Abstract
Background
Trapped fourth ventricle (TFV) is a rare and difficult to treat condition. Most patients have a past inciting event (infection, IVH, trauma) and history of prior CSF diversion. The symptoms are due to the mass effect on brainstem and cerebellum. Rarely, TFV can also be associated with syrinx formation due to a dissociated craniospinal CSF flow near the fourth ventricle outlets. We present our experience and outcomes of open posterior fenestration in 11 cases, along with an overview of the surgical management of TFV.
Methods
Between 2011 and 2018, 11 patients of TFV were operated by the posterior approach fenestration of the fourth ventricle outlets and arachnoid dissection. The clinical and radiological findings of the patients were retrieved from the hospital database. The surgical technique is described in detail. The patients’ neurological status and imaging findings in the follow-up were recorded and compared.
Results
The average age of the patients was 23.55 years. The most common presenting symptoms were headache (9/11) and gait imbalance (7), with TB meningitis being the commonest etiology. Ten patients had a history of prior CSF diversion with two presenting with shunt malfunction. Mean follow-up duration was 33.33 months. The improvement in neurological status was observed in 9/11 patients, 2 remained status quo. On follow-up imaging, 8/11 (72.72%) patients had a decrease in the size of TFV while syrinx improved in 3/5 (60%).
Conclusion
Multiple surgical approaches have been described for TFV. Endoscopic fourth ventriculostomy with aqueductoplasty is gaining popularity in the past two decades. However, an open posterior fenestration of the midline fourth ventricle outlet (magendieplasty) along with sharp arachnoid dissection (adhesiolysis) along the cerebello-medullary cisterns and paracervical gutters is relatively simple and provides physiological fourth ventricular CSF outflow. This is especially useful in TFV with syrinx as the craniospinal CSF circulation is established.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Trapped fourth ventricle (TFV) may occur as a sequalae to CSF diversion in cases of hydrocephalus. It is usually seen when hydrocephalus is caused by hemorrhage, chronic infection, or inflammation. This inflammation of ependyma following the primary event causes the obstruction of narrow CSF pathways and obstructive hydrocephalus [12]. As the supratentorial ventricular CSF is shunted, obstruction of the fourth ventricular inlet (Aqueduct) and outlets (Foramina of Luschka and Magendie) results in an isolated and dilated fourth ventricle [26]. Such dilated fourth ventricle acts as a posterior fossa mass lesion with symptoms of cerebellar and brainstem compression [8]. Multiple treatment strategies have been used in the management of TFV ranging from a posterior fossa shunt, open decompression with fenestration, and endoscopic procedures [12]. However, none of them had been proven to be distinctively superior over the others due to lack of sufficient data. Prompt radiological evaluation and strategically planned surgical intervention are the keys since most of the patients have a history of prior CSF diversion, multiple surgeries, and shunt malfunction. We have used open posterior fossa decompression with fenestration of fourth ventricle in our patients and present our experience with long term outcomes, especially in adult patients.
Methods
During the period between 2010 to 2018, 11 patients underwent surgery for TFV in National Institute of Mental Health and Neurosciences. Retrospective data including patient demographic profile, clinical presentation, imaging, and perioperative findings were collected from the hospital database. Along with it, the follow-up imaging and symptoms were analyzed (Table 1). Patients with progressive symptoms or deteriorating functional status (brainstem, cerebellar compression, or syrinx) with a prior inciting event, (CSF diversion procedure, trauma, IVH, or TB meningitis/arachnoiditis) consistent with an imaging finding of a TFV were considered for surgery. Ten patients had a history of previous CSF diversion procedure for hydrocephalus, 7 of them had undergone shunt revisions due to malfunction.
All the patients had symptoms of a “trapped fourth ventricular syndrome” as described further, with the cerebellar, brainstem, and/or cervico-medullary compression (Table 1). All the patients were evaluated with pre-operative MRI of the brain and T2WI screening of the cervical spine. CT brain was performed in patients with acute symptoms to rule out supratentorial shunt malfunction. Two patients underwent shunt revision following admission and before undergoing posterior fossa surgery.
Surgical procedure—technique and nuances
Surgery was done in prone position, head fixed with Mayfield clamps in flexion. Flexion was provided to open the space between foramen magnum and atlas. Intubation was done using a flexo-metallic endotracheal tube to avoid kinking during flexion. A midline suboccipital craniotomy was done in all the cases with excision of the posterior arch of atlas. All the procedures were done by the two senior authors (BID, NP).
Midline scalp incision was taken from inion to C4 spinous process. The suboccipital muscle was dissected in layers, and the squamous occiput was exposed along with the foramen magnum and atlas. A suboccipital craniotomy was performed with high speed drill with excision of the posterior arch of atlas with rongeurs. A “Y”-shaped durotomy was done under microscope to expose the cerebellar hemispheres and the cervico-medullary junction. A thickened layer of arachnoid with scarring with or without an enlarged cisterna magna was seen in all the cases (Fig. 1c, d). Adhesiolysis with sharp dissection was done along the arachnoid planes. The cisterna magna was opened, and the tonsils were retracted to reveal a scarred, closed foramen of Magendie. CSF was drained out of the fourth ventricle following release of these adhesions. The CSF flow channels were opened along the cerebello-medullary cisterns laterally and the subarachnoid spaces along the cervical cord. The excision of posterior arch of C-1 allowed a wide exposure of the subarachnoid spaces at the high cervico-medullary junction level in continuation with the arachnoid at the foramen magnum. In our opinion, the key to enabling flow of CSF from the opened fourth ventricle to the subarachnoid spaces is wide-opening of the arachnoid at these gutters bilaterally. The pulsating CSF flow was confirmed along the cerebello-medullary and upper cervical cisterns. An augmentative duroplasty was done with a pericranial graft to ensure watertight closure and avoid a pseudomeningocele formation. Bone-flap was replaced in 10 patients and fixed with non-absorbable sutures. Bone-flap was not replaced in case 5 (Table 1) owing to a prior asymmetric craniectomy defect and severe scarring in the posterior fossa. The scalp wound was closed in a layered fashion to avoid CSF leak. A subfascial vacuum assist drain was placed. All patients were kept under close neurological and vital monitoring for 24 h in recovery ward. A plain CT of the brain was done on post-op day (POD) 1 to rule out surgical site hematomas, subdural hematomas, and hydrocephalus. Surgical site drains were removed on post-op day 2 and drain site sutured. Patients were observed for 3–4 day post-op to rule out complications (pseudomenigocele, CSF leaks, deterioration in sensorium, or meningitis). Surgical site sutures were removed after 10 days.
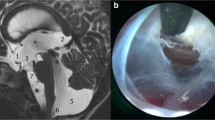
Case 1, a Axial T2WI shows dilated left lateral ventricle and asymmetric dilatation of the occipital horn on right side. VP Shunt in situ, tip in the right frontal horn. Periventricular T2 lucencies (PVL) or hyperdensities are suggestive of raised intraventricular pressure. b Dilated fourth ventricle with PVL, right cerebellopontine cistern is enlarged. c The TFV is compressing the brainstem anteriorly, the anterior CSF spaces are not seen, the cervico-medullary junction is stretched, with syrinx in cervical cord. d Post-op CT shows decrease in the size of fourth ventricle. e Intra-op image following durotomy shows closed fourth ventricular outlet with arachnoid scarring/plastering along the cerebellomedullary cistern and paracervical gutters. f The left cerebellar hemisphere is retracted to reveal the fourth ventricle following Magendieplasty (blue arrows). The arachnoid dissection along cerebellomedullary cisterns and paracervical gutters is the key to establish CSF flow
Results
Patient characteristics
Eleven patients underwent surgery for TFV during an 8-year period from 2011 to 2018 (Table 1). The mean age at presentation was 23.55 years (range 4 to 40 years). There were 4 females and 7 males. Ten patients had history of a VP shunt procedure for hydrocephalus. The most common etiology for TFV in our series was TBM (three cases), two post-op cases of trauma (one had cerebellar contusions while the other with acute subdural, contusions, and IVH) (Table 1). The cause of hydrocephalus and TFV was not known in three patients.
All the patients had symptoms due to a TFV, most common symptom being headache, and recurrent vomit episodes in 9 patients. Gait imbalance and ataxia were seen in 7 patients while limb weakness with signs of myelopathy (increased tone, exaggerated tendon reflexes, upgoing plantars) was present in 6 patients. Lower cranial nerve deficits were seen in two patients. Two patients presented with acute deterioration of symptoms due to shunt malfunction. They underwent a shunt revision procedure before definitive surgery.
Imaging
The classical imaging finding in TFV is the presence of a dilated fourth ventricle causing mass effect on brainstem anteriorly and cerebellum posteriorly. The lower medulla, cervico-medullary junction, and upper cord appear to be thinned out and stretched by the mass effect of the TFV (Figs. 1, 2 and 3). Crevico-dorsal syrinx was present in 5 patients. Periventricular lucencies (PVL) are seen on T2WI, especially the ones having a supratentorial ventriculomegaly (Fig. 1). Decreased or absent CSF flow across the aqueduct can be seen on CISS-3D images. The opening of the CSF spaces along the cervical cord and a decrease in the PVL is indirect signs of establishment of CSF outflow pathways and reduction in intraventricular pressure (Fig. 3). These findings are of important value in cases where there is no significant decrease in the size of the TFV.
Case 6, a and b Preoperative axial and sagittal T2WI, the TFV with mass effect on brainstem. Encysted CSF spaces enterior to a thinned out and stretched upper cervical cord. c Follow-up MRI shows no decrease in the size of the fourth ventricle; however, the absence of PVL may be indicative of decreased pressure in the ventricle. d Follow-up sagittal image, there is appearance of CSF posterior to the cord (white arrow) with decrease in the size of anterior encysted CSF space
Surgery and follow-up
All patients underwent the posterior fossa surgery as mentioned. There were no intra-operative complications. In the post-op period, one patient (case 9) developed multiple sequential supratentorial epidural hematomas (EDH) requiring surgical evacuation. [25] Another patient (case 2) required a shunt revision due to malfunction 10 days following surgery due to persisting supratentorial ventriculomegaly. Reduction in the size of the TFV was seen in 7 patients in the post-op CT.
An MRI of the brain with screening of the spine was performed at the first follow-up, and the clinical status was noted (usually 3–6 months). Mean follow-up duration was 33.33 months (median 37 months, range 6–53 months). Clinical improvement was seen in 9 out of the eleven patients. (Table 2). The neurological status remained stable for the remaining two patients at follow-up. Most marked improvement was seen in gait imbalance and ataxia. Signs of myelopathy were persistent in 5/11 (45.45%) patients.
On follow-up imaging, a reduction in the size of the TFV was noted in 8 patients (72.72%). All these cases showed improvement in the post-op CT and there was no case of recurrence of TFV following reduction in size. Syrinx improved in three of the five patients (60%) on follow-up imaging. Thereafter, follow-up clinical data was noted on subsequent visits and imaging repeated only in the event of onset of fresh symptoms or neurological deficits.
Discussion
Foramina of Luschka, and Magendie act as outlet channels for CSF to pass from fourth ventricle to the cisternal spaces and maintain the patency of CSF circulation. A variety of pathological processes can cause obstruction of these outlets, viz., infection, post IVH, congenital–atretic, trauma even iatrogenic. [12] Continuous CSF production by the choroid plexus of fourth ventricle causes the accumulation of CSF and dilatation of the fourth ventricle. A patent aqueduct decompresses this excess fluid into lateral and third ventricles. In the event of an added aqueductal stenosis, the fourth ventricle becomes trapped with no outlet for CSF egress. Raimondi et al. mentioned that superior displacement of the vermis by an enlarging fourth ventricle can directly compress the aqueduct at the incisura. [22] CSF diversion from lateral ventricles with a shunt reduces the flow across the aqueduct promoting further narrowing. Finally, the ependymal inflammation and scarring caused by the underlying pathology result in aqueductal obstruction and a TFV. This appears to be a fair enough theory for the etiology of TFV. However, Ferrer’s mention of a “functional TFV” based on their operative findings and dynamic magnetic resonance imaging shows an absence of physical obstruction with an altered CSF flow pattern. [5]
The formation of this large ball of CSF in a crowded posterior fossa causes mass effects on the other occupants. Symptoms are related to raised intracranial pressure (ICP); compression of cerebellum, brainstem, tonsillar descent, and cerebello-medullary junction. Headache, visual obscuration, and vomit are the early symptoms. Gait and limb ataxia, dysarthria, cranial nerves dysfunction occur frequently. Increase in the cyst size may lead to lethargy, altered consciousness, bradycardia, and posturing. In the pediatric age group, a common presentation is of an infant with premature birth, intra ventricular hemorrhage (IVH), and a history of shunt procedure for hydrocephalus. In 1966, Foltz et al. described the occurrence of aqueductal stenosis following shunt in children with history of perinatal IVH and hydrocephalus. [6] However, not all infants with IVH go on to have TFV. In a review 761 cases of neonatal IVH, TFV occurred in less than 1% (6 patients). [11]
The first mention of a dilated, isolated fourth ventricle was made by Hawkins et al. in 1978, in post shunt patients. [13] Dandy was the first to describe inflammatory fourth ventricle outlet obstruction in adults. He differentiated the condition from the congenital atretic type of occlusion. [3]
Treatment options—from rubber catheters to complex endoscopy
Multiple approaches have been described for the treatment of a TFV, ranging from simple CSF diversion procedures to complex endoscopic aqueductoplasty and open fenestration or “magendieplasty.” However, the ideal treatment with minimal complications and good outcomes remain elusive. Many a times, a combination of these options must be chosen for individual cases.
In 1920, Walter Dandy did a retrograde recanalisation of the aqueduct with a rubber catheter through the fourth ventricle. [3] Lars Leksell described the use of tantalum wire spiral tube for aqueductal fenestration via the suboccipital route. A fourth ventricular shunt can be placed to decompress a TFV by a lateral trans-cerebellar route or by a midline transforaminal route. [12] Such shunt can be connected to a separate distal system or incorporated along with an existing lateral ventricular shunt system. What appears to be a simple and effective method of TFV decompression is not devoid of complications. Herniation (both upwards and downwards) across the tentorium can occur due to altered pressure gradient in both the compartments. [12] Injuries to the brainstem while insertion or following decompression, IVH, and shunt malfunction requiring revision are known to occur. [19] The open technique of placing the shunt in the fourth ventricle requires a suboccipital craniotomy and opening the foramen of Magendie. The placement of the peritoneal end may also require turning the patient in supine position during the procedure. Efforts should be made to ensure the supratentorial ventricular system is decompressed either by an existing functioning or a simultaneously placed lateral ventricle shunt. This can also be achieved by doing a third ventriculostomy or aqueductoplasty.
To reduce the risk of neural injury, the shunt tube can be placed into the fourth ventricle through a supratentorial entry point. A transcortical transtentorial or a transventricular–transaqueductal approach can be used with the help of ventriculoscopy, intra-op ultrasound, stereotaxy, or neuronavigation. [2, 12, 20] The trajectory is parallel to the fourth ventricular axis, but the procedure could be technically demanding. Shunts can also be placed by an open technique into the fourth ventricle or through the aqueduct. These catheters can be communicated to the subarachnoid spaces, thereby avoiding the need for a distal catheter. [4, 26] Garber et al. stated that stereotactically placing a fourth ventricle shunt via parietal transtentorial route had significantly better shunt survival times and lower rates of revision than the traditional suboccipital approach. [9] Overall, shunts placed for TFV have inferior patency rates than simple shunts in the lateral ventricles. [12, 15]
Endoscopic approaches for internal CSF diversion were developed to avoid an external shunt. Fourth ventricle can be approached via a supra-tentorial or infratentorial endoscopic approach. The obstruction can be relieved by an aqueductoplasty or a stent placed to open the flow. [8] Aqueductoplasty precludes the need of a foreign body but still carries the risk of restenosis, especially, in cases who have had an IVH. Fritsch et al. found 73% closure rates for aqueductoplasty. [7] In a review by Gallo et al., there was no incidence of re-stenosis following stenting when compared with 53% in cases of aqueductoplasty. [8] However, the introduction of an implant with risk of infection and migration of the stent cannot be overlooked. Transient ophthalamoparesis due to manipulation of the tectal region with pressure on periaqueductal gray occurs frequently (~ 10% cases). [24] Not to mention, the expertise and the learning curve required to maneuver the endoscope through the foramen of Monroe to the posteriorly located aqueduct, with the risk of injury to the structures along the way are major shortcomings of the endoscopic approach.
The advent of endoscopic approaches to the third ventricle made the open retrograde cannulation of the aqueduct obsolete, but as surgeons gained expertise, they re-explored the old route mentioned by Dandy with an endoscope. In the 1990s, multiple reports mentioned good outcomes via the infra-tentorial endoscopic approach. [18, 24]
Endoscope-assisted pan ventricular catheter, from a pre-frontal burr hole spanning through the foramen of Monroe, aqueduct into the fourth ventricle with multiple perforations across the shunt, has also been described. The technique has been used by a single or two burr holes with satisfactory outcomes. [1, 7, 27] Such technique has the advantage of putting the shunt axis parallel to fourth ventricle, thus avoiding brainstem injury and decompressing multiple compartments.
Tubercular meningitis and TFV
Tubercular meningitis (TBM) continues to be a major cause of adult hydrocephalus in the developing world. TBM can cause a communicating hydrocephalus in more than 80% of the cases. However, the chronic arachnoiditis with exudates and scarring of the meninges can cause obliteration of the fourth ventricles outlets resulting in an obstructive hydrocephalus. [23] As mentioned above a shunt procedure in the presence of an aqueductal stenosis can cause a TFV. Three of our patients had a history of TBM, and all of them have undergone a previous VP shunt.
TFV with syrinx
Although rare, a syrinx can form as a sequel of arachnoiditis across the cervico-medullary junction. This can occur following trauma [29], chronic inflammation, or infection. TB arachnoiditis can also cause syrinx formation in up to 15% cases as a delayed manifestation. [10] The arachnoid adhesions and scarring obstruct the CSF flow around cerebello-medullary cisterns. [14] Simultaneous association of tonsillar herniation due to hydrocephalus makes the picture similar to Chiari I malformation. Open exploration with a suboccipital craniotomy and exploration of the membranous obstruction of the cisterns thus appears to be a more physiological approach in such situation. In our series, syrinx was present in 5 patients, and improved in 3 following surgery. There was no progression of syrinx in the remaining two patients. All patients had a favorable neurological outcome following the procedure.
Open posterior fenestration
Dandy described the procedure of opening the fourth ventricle outlets, foramen of Magendie blocked by inflammation. [3] Open surgical fenestration of the fourth ventricle was also recommended by Villavicencio et al., for posterior fossa cysts including TFV with good outcomes. [28] This wide fenestration of fourth ventricle from midline or “magendieplasty” with release of arachnoid adhesions opens up the CSF channels. [16] CSF can now flow out of the fourth ventricle into the cerebello-medullary cisterns, cisterna magna, and subarachnoid space around the cervical spine. By excising the posterior arch of C1 and adding a duroplasty, we intend to take care of the herniated tonsils and the syrinx. This has been seen on the follow-up imaging of our patient wherein both the fourth ventricle and syrinx have reduced in size and the fourth ventricle. Similar results were reported by Orakdogen et al., in four patients. [21] They used a direct fourth ventricle to subarachnoid space catheter following open decompression. Others have described the placement of a fourth ventricle to subarachnoid stent/shunt with open decompression. [4, 26] The efficacy of the shunt has not been clearly established with the limited follow-up data, and there is no clear advantage. We believe that introducing an implant could add to the morbidity with infection, migration, scarring etc. As already mentioned, these patients have had multiple procedures and might have an ongoing local chronic inflammatory process, so the introduction of a foreign body could just be avoided.
Most of the patients in our series had a functioning supratentorial shunt system which made the lateral and third ventricles small, so in our opinion, a suboccipital approach to the fourth ventricle seems more logical and less risky. Also, the key to internally decompress the fourth ventricle into the posterior cisternal spaces is a wide exposure and diligent arachnoid dissection.
The fenestration of fourth ventricle has been successfully attempted with endoscopy, especially in pediatric cases with primary fourth ventricle obstruction. [12, 16, 17] However, in patients with severe adhesions following arachnoiditis open microsurgical arachnoid dissection provides a wider view and better accessibility at opening multiple outlets by arachnoid dissection. We believe by opting for microsurgical opening of the foramen of Magendie and cisternal dissection, a fourth ventricle shunt or a complicated endoscopy with aqueductal stent can be avoided.
Only one patient developed a pseudomeningocele following surgery which was managed non-surgically. None of our patients developed wound dehiscence or infection following surgery. We did not observe any perioperative complications during the hospital stay as seen with shunts (brainstem injury or hemorrhage) or aqueductoplasty (ophthalam-oparesis). There were no recurrences in the follow-up period.
On follow-up, clinical improvement was noted in 9/11 patients (81.81%). The decompression provided by craniectomy and C1 arch excision help in alleviating the progression of syrinx. Post-operative reduction in the size of the fourth ventricle on imaging has been observed only in three-fourths of the patients. [8, 26] The elasticity of the ventricular walls may decrease over time, and with prolonged compression, TFV can also cause loss of cerebellar volume. These might explain the lack of reduction in ventricle size. In our series, the ventricular size decreased in 72.72% patients. However, once the patency of the fourth ventricular outlets is established, the intraventricular pressure decreases with resultant reduction in the mass effect over the brainstem. The symptomatic and neurological improvement is a testimony to this theory. [8]
The limitation of our study is the small sample size of the cases. Due to the rarity of the disease, it is difficult to elucidate the natural course and outcome comparisons with different surgical strategies. Long term follow-up studies are required comparing the advantages of endoscopy versus open surgery in both pediatric and adult subjects.
Conclusion
TFV is a rare but difficult condition to treat. Most patients have a history of prior CSF diversion procedure. The presence of associated syrinx presents a further surgical challenge. A variety of treatment options have been described in literature with varying outcomes. We believe an open posterior fenestration of the fourth ventricle provides a technically simple, safe, effective, and a more physiological option for TFV. The addition of C1 arch decompression is a key in cases with associated syrinx.
References
Cinalli G (2014) Endoscopic aqueductoplasty and placement of a stent in the cerebral aqueduct in the management of isolated fourth ventricle in children. https://doi.org/10.3171/ped.2006.104.1.21
Colpan ME, Savas A, Egemen N, Kanpolat Y (2003) Stereotactically-guided fourth ventriculo-peritoneal shunting for the isolated fourth ventricle. Minim Invasive Neurosurg 46(1):57–60
Dandy EW (1920) The diagnosis and treatment of hydrocephalus resulting from strictures of the aqueduct of Sylvius. SurgGynecolObstet 31:340–345
Dollo C, Kanner A, Siomin V, Ben-Sira L, Sivan J, Constantini S (2001) Outlet fenestration for isolated fourth ventricle with and without an internal shunt. Childs Nerv Syst 17(8):483–486
Ferrer E, de Notaris M (2013) Third ventriculostomy and fourth ventricle outlets obstruction. World Neurosurg 79(2):S20.e9–S20.e13
Foltz EL, Shurtleff DB (1966) Conversion of communicating hydrocephalus to stenosis or occlusion of the aqueduct during ventricular shunt. J Neurosurg 24(2):520–529
Fritsch MJ, Kienke S, Manwaring KH, Mehdorn HM (2004) Endoscopic aqueductoplasty and interventriculostomy for the treatment of isolated fourth ventricle in children. Neurosurgery 55(2):372–377 discussion 377-9
Gallo P, Hermier M, Mottolese C, Ricci-Franchi A-C, Rousselle C, Simon E, Szathmari A (2012) The endoscopic trans-fourth ventricle aqueductoplasty and stent placement for the treatment of trapped fourth ventricle: long-term results in a series of 18 consecutive patients. Neurol India 60(3):271
Garber ST, Riva-Cambrin J, Bishop FS, Brockmeyer DL (2013) Comparing fourth ventricle shunt survival after placement via stereotactic transtentorial and suboccipital approaches. J Neurosurg Pediatr 11(6):623–629
Garg RK, Malhotra HS, Gupta R (2015) Spinal cord involvement in tuberculous meningitis. Spinal Cord 53:649–657
Hall TR, Choi A, Schellinger D, Grant EG (1992) Isolation of the fourth ventricle causing transtentorial herniation: neurosonographic findings in premature infants. Am J Roentgenol 159(4):811–815
Harter D (2004) Management strategies for treatment of the trapped fourth ventricle. Childs Nerv Syst 20(10):710–716
Hawkins JC, Hoffman HJ, Humphreys RP (1978) Isolated fourth ventricle as a complication of ventricular shunting. J Neurosurg 49(6):910–913
Kaynar MY, Koçer N, Gençosmanoğlu BE, Hancı M (2000) Syringomyelia - as a late complication of tuberculous meningitis. Acta Neurochir 142(8):935–939
Lee M, Leahu D, Weiner HL, Abbott R, Wisoff JH, Epstein FJ (1995) Complications of fourth-ventricular shunts. Pediatr Neurosurg 22(6):309–314
Longatti P, Fiorindi A, Feletti A, Baratto V (2006) Endoscopic opening of the foramen of Magendie using transaqueductal navigation for membrane obstruction of the fourth ventricle outlets. J Neurosurg 105(6):924–927
Longatti P, Fiorindi A, Martinuzzi A, Feletti A (2009) Primary obstruction of the fourth ventricle outlets. Neurosurgery 65(6):1078–1086
Matula C, Reinprecht A, Roessler K, Tschabitscher M, Koos WT (1996) Endoscopic exploration of the IVth ventricle. Minim Invasive Neurosurg 39(3):86–92
Mohanty A, Manwaring K (2018) Isolated fourth ventricle: to shunt or stent. Oper Neurosurg 14(5):483–493
Montes JL, Clarke DB, Farmer JP (1994) Stereotactic transtentorial hiatus ventriculoperitoneal shunting for the sequestered fourth ventricle. J Neurosurg 80(4):759–761
Orakdogen M, Emon ST, Erdogan B, Somay H (2015) Fourth ventriculostomy in occlusion of the foramen of magendie associated with Chiari Malformation and Syringomyelia. NMC Case Rep J 2(2):72
Raimondi AJ (1987) Pediatric neurosurgery : theoretical principles art of surgical techniques. Springer, New York
Rajshekhar V (2009) Management of hydrocephalus in patients with tuberculous meningitis. Neurol India 57(4):368–374
Teo C, Burson T, Misra S (1999) Endoscopic treatment of the trapped fourth ventricle. Neurosurgery 44(6):1257–1262
Tyagi G, Bhat DI, Devi BI, Shukla D (2019) Multiple remote sequential supratentorial epidural hematomas—an unusual and rare complication after posterior fossa surgery. World Neurosurg 128:83–90
Udayakumaran S, Biyani N, Rosenbaum DP, Ben-Sira L, Constantini S, Beni-Adani L (2011) Posterior fossa craniotomy for trapped fourth ventricle in shunt-treated hydrocephalic children: long-term outcome. J Neurosurg Pediatr 7(1):52–63
Upchurch K, Raifu M, Bergsneider M (2007) Endoscope-assisted placement of a multiperforated shunt catheter into the fourth ventricle via a frontal transventricular approach. Neurosurg Focus 22(4):E8
Villavicencio AT, Wellons JC III, George TM (1998) Avoiding complicated shunt systems by open fenestration of symptomatic fourth ventricular cysts associated with hydrocephalus. Pediatr Neurosurg 29(6):314–319
Williams B (1990) Paraplegia post-traumatic syringomyelia, an update. ParaplegitJ 28:296–313
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this type (retrospective) of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Comments
The authors retrospectively review a single-centre case series of 11 patients who underwent surgery for symptomatic trapped fourth ventricle between 2010 and 2018. All underwent an open procedure involving a posterior fossa craniotomy and removal of the posterior arch of C1, with extensive adhesiolysis along the cerebellomedullary fissure, foramen of Magendie and upper cervical spine, and expansile duraplasty. Mean follow up was 33.3 months (all over six months) with clinical improvement in nine patients. The authors conclude that open surgery is an effective and safe method to treat a trapped fourth ventricle. While the surgical risk of an extensive open procedure in a chronically scarred posterior fossa and craniocervical junction may be considered to be higher than in image-guided shunting of the fourth ventricle, this technique allows some restoration of normal anatomy and CSF flow. This study offers further insight into a rare neurosurgical problem and is a valuable contribution to the existing literature.
Kristian Aquilina
London,UK
This article is part of the Topical Collection on CSF Circulation
Rights and permissions
About this article
Cite this article
Tyagi, G., Singh, P., Bhat, D.I. et al. Trapped fourth ventricle—treatment options and the role of open posterior fenestration in the surgical management. Acta Neurochir 162, 2441–2449 (2020). https://doi.org/10.1007/s00701-020-04352-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-020-04352-3