Abstract
A series of 5 patients treated with the fourth ventricle to spinal subarachnoid space stent (FVSSS) is presented. Indication for surgery, surgical technique, pre-operative and post-operative images, and outcome are analyzed. A systematic review of the pertinent literature has also been performed. This is a retrospective cohort review of a series of 5 consecutive patients with refractory syringomyelia who underwent a fourth ventricle to spinal subarachnoid space shunt surgery. The surgical indication was based on the presence of refractory syringomyelia in patients already treated for Chiari malformation or in patients who developed scarring at the level of the outlets of the fourth ventricle following posterior fossa tumor surgery. The mean age at FVSSS was 11.30 ± 5.88 years. Cerebral MRI revealed crowded posterior fossa, with a membrane at the level of the foramen of Magendie. Spinal MRI showed syringomyelia in all patients. Before surgery, the averages of the craniocaudal and the anteroposterior diameter were 22.66 and 1.01 cm, respectively, whereas the volume was 28.16 cm3. The post-operative period was uneventful in 4 out of 5 patients; one child died on the 1st post-operative day due to complications unrelated to surgery. In remaining cases, syrinx marked improvement. The post-operative volume was 1.47 cm3 with an overall reduction of 97.61%. With regard to literature, 7 articles with a total of 43 patients were analyzed. After FVSSS, syringomyelia reduction was observed in 86.04% of cases. Three patients underwent reoperation due to syrinx recurrence. Four patients presented a catheter displacement, one a wound infection and meningitis and one CSF leak requiring placement of a lumbar drain. FVSSS is highly effective in restoring CSF dynamics, with dramatic improvement of syringomyelia. In all our cases, the volume of the syrinx was reduced by at least 90%, with improvement/resolution of accompanying symptomatology. This procedure should be reserved to patients in which other causes of gradient pressure between the fourth ventricle and subarachnoid space are excluded, for example, tetraventricular hydrocephalus. Surgical procedure is not simple, because it requires meticulous microdissection of cerebello-medullary fissure and upper cervical spine, in already operated patients. To avoid migration of the stent, it should be carefully sutured to the dura mater or thick arachnoid membrane.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Syrinx is the Greek name for a cavity of tubular shape. Ollivier D’Angers firstly introduced this term in 1827 for cystic cavitations of the spinal canal [1]. Syringomyelia is a disorder characterized by the presence of cystic cavities in the spinal cord containing extracellular or cerebrospinal fluid (CSF). It occurs most commonly in association with Chiari malformation but also can be the result of spinal cord injury, intramedullary spinal tumor, spinal dysraphism and spinal arachnoiditis. The pathophysiology of syrinx formation is uncertain, and the appropriate management is unclear. The present study aimed to present a case series of 5 patients treated with the fourth ventricle to spinal subarachnoid space stent (FVSSS) because refractory syringomyelia, with a closer analysis of indication for surgery, surgical technique, pre-operative and post-operative images, and outcome. A systematic review of the pertinent literature has also been performed.
Methods
This was a retrospective cohort review of a series of 5 consecutive patients with refractory syringomyelia who underwent a fourth ventricle to spinal subarachnoid space shunt surgery during the last decade. The surgical indication was based on the presence of refractory syringomyelia in patients already treated for Chiari malformation type 1 (2 patients) or type 2 (1 patient), or in patients who developed scarring at the level of the outlets of the fourth ventricle following posterior fossa surgery. In all cases, radiological investigation showed a clear obstruction between the fourth ventricle and subarachnoid space of the cisterna magna. All patients had been previously treated for hydrocephalus and had a ventriculo-peritoneal shunt (4 patients) or spino-peritoneal shunt (1 patient) in situ. All patients were evaluated with craniospinal magnetic resonance imaging (MR) before shunt surgery, as well the day after, in association with computed tomography (CT) and at regular follow-up. Syringomyelia was diagnosed by the presence of intramedullary hypointensity in T1-weighted midsagittal MRI and hyperintensity in T2-weighted MRI, extending over more than 1 spinal segment with distension of spinal cord. The maximum craniocaudal and anteroposterior syringomyelia diameter were calculated before and after the shunt procedure. The spinal syrinx volume was calculated in cubic centimeters for each patient by using a freely available segmentation software (HOROS Project, GNU Lesser General Public License version 3.0).
Written informed consent was obtained from all the parents of the patients for publication of this case series report and any accompanying images. All identifying information was stripped off. The manuscript was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Online databases MEDLINE (PubMed) and SCOPUS were searched for English-language articles published between January 1976 and December 2022 containing the following keywords alone or in combination: ‘”fourth ventricle,” “subarachnoid space,” “shunt,” “Chiari malformation,” and “syringomyelia.” As recommended in the PRISMA statement, we reviewed all abstracts, and each article of interest was marked for further review. The full text of the marked studies was retrieved, and studies that satisfied our inclusion criteria were included in this review. The references listed in each article of interest were also reviewed for pertinent articles.
Results
The clinical details of the 5 patients are listed in Table 1. Three patients had Chiari malformation as initial diagnosis (Figs. 1–2). Two patients developed syringomyelia following posterior fossa tumor surgery (foramen magnum meningioma and acoustic neurinomas in one patient, affected by neurofibromatosis type 2 (Fig. 3) and medulloblastoma in the other (Fig. 4)).
A: MRI of a patient with GH deficiency and Chiari malformation and syringomyelia at first presentation; the patient underwent suboccipital craniectomy, laminectomy of C1, and removal of the outer layer of the dura. B: Following stability of syrinx for 3 years, the cavity enlarged again, and the patient underwent a second operation (broader opening of the bone and dura expansion using autologous pericranial graft). C: The syrinx continued to enlarge, the patient developed hydrocephalus and was shunted. Finally, he underwent stenting from the fourth ventricle to the spinal subarachnoid space, with resolution of the syrinx (D) (Patient 2, in Table 1)
A, B: Recurrence of syringomyelia following foramen magnum decompression and duraplasty with non-absorbable dural patch, in a patient operated at birth for lypomyelomeningocele. C, D: Marked improvement of the syrinx following FVSSS (C, D) (Patient 1, in Table 1)
A: Appearing of holo-cord syringomyelia in 18-year-old NF2 patient operated for foramen magnum meningioma at the age of 7, removal of left acoustic neurinoma at the ages of 13 and 16, and removal of right acoustic neurinoma at the age of 17. B: Almost complete resolution of the syrinx 3 months following FVSSS. C, D: Sagittal CT scan showing position of the stent in the IV ventricle (arrow in C) and spinal subarachnoid space (arrow in D) (Patient 3, in Table 1)
A: Appearing of rosary bead-like multiloculated syringomyelia 1 year following removal of medulloblastoma. The patient underwent FVSSS. B: Reduction of the syrinx immediately post-op. C: Complete resolution at 3-month follow-up. (Patient 4, in Table 1)
The mean age at FVSSS was 11.30 ± 5.88 years (2.2–17.2). The average of months between previous posterior fossa surgery and FVSSS was 18 ± 10.56 (7–35). Standard surgical procedure for initial posterior fossa decompression performed at our institution included occipital craniectomy with C1 posterior arch ablation, extending beyond the lateral aspect of the spinal cord, removal of the outer layer of the dura, or in alternative, opening of the dura, duraplasty with either autologous pericranium or Neuro-Patch® (B-Braun, Saint-Cloud, France). Three patients had single posterior fossa operation before stenting (2 Chiari patients, and 1 medulloblastoma patient). The remaining patients underwent multiple surgeries (Fig. 1). All patients had already been shunted for hydrocephalus at time of stenting: four with ventriculo-peritoneal shunts and one with third ventriculo-cisternostomy plus lumboperitoneal shunt.
At time of stent placement one patient presented progressive worsening of pre-existing paraparesis (lipomyelomeningocele patient), one patient worsening of pre-existing bilateral facial nerve palsies, two patients asthenia and mild motor difficulties, and one was completely asymptomatic.
Cerebral MRI revealed crowded posterior fossa, with a recognizable membrane at the level of the foramen of Magendie. In all cases, the fourth ventricle was moderately enlarged, despite a working shunt. Spinal MRI showed syringomyelia between cervico-dorsal segment in 4 patients (C1-D7; C2-D10; C1-D9; C2-D4) and cervico-dorsal-lombar in another one (C1-L1), with a mean of 15 ± 3.74 levels.
Before surgery, the averages of the craniocaudal and the anteroposterior diameter were 22.66 cm ± 11.02 cm (11.03–37) and 1.01 cm ± 0.26 cm (0.8–1.4), respectively, whereas the volume was 28.16 cm3 ± 27.40 cm3 (9.45–75.94) (Table 2).
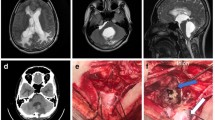
Surgical technique (Fig. 5; Video 1)
Under general anesthesia, in a prone position, with the head positioned in a horseshoe headrest, the previous midline skin incision, extending from the inion to C7, was reopened. The muscular masses were dissected along the avascular white line. Suboccipital craniotomy was enlarged to expose at least 4 cm × 4 cm of the posterior fossa dura. The cervical dura was exposed removing the posterior arch of C1 (if not already performed at previous surgery). A linear-shaped durotomy (or opening of a dural patch, positioned at previous surgery) was done to expose the cerebellar tonsils and the cisterns surrounding the upper spinal cord. Dissection was extended caudally, until normal arachnoid was found. In two cases, it was necessary to remove also the posterior arch of C2 to expose normal arachnoid. In all patients, arachnoid adhesions were observed between the dura mater and the cerebellar tonsils/cranio bulbar junction. To avoid damage of posteroinferior cerebellar artery (PICA) or the floor of the 4th ventricle, under microscopic magnification, arachnoidolysis with sharp dissection was done to identify the foramen of Magendie (Fig. 5A). At least from one side, dissection continued caudally in the cerebello medullary fissure to create space for placing the distal end of the catheter in a subarachnoid space where CSF can freely flow (Fig. 5B): clear visualization of posterior inferior cerebellar artery, lower cranial nerves, and superior cervical roots are necessary. The obstructed foramen of Magendie was reopened and IV ventricle was assessed. A 7-cm stent, composed by a fragment of standard ventricular catheter as proximal end, connected to a fragment of spinal catheter (as distal end), was harvested (Fig. 5D). The proximal end was positioned inside the fourth ventricle, through the re-opened Magendie foramen. The distal end was placed down to the upper cervical subarachnoid space, laterally to the spinal cord. The stent was fixed with non-absorbable sutures to the dura mater or thickened arachnoid membrane (Fig. 5C). Dura mater or pre-existing dural patches were re-closed. Layered-fashioned standard closure was achieved. In one case, this procedure was associated with revision of the occipito-cervical fixation system, with repositioning of screws and bar from one side (Video 1).
Intraoperative images. A: Placement of the proximal end of the stent into the fourth ventricle through the foramen of Magendie. B: Placement of the distal end in the lateral cervical subarachnoid space. C: Final position of the stent, fixed to a thicker arachnoid membrane. D: The stent is composed by a standard 7Fr ventricular catheter as proximal end, and a 4 Fr lumbar catheter as distal end
Outcome
The post-operative period was uneventful in 4 out of 5 patients. The patient, in which the occipito-cervical fixation system was revised, died on the 1st post-operative day due to complications unrelated to surgery (accidental tracheostomy dislodgement).
In remaining cases, syrinx marked improvement, with almost complete resolution in two. At post-operative MRI, syringomyelia levels decreased by 71.59% with an average of 4.75 ± 4.86 levels. The mean of the craniocaudal and the anteroposterior diameter were 8.53 cm ± 10.78 cm (0–24) and 0.2 cm ± 0.2 cm (0–0.47), with an average decrease of 72.46% for the former and 81.58% for the latter, whereas the volume was 1.47 cm3 ± 2.72 cm3 (0–5.55) with an overall reduction of 97.61%.
The average follow-up was 31.25 ± 20.50 months ranging between 13 and 59. No complications such as ventricular infection or catheter migration were reported over the whole time-frame (Table 2). Symptoms improved in two patients, and disappeared in one.
After screening 24 articles, 7 were analyzed, having satisfied our inclusion criteria [2,3,4,5,6,7,8]. The main features of 43 patients are summarized in Table 3.
Thirty-six patients had Chiari malformation as initial diagnosis. Four patients developed syringomyelia following a head trauma; one had a neonatal ventricular hemorrhage; one had a posterior fossa surgery for an aneurysm; and one developed a lumbar spinal arachnoiditis, following an epidural anesthesia for childbirth.
After FVSSS, syringomyelia reduction was observed in 37 out of 43 patients (86.04%), whereas it was unchanged in the others. Three patients underwent reoperation due to syrinx recurrence. Four patients presented a catheter displacement, one a wound infection and meningitis, and one CSF leak requiring placement of a lumbar drain.
Discussion
Fourth ventricle to cervical spinal subarachnoid space stenting is a procedure rarely reported in literature with only 43 cases (Table 3), that include only two large series. These have been recently published by Riordan and Scott (consisting of 14 pediatric patients) [3] and Lou et al. [8] (consisting of 15 adults). FVSSS is indicated in the case of recurrent, persisting, or expanding syringomyelia, mainly in patients with Chiari malformation, who had already undergone cranio-cervical decompression. Less common indication is syringomyelia, secondary to arachnoiditis at the craniocervical junction (post-traumatic, post-infective or post-hemorrhagic) [2, 9]. We used FVSSS also in two cases of patients previously operated for posterior fossa tumors. Common link between these conditions is the presence of an obstruction to CSF flow from the fourth ventricle to the spinal subarachnoid space. Diagnosis is often presumptive, based on pre-operative MRI that can show attenuation of the cisterna magna and foramen magnum subarachnoid space, cerebellar ectopia, and entrapment of the fourth ventricle. Dynamic sequences may demonstrate tethering of posterior fossa structures to the overlying dura, as well as limited movement of the cerebellar tonsils and no evidence of CSF flow in the dorsal subarachnoid space at the level of the cerebellar tonsils [2]. However, in most cases, the diagnosis is only intraoperatively confirmed with the finding of thick arachnoid bands. Riordan and Scott [3] reported that their decision to place a stent at re-do surgery was based on the reported findings at the primary procedure, knowledge of any complications occurred after the first operation, (infections or bleeding) and the intraoperative finding of fourth ventricle outflow obstruction, which was believed to likely recur if simply lysed. Our experience suggests that FVSSS is highly effective in restoring CSF dynamics, with dramatic improvement of syringomyelia on post-operative imaging. In all our cases the volume of the syrinx was reduced by at least 90%, with improvement/resolution of accompanying symptomatology. Data from the literature also confirms this data: Riordan and Scott [3] reported complete resolution of the syrinx in 93% of patients. Lou et al. [8] reported significant reduction of syrinx on 13 out of 15 patients. No complications occurred in our series. In literature, the rate of complication is low: 3 catheter displacements (7%), 1 infection, and 1 CSF leak. Syringomyelia recurred in 3 patients, in addition to those with stent displacement. Further surgery for recurrence/complication was performed in 7 out of 45 cases (including our patients). Nevertheless, this should be considered a complex surgery and only performed by an expert surgeon in order to avoid damaging PICA or the floor of the 4th.
Other surgical strategies have been proposed to treat refractory syringomyelia. Actually, literature focusing on revision surgery is scarce. In a systematic review, including a total of 616 patients undergoing foramen magnum decompression for Chiari malformation, Schuster et al. found a 6.7% rate of persistent or recurring syringomyelia [10]. Most patients have been treated with revision surgery at the level of the foramen magnum, with a broader opening of the bone and/or dura expansion (using a graft), with/without tonsillar shrinking or resection, or directly shunting the syrinx, by the means of syringo-peritoneal, syringo-pleural, or syringo-subarachnoid shunting [11, 12]. Extra-axial shunting of the syrinx (syringo-pleural or syringo-peritoneal shunt) appears to be burdened by a high rate of dysfunction [8, 11]. Syringo-subarachnoid shunting has been proposed by Soleman et al. as a safe and effective strategy [11]. They suggest this operation after having ruled out all obvious reasons for a persistent syrinx (e.g., instability of the spine, tethered cord, prominent retroflexion of the dens, basilar invagination, and hydrocephalus) that should be treated accordingly. We agree with this point of view, but encourage to stress the pathogenetic role of fourth ventricle outflow obstruction in pathogenesis of syringomyelia and to carefully evaluate pre-operative MR images in order to identify this condition, using very thin slides T2 or dynamic sequences [4]. Only in the case of anatomical contraindication to FVSSS, the patient should undergo syringo-subarachnoid shunt. This procedure appears to be less physiologic and (at least theoretically) more invasive as it requires violation of the neural tissue. In fact, the proximal end of the catheter is placed inside the syrinx through a dorsal myelotomy.
Future trend is to correctly understand pathogenesis of syringomyelia, so as to adequately treat each case at first surgery and avoid recurrences. Patients with atlanto-axial instability, for example, should be recognized and treated with C1-C2 fixation, avoiding intradural dissection that may contribute to development of scarring at the level of the fourth ventricle outlets, worsening CSF dynamics [13].
Conclusions
FVSSS is an effective strategy to treat refractory syringomyelia, not only in patients with Chiari malformation, but also in other conditions that affect the free flow of CSF from the fourth ventricle to spinal subarachnoid space. This procedure should be reserved to patients in which other causes of gradient pressure between the fourth ventricle and subarachnoid space are excluded, for example, tetraventricular hydrocephalus. Surgical procedure is not simple, because it requires meticulous microdissection of cerebello-medullary fissure and upper cervical spine, in already operated patients. To avoid migration of the stent, it should be carefully sutured to the dura mater or thick arachnoid membrane.
Data availability
Not applicable.
References
Ollivier d'Angers PC (1827) Traite de la moelle epiniere et de ses maladies; contenant l'histoire anatomique, physiologique et pathologique de ce centre nerveux chez l'homme, vol 2. Crevot, Paris, p 116
Davidoff CL et al (2017) Treatment of syringomyelia in patients with arachnoiditis at the craniocervical junction. World Neurosurg 107:565–573
Riordan CP, Scott RM (2018) Fourth ventricle stent placement for treatment of recurrent syringomyelia in patients with type I Chiari malformations. J Neurosurg Pediatr 23(2):164–170
Knafo S et al. (2022) Surgical management after Chiari decompression failure: craniovertebral junction revision versus shunting strategies. J Clin Med 11(12)
Orakdogen M et al (2015) Fourth ventriculostomy in occlusion of the foramen of Magendie associated with Chiari malformation and syringomyelia. NMC Case Rep J 2(2):72–75
Champeaux-Depond C, Froelich S, Parker F, Birladeanu A (2022) Magendie’s foramen debridement and catheterisation for the treatment of syringomyelia due to diffuse craniocervical junction arachnoiditis. A case report and technical note. Neurochirurgie 68(6):674–678. https://doi.org/10.1016/j.neuchi.2022.05.007
Serratrice N et al (2021) Case report: a rare case of fourth ventricle to spinal subarachnoid space shunt migration: surgical pearl and literature review. Front Surg 8:696457
Lou Y et al (2022) A clinical study on the treatment of recurrent Chiari (type I) malformation with syringomyelia based on the dynamics of cerebrospinal fluid. Biomed Res Int 2022:9770323
Klekamp J et al (2002) Syringomyelia associated with foramen magnum arachnoiditis. J Neurosurg 97(3 Suppl):317–322
Schuster JM et al (2013) Persistent/recurrent syringomyelia after Chiari decompression-natural history and management strategies: a systematic review. Evid Based Spine Care J 4(2):116–125
Soleman J et al (2017) Syringo-subarachnoid shunt for the treatment of persistent syringomyelia following decompression for Chiari type I malformation: surgical results. World Neurosurg 108:836–843
Menezes AH (1991) Chiari I malformations and hydromyelia–complications. Pediatr Neurosurg 17(3):146–154
Goel A et al (2020) Chiari 1 formation redefined-clinical and radiographic observations in 388 surgically treated patients. World Neurosurg 141:e921–e934
Author information
Authors and Affiliations
Contributions
All the authors whose names appear on the submission made substantial contributions to the conception and design of the work, the acquisition, analysis, and interpretation of the data. They approved the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethical approval
Written informed consent was obtained from all the parents of the patients for publication of this case series report and any accompanying images. All identifying information was stripped off. The manuscript was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MOV 96949 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Spennato, P., Vitulli, F., Tafuto, R. et al. Fourth ventricle to spinal subarachnoid space stenting in pediatric patients with refractory syringomyelia: case series and systematic review. Neurosurg Rev 46, 67 (2023). https://doi.org/10.1007/s10143-023-01972-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-023-01972-y