, 15, 21,22,23]. Therefore, we hypothesize that Type 4 is present in the fetal population and remains stable until adulthood. In addition, the fetal and adult studies are studies of Asian ethnicity; thus, there is an Asian influence on the cadaveric sample studied. Only one study [7] – with a European adult population and anatomical assessment by ultrasound – showed a lower frequency of Type 4.
In the current study, Type 1 was the second most frequent pattern, as in all previous studies analyzed with fetal populations [5, 24]. However, Type 1 had a lower prevalence in the adult population, according to Olave et al. [1] and Popieluszko et al. [7], but had a high prevalence in the studies by Mestdagh et al. [20], Nieto et al. [12], and Sekiya et al. [25]. Thus, LSCN may likely present branching like FCB, with a higher prevalence in fetal samples.
Type 2 anatomy (2.5%) was not found in any previous anatomical study. This may indicate that this variation is new and may be being reported for the first time in a Brazilian population.
Type 6 was reported in all previous articles in both fetal and adult populations. This pattern was found in seven studies with Asian samples [3, 4, 8, 10, 22, 26, 27] and five studies with Eurasian ethnicity, all from Turkey [2, 5, 9, 16, 28]. However, only three European studies showed Type 6 [7, 20, 24]. Type 6 may be present in the Asian, European, and South American (Brazilian) populations.
Bilateral symmetry of the SN formation pattern was highly prevalent in previous studies [5, 9, 15, 26, 27]. This is consistent with our results, which showed high symmetry between the patterns of nerve formation. However, Mahakkanukrauh et al. found a high bilateral asymmetry in their study [10]. This difference, compared to the higher prevalence of symmetry in the other articles, may be related to the Asian geographic subgroup or the sample size studied in this single article with high asymmetry. Thus, the existence of bilateral asymmetrical distribution requires researchers to evaluate both lower limbs in future anatomical studies [7].
Several studies have described variations in SN anatomy, focusing mainly on the SN location for surgical procedures [1, 2, 11, 14, 29, 30]. However, in the clinical area, this nerve is widely used for diagnostic, such as in nerve conduction studies (NCS) and biopsies [10].
During electrodiagnostic testing, the antidromic technique of NSC applied to the SN is useful to diagnose generalized or focal neuropathies [23], such as compressive, post-traumatic, diffuse polyneuropathic, lumbosacral plexus, and sciatic or tibial nerve injuries [31]. Sural nerve variations may influence the stimulation of the SN complex and affect the amplitude and latency parameters of the sensory nerve action potential, leading to erroneous and misinterpreted diagnostic findings [23]. Sural nerve biopsy is a valuable method to investigate the cause of peripheral neuropathies [3].
The site of SN formation was also investigated. According to Uluutku et al. [32], the most common site of SN formation was the middle third of the leg. Büyükmumcu et al. [16] reported similar results. Mestdagh et al. [20] observed a higher incidence of nerve branch communication in the distal half of the leg. Nuri et al. [22] found that the majority of medial and lateral branch connections were found in the distal leg, while Eid et al. [6] reported a higher frequency of connection between MSCN and FCB in the distal leg. Similarly, our study showed a higher frequency of branch connections in the distal leg.
Surgeons recognize the SN and its components as ideal for grafting [8]. The use of the SN graft in the treatment of peripheral nerve injuries is extensive for facial paralysis, post-obstetric brachial plexus palsy, and post-traumatic injuries [16, 32]. Depending on anatomical variations, surgical procedures or incisions for reconstruction or repair of CT, subtalar arthrodesis, and distal fibula fractures [2] may injure the SN or its branches. Therefore, researchers report the need to use anatomical knowledge and preoperative ultrasound for the surgical success of procedures [4, 7, 11, 14].
The present study was limited in the analysis of the course of the SSV in relation to the SN because of the sample size (40 lower limbs). This small sample was due to the difficulty in visualizing the SSV in fetuses of smaller gestational age and the ease of venous injury during dissection.
Although our study did not evaluate different geographic groups or ethnicities, we hypothesize that these factors influence the distribution of the anatomical patterns mentioned here.
We believe our anatomical classification is the most complete currently available.
Conclusion
The results suggest that the SN is most commonly formed by the joining of the MSCN with the LSCN. The joining site most often occurs in the distal leg. In addition, the SN (or its branches) travels near the SSV, crosses from the medial to the lateral CT, and runs posteroinferior to the LM until it reaches the dorsolateral border of the foot. This study contributes to the field by providing a new classification for the anatomical patterns of SN.
Data availability
Not applicable.
References
Olave E, Cruzat C, Retamal P, Galaz C (2010) Formación del Nervio Sural en Individuos Chilenos. Int J Morphol 28:273–276
Aktan Ikiz ZA, Uçerler H, Bilge O (2005) The anatomic features of the sural nerve with an emphasis on its clinical importance. Foot Ankle Int 26:560–567. https://doi.org/10.1177/107110070502600712
Kavyashree AN, Lakshmi Prabha S, Asha KR, Bindu Rani MK (2013) Anatomical variations in formation of sural nerve in adult Indian cadavers. J Clin Diagnostic Res 7:1838–1841. https://doi.org/10.7860/JCDR/2013/6633.3328
Zhu J, Li D, Shao J, Hu B (2011) An ultrasound study of anatomic variants of the sural nerve. Muscle Nerve 43:560–562. https://doi.org/10.1002/mus.21918
Desdicioglu K, Malas MA, Bahceci S, Simsek F, Polat AG (2017) Anatomical and histological morphometry of the sural nerve in human fetuses. J Anat Soc India 66:37–42. https://doi.org/10.1016/j.jasi.2017.05.005
Eid EM, Hegazy AMS (2011) Anatomical variations of the human sural nerve and its role in clinical and surgical procedures. Clin Anat 24:237–245. https://doi.org/10.1002/ca.21068
Popieluszko P, Mizia E, Henry BM, PĘkala PA, Sanna B, Roy J, Loukas M, Tomaszewski KA (2018) The surgical anatomy of the sural nerve: An ultrasound study. Clin Anat 31:450–455. https://doi.org/10.1002/ca.22997
Seema SR (2013) Study of sural nerve complex in human cadavers. ISRN Anat 2013:827276. https://doi.org/10.5402/2013/827276
Ulcay T, Uzun A (2018) Anatomical variations of the formation of human sural nerve in stillborns. J Anat Soc India 67:50–54. https://doi.org/10.1016/j.jasi.2018.04.001
Mahakkanukrauh P, Chomsung R (2002) Anatomical variations of the sural nerve. Clin Anat 15:263–266. https://doi.org/10.1002/ca.10016
Kammar H, Carmont MR, Kots E, Laver L, Mann G, Nyska M, Mei-Dan O (2014) Anatomy of the sural nerve and its relation to the achilles tendon by ultrasound examination. Orthopedics 37:e298-301. https://doi.org/10.3928/01477447-20140225-64
Nieto JL, Vergara Amador E, Amador JA (2009) Sural nerve: anatomical study and clinical aspects. Colomb Med 40:252–258. https://doi.org/10.25100/cm.v40i3.653
Park J-H, Park K-R, Kim D, Kwon H-W, Lee M, Choi Y-J, Kim Y-B, Park S, Yang J, Cho J (2019) The incision strategy for minimizing sural nerve injury in medial displacement calcaneal osteotomy: a cadaveric study. J Orthop Surg Res 14:356. https://doi.org/10.1186/s13018-019-1411-7
Apaydin N, Bozkurt M, Loukas M, Vefali H, Tubbs RS, Esmer AF (2009) Relationships of the sural nerve with the calcaneal tendon: an anatomical study with surgical and clinical implications. Surg Radiol Anat 31:775–780. https://doi.org/10.1007/s00276-009-0520-0
Choi H, Chung SY, Kang S, Son S-H, Yoon JS (2019) Could Ultrasound-Guided Stimulation of Sural Nerve Affect Nerve Conduction Study? Ann Rehabil Med 43:74–80. https://doi.org/10.5535/arm.2019.43.1.74
Büyükmumcu M, Aydin Kabakçi AD, Akin Saygin D, Yilmaz MT, Şeker M (2021) Sural nerve harvest for infants: Integrated with information based on anatomical dissections. Turkish J Med Sci 51:473–482. https://doi.org/10.3906/sag-2005-225
Kumar GP, Kumar UK (1994) Estimation of Gestational Age from Hand and Foot Length. Med Sci Law 34:48–50. https://doi.org/10.1177/002580249403400106
Iwanaga J, Singh V, Takeda S, Ogeng’o J, Kim H-J, Moryś J, Ravi KS, Ribatti D, Trainor PA, Sañudo JR, Apaydin N, Sharma A, Smith HF, Walocha JA, Hegazy AMS, Duparc F, Paulsen F, Del Sol M, Adds P, Louryan S, Fazan VPS, Boddeti RK, Tubbs RS (2022) Standardized statement for the ethical use of human cadaveric tissues in anatomy research papers: Recommendations from Anatomical Journal Editors-in-Chief. Clin Anat 35:526–528. https://doi.org/10.1002/ca.23849
Shield LK, King RH, Thomas PK (1986) A morphometric study of human fetal sural nerve. Acta Neuropathol 70:60–70. https://doi.org/10.1007/BF00689515
Mestdagh H, Drizenko A, Maynou C, Demondion X, Monier R (2001) Origin and make up of the human sural nerve. Surg Radiol Anat 23:307–312. https://doi.org/10.1007/s00276-001-0307-4
Dangintawat P, Huanmanop T, Agthong S, Chentanez V (2016) Anatomy of the Sural Nerve Related to Calcaneal Tendon, Intermalleolar Line and Small Saphenous Vein. Int J Morphol 34:380–384. https://doi.org/10.4067/s0717-95022016000100055
Nuri T, Ueda K, Maeda S, Otsuki Y (2012) Anatomical study of medial and lateral sural cutaneous nerve: implications for innervated distally-based superficial sural artery flap. J Plast Surg Hand Surg 46:8–12. https://doi.org/10.3109/2000656X.2011.644720
Pyun SB, Kwon HK (2008) The effect of anatomical variation of the Sural nerve on nerve conduction studies. Am J Phys Med Rehabil 87:438–442. https://doi.org/10.1097/PHM.0b013e318174e569
Ugrenovic S, Vasovic L, Jovanovic I, Stefanovic N (2005) Peculiarities of the sural nerve complex morphologic types in human fetuses. Surg Radiol Anat 27:25–29. https://doi.org/10.1007/s00276-004-0276-5
Sekiya S, Suzuki R, Miyawaki M, Chiba S, Kumaki K (2006) Formation and distribution of the sural nerve based on nerve fascicle and nerve fiber analyses. Anat Sci Int 81:84–91. https://doi.org/10.1111/j.1447-073x.2006.00135.x
Jeon SK, Paik D-J, Hwang Y-I (2017) Variations in sural nerve formation pattern and distribution on the dorsum of the foot. Clin Anat 30:525–532. https://doi.org/10.1002/ca.22873
Shankar N, Selvam RP, Dhanpal N, Reddy R, Alapati A (2010) Anatomical variations of the sural nerve in the leg: a fetal study. Neurol India 58:24–28. https://doi.org/10.4103/0028-3886.60390
Albay S, Sakalli B, Kastamoni Y, Candan IA, Kocabiyik N (2012) Formation of the sural nerve in foetal cadavers. Folia Morphol (Warsz) 71:221–227
Park H-D, Kwak H-H, Hu K-S, Han S-H, Fontaine C, Kim H-J (2007) Topographic and histologic characteristics of the sural nerve for use in nerve grafting. J Craniofac Surg 18:1434–1438. https://doi.org/10.1097/scs.0b013e3181534a4d
Porter KJ, Robati S, Karia P, Portet M, Szarko M, Amin A (2014) An anatomical and cadaveric study examining the risk of sural nerve injury in percutaneous Achilles tendon repair using the Achillon device. Foot Ankle Surg 20:90–93. https://doi.org/10.1016/j.fas.2013.11.005
Tankisi H, Pugdahl K, Otto M, Fuglsang-Frederiksen A (2014) Misinterpretation of sural nerve conduction studies due to anatomical variation. Clin Neurophysiol 125:2115–2121. https://doi.org/10.1016/j.clinph.2014.01.030
Uluutku H, Can MA, Kurtoglu Z (2000) Formation and location of the sural nerve in the newborn. Surg Radiol Anat 22:97–100. https://doi.org/10.1007/s00276-000-0097-0
Acknowledgements
We thank Alisson Correia (ORCID ID: 0000-0002-7599-5124) for his dedication and commitment to this project, which enabled us to go international with our paper. Thank you, Alisson, for your contributions throughout this process.
Author information
Authors and Affiliations
Contributions
All authors significantly contributed to performing this study.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Human Research Ethics Committee, Federal University of Sergipe (no. 79260417.0.0000.5546).
Consent for publication
Not applicable.
Competing interests
No conflict of interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Garção, D.C., de Souza Paiva, M.S. & Corcinio, K.S. Variations in sural nerve formation and course in fetuses. Neurosurg Rev 46, 189 (2023). https://doi.org/10.1007/s10143-023-02098-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-023-02098-x