Abstract
Treatment-refractory meningiomas have a dismal prognosis and limited treatment options. Meningiomas express high-densities of somatostatin receptors (SSTR), thus potentially susceptible to antitumorigenic effects of somatostatin analogues (SSA). Evidence for SSA in meningiomas is scarce, and it is unclear if published literature would either (1) support wider use of SSA, if (2) more evidence is desirable, or if (3) available evidence is sufficient to discard SSA. We addressed the need for more evidence with a systematic review and meta-analysis. We performed an individual patient data (IPD) meta-analysis. Main outcomes were toxicity, best radiological response, progression-free survival, and overall survival. We applied multivariable logistic regression models to estimate the effect of SSA on the probability of obtaining radiological disease control. The predictive performance was evaluated using area under the curve and Brier scores. We included 16 studies and compiled IPD from 8/9 of all previous cohorts. Quality of evidence was overall ranked “very low.” Stable disease was reported in 58% of patients as best radiological response. Per 100 mg increase in total SSA dosage, the odds ratios for obtaining radiological disease control was 1.42 (1.11 to 1.81, P = 0.005) and 1.44 (1.00 to 2.08, P = 0.05) for patients treated with SSA as monodrug therapy vs SSA in combination with everolimus, respectively. Low quality of evidence impeded exact quantification of treatment efficacy, and the association between response and treatment may represent reverse causality. Yet, the SSA treatment was well tolerated, and beneficial effect cannot be disqualified. A prospective trial without bias from inconsistent study designs is warranted to assess SSA therapy for well-defined meningioma subgroups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meningiomas are classified according to the WHO classification of tumors of the central nervous system (CNS) and constitute the most prevalent primary intracranial neoplasm in adults [31, 43]. The majority of lesions harbor benign molecular and epigenetic properties leading to an indolent clinical course [7, 47]. The primary treatments comprise follow-up, surgery, and possibly radiotherapy depending on the histological grade and residual tumor volume. However, a subset of meningiomas elicit a particular aggressive behavior irrespective of the WHO grade, which may often be linked to distinct alterations, such as TERT promoter mutations or CDKN2A/B homozygous deletions [32, 33, 35, 50, 56]. Aggressive subtypes are associated with higher rates of recurrence, progression, and ultimately treatment-refractory disease leading to a dismal prognosis. Therapeutic options are then limited to renewed surgery, radio- or cytotoxic chemotherapy without established efficacy [12, 28]. Thus, new treatment options are needed.
The somatostatin receptor (SSTR) represents a potential target, as various SSTR subtypes are expressed with high-densities on almost all meningioma cells [3]. Somatostatin analogues (SSA) are used for treatment of growth hormone-producing pituitary adenomas and neuroendocrine tumors that also express SSTR [29, 48, 60]. The antitumorigenic effects of SSTR-binding properties could, therefore, be exploited therapeutically for meningiomas as well [34]. Hitherto published cohorts investigating SSA in meningioma were limited to progressive meningiomas and small sample sizes unfeasible for deriving generalized conclusions. It is virtually impossible to conduct prospective trials for small subgroups of meningiomas that were refractory to surgery and radiation. We can thus not know if SSA is a potentially useful treatment for any group of meningiomas. We suggest that compiling data from previously published cohorts ameliorate limitations inherent from small cohorts, thus enabling a more valid assessment of the effect and toxicity of SSA therapy in meningioma patients. A systematic and critical analysis of evidence will indicate if available evidence would either (1) support continued compassionate use, or (2) support removal of SSA from potential meningioma treatments, or (3) justify search of more evidence.
This study aims to evaluate evidence for treatment of meningioma with SSA systematically and at the individual patient level by analyzing toxicities, response to treatment, radiological response, progression-free survival (PFS), and overall survival (OS). The analyses were enabled by compiling data from all meningioma patients subjected to SSA who are available in the published literature, i.e., a systematic review with a meta-analysis of individual patient data.
Methods
The present study constitutes a part of larger international collaboration investigating the effects of radiolabeled and non-radiolabeled somatostatin analogues in treatment-refractory meningioma, which has been PROSPERO-registered on the 30th January 2019 (CRD42019119140). We adhered to the PRISMA-IPD guidelines (Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data) [59]. In this context, the one-stage approach for the individual patient data meta-analysis was used for synthesis, i.e., data was compiled and analyzed simultaneously.
We included and compiled untraceable, anonymized patient data that already has been published previously, thus not requiring Institutional Review Board approval by Danish law.
Literature search
The PubMed, Embase, and Cochrane Library databases were systematically surveyed the 5th of May 2021 using the following keywords and MeSH-terms: “meningioma” in combination with “somatostatin analogue,” “octreotide,” “pasireotide,” “sandostatin,” or “lanreotide” (Meningioma AND (octreotide OR somatostatin analogue OR pasireotide OR lanreotide)).
Study selection, outcomes, and data extraction
Eligible studies comprised investigations of SSA applied to treatment-refractory meningiomas. We excluded case reports (n = 7) [11, 24, 42, 46, 49, 51, 55] and abstracts (n = 0) from the quantitative synthesis, i.e., the meta-analysis, but imposed no restrictions on study design or language for the qualitative synthesis, i.e., the systematic review. Treatment-refractory was defined across each study as failed tumor control despite multiple attempts with conventional treatment modalities including surgery, radiotherapy, and medication of any kind. Therapeutic options were considered depleted by the treating physicians prior to initiation of SSA treatment, which comprised surgery and radiotherapy for the vast majority and cytotoxic therapy for additionally ~ 15% of the cases. SSA were either administered as monodrug therapy or in combination with everolimus.
Outcomes comprised toxicity, response to treatment, radiological response,, and OS. All corresponding authors of eligible papers were contacted to request individual patient data, in cases where the data was not already available in the publication. The requested data comprised age, sex, WHO tumor grade, the specific SSA analogue and the exact applied dosage, the number of treatment cycles, best obtained radiologic treatment response, toxicity, PFS, and OS. Screening, data extraction, and management were performed independently by two authors (LRJ and CM) and subsequently compared.
Three research groups continued to follow the patients subsequent to the publication and/or enrolled additional patients, which we included herein and thereby augmented the data compilation compared with three of the original publications, including five non-skull base treatment-refractory meningiomas (with missing data on complete treatment history) [53]; updated follow-up time [26], and updated best radiological response [5].
Quality of evidence and risk of bias
We applied GRADE (Grading of Recommendations, Assessment, Development and Evaluations) to rate quality of evidence [20], while ROBINS-I (“Risk of Bias in Non-randomized Studies –of Interventions”), developed by Cochrane, was used to assess risk of bias [58].
Toxicity
Included studies applied different criteria systems, predominantly the Common Terminology Criteria for Adverse Events (CTCAE) version 1.0, 3.0, or 4.0 [38, 39]. CTCAE v. 1.0, v. 3.0, and v. 4.0 criteria for hematotoxicity are identical 1:1, and therefore comparable, except limit values for lymphocytopenia which were slightly different in v.1.0, exclusively.
Data management and statistics
In total, SSA was administered as monodrug therapy in 99 patients (74.4%), while 34 patients received everolimus concomitantly. To account for effects attributable to each treatment modality, we separated data into two distinct datasets comprising patients treated with (1) SSA as monodrug therapy vs (2) SSA combined with everolimus.
The included patients received SSA on a monthly basis. Hence, the total SSA dosage would increase as function of follow-up time (Supplementary Fig. 1), which consequently implicate a strong correlation between survival failure and cumulative SSA dosage in a time-to-event analysis—regardless of true effect. Therefore, it was unfeasible to quantify the effect of SSA treatment on progression and death using standard time-to-event analysis. As second choice, we considered best radiological response obtained as proxy for disease control, which was defined as either stable disease, partial or complete response on MRI (contrarily to radiological progressive disease). The best radiological response was evaluated using different algorithms, including RANO, RESIST v1.1, and Macdonald.
Subsequently, we estimated the probability of disease control in the separated cohorts encompassing (1) SSA monodrug therapy vs (2) SSA with everolimus by applying multivariable logistic regression adjusted to Total-SSA (cumulative SSA dosage applied in mg), age, sex, and WHO grade. Total-SSA and age were included as continuous covariates. The predictive performance was evaluated in both cohorts using the area under the receiver operating characteristics curve (AUC, a higher score indicates a better model) and the Brier score (a lower score indicates a better model).
Finally, progression-free and overall survival probabilities were reported. The absolute risk of progression was estimated using a competing risk approach, as progression-free death would preclude the event of progression. Here, patients were censored either at the time of progression-free death or alive and progression-free at the end of follow-up. The absolute risk of progression was subsequently estimated using the Aalen-Johansen method with Gray’s test applied for testing of significant differences in absolute risks. Contrarily, all-cause death implied no competing risk scenarios and patients were therefore censored if alive at the end of follow-up. Hence, the probability of survival failure was estimated using the Kaplan–Meier method with the log-rank test applied for testing of significantly different overall survival probabilities.
We considered two-sided P values < 0.05 significant. The statistical software R v. 4.2.0 was used.
Results
Search strategy and eligible studies
A detailed PRISMA-IPD search diagram can be found in Fig. 1. The preliminary search identified 504 publications, which were subjected to individual assessment for eligibility. Finally, we identified nine eligible studies [5, 6, 10, 16, 23, 26, 40, 53, 57]. The authors of one study declined data contribution [40], and their study was therefore included for the qualitative synthesis, exclusively. In contrast, individual patient data was retrieved online from one study [6], and received from the remaining seven authors who agreed to contribute [5, 10, 16, 23, 26, 53, 57].
Study characteristics
The individual patient data meta-analysis encompassed data from eight out of nine hitherto published cohorts (~ 89%) and data from five patients not previously disclosed [53]. There were no disagreements in data extraction or management between the study authors (LRJ and CM). Study designs comprised four retrospective studies [5, 10, 23, 53], three phase II clinical trials [16, 26, 57], and one prospective study [6] (Table 1).
The qualitative synthesis comprised the nine cohort studies and additionally seven case reports identified from the search. Case report characteristics were summarized in Supplementary Table 1.
Quality of evidence and risk of bias
We rated the quality of evidence “very low” for all included studies. In addition to study heterogeneity, the predominant contributors of downvoting the studies were non-randomization and lack of head-to-head comparisons [18, 19] (Supplementary Table 2).
We associated all included studies with an increased risk of bias, which we rated as “moderate.” Study design, heterogeneity, and small cohorts constituted the greatest risks of bias (Supplementary Table 3).
Toxicity
One study applied CTCAE v.1 [26], two v.3 [6, 57], and three v.4 [5, 16, 23]. One study did not distinguish between grades 1 and 2 using CTCAE v. 4. Two studies with none or few adverse events reported did not apply any assessment schemes [10, 53]. Diarrhea (30%), fatigue (19%), and headache (11%) comprised the most frequent adverse event during the SSA treatment. Most adverse events were grades 1 and 2, while nine (7%) and one (0.7%) patient experienced a grade 3 and grade 4 adverse events. A complete list of reported adverse events was shown in Supplementary Table 4.
Patient characteristics
A total of 133 patients with treatment-refractory meningioma were treated between 1996 and 2019, including 55 WHO-1, 41 WHO-2, and 37 WHO-3 meningioma patients. The median follow-up was 19.0 months (range: 1 to 227), which corresponded to a total follow-up of 263 person-years. The SSA comprised octreotide (n = 132) [5, 6, 10, 16, 23, 26, 53, 57] and lanreotide (n = 1) [10]. Two studies combined SSA with everolimus (n = 34) [5, 16]. A detailed overview is shown in Table 1.
In particular, the authors of Schulz et al. originally published data on eight out of the 13 patients included herein [53], meaning that the authors supplied outcome data on the remaining five for our analyses. The data of these five patients was previously not published, why we performed a sensitivity analysis with and without these data (Supplementary Fig. 2: adding the five additional patients did not affect the predictive performance negatively, thus reasonably justifying inclusion for further analysis).
Individual patient data and radiological evaluation
Five studies applied RANO [5, 16, 23, 26, 57], one RECIST 1.1 [10], and one Macdonald [6] as radiological response criteria. One study defined growth progression as any increase in size detectable on MRI [53] (Table 1). Of the 133 treated patients, 79 patients continued to have progressive disease (59.4%), 48 patients died (36.0%) (including seven progression-free deaths) while 39 patients (29.3%) were censored alive at the end of follow-up. Figure 2A depicts the best radiological response obtained on MRI scans. Here, 63 patients (47.4%) obtained stable disease as best radiological response, while nine patients (6.8%) and five (3.8%) patients obtained partial response and complete response, exclusively. The remaining 56 (42.1%) patients had progressive disease as best radiological response (Fig. 2B).
Radiological disease control: odds ratios and predictive performance
In the cohort comprising 99 patients treated with SSA as monodrug therapy, the odds ratio for obtaining disease control as best radiological response was 1.42 (95% CI: 1.11 to 1.81, P = 0.005) for each 100 mg increase in Total-SSA (Table 2). In comparison to WHO-1 lesions, the odds ratios decreased to 0.31 (95% CI: 0.09 to 1.05, P = 0.06) and 0.08 (95% CI: 0.02 to 0.33, P < 0.001) for WHO-2 and WHO-3 lesions, respectively. The performance was AUC 0.84 (95% CI 0.77 to 0.92) and Brier 0.15 (95% CI: 0.11 to 0.19) for the logistic regression model in predicting disease control as best radiological response (Fig. 3A). Furthermore, the agreement between predicted probability and actual frequency of obtaining disease control was well calibrated (Fig. 3B).
For the remaining 34 patients receiving SSA concomitant to everolimus, the odds ratio for obtaining disease control remained 1.44 (95% CI: 1.00 to 2.08, P = 0.05). However, WHO grade did not harbor similar characteristics. In reference to WHO-1, the odds ratios for obtaining disease control were 0.84 (95% CI: 0.03 to 20.94, P = 0.9) and 2.86 (95% CI: 0.11 to 76.1, P = 0.5). The logistic regression model performed considerably worse in terms of predicting disease control with AUC 0.71 (95% CI: 0.62 to 0.79) and Brier 0.24 (95% CI: 0.19 to 0.28) (Fig. 3C). The calibration was negatively affected with a tendency to underestimate the probability of obtaining disease control. Furthermore, the cohort combining SSA with everolimus included only two WHO-1 patients, who had stable and progressive disease, respectively. The remaining patient had WHO-2 or WHO-3 lesions, but obtained partial and complete response as best radiological evaluation—thus, partially explaining the decreased predictive performance and calibration.
Probability of progression-free and overall survival
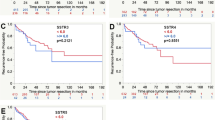
The absolute risk of progression and probability of survival failure increased significantly corresponding to each WHO grade. However, the absolute risk of progression reached a plateau for both WHO-1 and -2 meningiomas after approximately 3 and 5 years (Fig. 4A and B).
Qualitative synthesis: cases not included in the individual patient data meta-analysis
Cohorts
One phase II study, which applied pasireotide to 18 progressive meningiomas, did not contribute with data for the quantitative synthesis [40]. The SSA treatment was well tolerated with transient and manageable toxicities including fatigue, nausea, and diarrhea. Grades 3 and 4 toxicities comprised hyperglycemia, hypoglycemia, elevated amylase, elevated lipase, fatigue, and hypokalemia. The median PFS and OS comprised 15 and 104 weeks, respectively. The best radiographic response obtained was not reported.
Case reports
Four case studies reported positive effects from SSA treatment, including a 2-year progression-free survival [42] and stable disease [11, 46], including partial remission reported. Also, visual improvement in a episellar meningioma [24] and favorable acute and chronic effects on meningioma tumor size on MRI were reported [49]. The applied radiological protocol was not reported.
One study reported no growth inhibition in a patient with simultaneous pituitary acromegaly [55]. Finally, one study reported a case of multifocal demyelination after octreotide treatment in a patient with metastatic meningioma [51].
Discussion
We herein present a systematic review with a meta-analysis of individual patient data compiled from eight out of nine hitherto published cohorts. The effect of SSA on progression and death could not be quantified using standard time-to-event analysis. The SSA treatment was applied as salvage treatment and administered on a monthly basis. Consequently, unlimited treatment cycles were allowed and only discontinued in case of deterioration. Therefore, the cumulative SSA dosage received was highly correlated to (1) length of overall survival per default and (2) progression-free survival, as treatment was terminated in progressive lesions, thus yielding lower cumulative dosages in patients with a progression. Subsequently, the association between outcomes and cumulative dose may reflect reversed causality, and the quantification of odds for achieving disease control using a multivariable logistic regression analysis may be correspondingly biased. The applicability of odds for obtaining disease control is limited to generation and calibration of hypotheses for prospective trials.
SSA therapy has well-established antitumorigenic effects in vitro [1, 13,14,15], and SSA therapy is an established treatment for other SSTR positive tumors [29, 41, 48, 60]. We therefore expected to find supporting evidence also for meningioma through our review and meta-analysis. Some results suggested benefit for selected patients: ~ 11% of all included patients obtained partial or better “best radiological response” and an additional 47% obtained “stable disease.” Next, the regression model was applied in the separate cohorts comprising patients receiving (1) SSA as monodrug therapy vs (2) SSA combined with everolimus. The odds ratios showed statistically significant radiological disease control with 1.42 and 1.44 per 100 mg increase in Total-SSA in both cohorts. The predictive performance of the logistic regression model showed good agreement between predicted vs observed frequency of disease control in the SSA monodrug therapy cohort. In contrast, the prediction model underestimated the probability of disease control in the combined treatment cohort. This result suggested an added effect of everolimus, but reflected relatively few patients and should be viewed with caution. This result could also be attributed to the extensive heterogeneity between the individual patients and cohorts—e.g., the cohort combining SSA with everolimus included two WHO-1 patients, while complete response to treatment was reported for WHO-3 lesions. In contrast, WHO-1 patients were predominant in the cohort applying SSA as monodrug therapy, while no case complete response was reported for WHO-3 lesions. The meta-analysis was, however, compromised by the very low quality of evidence in included studies. The individual studies did not measure outcomes following a fixed SSA dosage, but allowed for unlimited treatment cycles over time. Evaluation of treatment effects were, therefore, complicated by well-responding patients receiving more SSA than non-responders, allowing for reverse causality. SSA treatment for meningioma was neither supported nor disqualified by our meta-analysis.
Another issue was generalizability and external validity, which would require well defined treatment groups. The term “treatment-refractory” does not constitute a universal clinical and traceable definition. Still, the relation between risks was usually associated with each individual WHO grade and was preserved as demonstrated by the Aalen-Johansen method for absolute risk of progression and the Kaplan–Meier method for overall survival probabilities. Thus, “treatment-refractory” was interpreted to denote meningiomas with a particularly aggressive phenotype within each WHO grade rather than a specific subgroup of aggressive meningiomas, but still remains undefined.
Moreover, the studies comprised vastly heterogenous cohorts dominated by patients with dismal prognoses at baseline. Interpretation of results is hampered not only by such heterogeneity but also by the fact that “treatment refractory meningiomas” may be particularly difficult to treat with any therapy, and that less aggressive meningiomas could be better treated.
Published data were inconclusive as they reflected either causality or confounders; SSA treatment for meningioma was neither supported nor disqualified. Considering its low toxicity and the analogy between meningiomas and other somatostatin-receptor positive tumors [41, 48], SSA remains a potential future treatment for meningioma.
It follows that prospective trials are required to resolve whether SSA is useful for therapy of meningiomas. It could be speculated that less aggressive tumors and lower tumor burden may associate with better detectable treatment responses. In this context, several essential aspects remain unresolved. Currently, five unique SSTR-subtypes have been described. While SSTR-2a may be expressed predominantly on de novo meningioma tumor cells, it has been shown that recurrent lesions and previous treatment with radiotherapy affect the SSTR subtypes 1, 3, and 5 [2]. How these alterations may influence antitumorigenic properties of SSA is unknown but crucial to determine therapeutic potentials of SSA treatment. Furthermore, it has been demonstrated that 68 Ga-DOTATATE/-TOC uptake on PET/CT scans correlated with benefit from SSTR-targeted peptide radionuclide receptor therapy (PRRT) [54]. That treatment also utilizes somatostatin analogues, indicating that scintigraphy and PET technologies targeting SSTR may allow selection of patients for SSA treatment. Finally, molecular characterization and methylation-based classification offers improved risk stratification of meningioma [37, 50, 64]. Thus, it is expected that future studies could select patients from molecular and epigenetic profiles for SSA treatment.
SSA have plausible mechanistic effects on meningioma, and they are established therapy for other tumors that express SSTR. In this context, the benefit suggested by the reviewed articles may well reflect a true effect. We conclude that we (1) cannot discard an effect SSA applied to treatment of meningiomas and (2) evidence is currently insufficient to support other than experimental use. Thus, search for better evidence is warranted.
Comparison to cases not included in the quantitative synthesis
Data from one phase II study were not compiled in the quantitative synthesis [40]. The reported adverse events are similar to the presented individual patient data meta-analysis with predominantly transient and manageable toxicities comprising mainly gastrointestinal discomfort, and only few severe toxicities. The endpoints comprising PFS and OS were comparable to the results obtained from the individual patient data meta-analysis as the 18 subjects suffered from recurrent or progressive WHO-2 and -3 meningiomas. We, therefore, consider that the reported toxicities and time-to-event endpoints support the primary results presented herein.
Previous case reports have investigated the use of SSA in treatment-refractory meningioma. These did not report consistent results; five case reports reported favorable outcomes in the form of stable disease or regression [11, 24, 42, 46, 49], one study reported progression, and one study an unexpected complication [51, 55]. Except one severe adverse effect consisting of multifocal demyelination, the case reports overall outline a safe use of SSA.
The somatostatin receptor and antitumorigenic effects
Biological pathways exerting antitumorigenic effects are initiated via SSTR 1–5 agonism, and mainly comprise induced apoptosis, inhibited proliferation, and inhibited hormone secretion (Fig. 5 details a graphic overview). The intracellular effects mediated by SSTR 1 through 5 may be receptor subtype selective, but currently remain incompletely mapped [22].
Graphic overview of antitumorigenic pathways initiated by the somatostatin receptors. Apoptosis is activated by SHP-1 leading to p53-dependent apoptosis executed by caspases [43,44,45], and NF-κB-mediated control of JNK-cascade [46, 47]. Antiproliferative effects involve PI3K/Akt and Ras-Raf-MEK-ERK pathways activated by PTP [48]. Inhibition of proliferation occurs through upregulation of cyclin-dependent kinase inhibitors (e.g., p21 and p27). Antitumorigenic effects occur through inhibition of adenylate cyclase leading to reduced levels of cAMP and Ca2 + that inhibits secretion of growth factors and hormones, such as VEGF [51, 52]. SSA may induce immunomodulatory mechanisms facilitating antitumorigenic effects [53]
Induced apoptosis is activated by the protein-tyrosine phosphatase SHP-1 leading to p53-dependent apoptosis executed by caspases [30, 52, 61], and NF-\(\kappa\) B-mediated control of JNK-cascade that induces apoptosis [17, 44]. The main pathway mediating antiproliferative effects involves phosphotyrosine phosphatases (PTP), that transduce the activity of downstream signaling molecules including the PI3K/Akt and Ras-Raf-MEK-ERK pathways [9]. Ultimately, cyclin-dependent kinase inhibitors, such as p21 and p27, are upregulated leading to inhibition of cell proliferation [8, 63]. Furthermore, SSTR also initiate antitumorigenic effects indirectly through inhibition of adenylate cyclase leading to reduced levels of cAMP and Ca2+ that inhibit the secretion of tumorigenic growth factors and hormones, such as VEGF [27, 62]. Finally, SSA may induce immunomodulatory mechanisms facilitating antitumorigenic effects [45].
In vitro, an antiproliferative activity of octreotide and pasireotide has been documented on meningioma cells, both solitarily and in combination with everolimus [1, 13,14,15]. We could, however, not detect a statistical interaction between SSA and everolimus with effect on the progression and mortality rate. It is probable that selection of tumors defined as treatment-refractory provides a cohort of patients where long-lasting effects are unlikely and cure impossible. Furthermore, distribution and expression of different SSTR subtypes might change in recurring meningiomas. This could impact the efficacy of SSAs with affinity to mainly SSTR2 and SSTR5, and explain escape from response [22]. Recent insights to SSTR 1–5 expression in meningioma show overall lower SSTR expression scores associated with higher WHO grade and differences in SSTR1-5 distribution among meningioma subgroups [3]. Receptor type differences could be a result of the underlying heterogeneous mutational landscape across recurrences due to geographic heterogeneity of the primary tumor [3, 4]. It remains to be resolved how this may affect the antitumorigenic properties of treatment with SSA.
Strengths and limitations
The major strength was the inclusion of ~ 89% of individual patient data from previously published cohorts, thus enabling unique exploration at a personal level comprising most of hitherto SSA-treated meningioma patients. As elaborated above, the primary weaknesses are the small number of patients, a non-randomized study design with no head-to-head comparisons, study, and patient heterogeneity. Notably, none of the currently published studies reported clinicopathologic features and only provided limited data on previous treatment history, which prevented us from identifying features that might predict response. Upcoming studies are encouraged to report these features.
Furthermore, although the radiological assessment protocols differed across the studies, the applied protocols independently constitute acknowledged and widely used assessment schemes within neuro-oncology. Also, the included patients were graded according to the 2007 (n = 58) versus 2016 (n = 77) editions of the WHO classification of CNS tumors. The only difference between the two versions, however, encompasses brain invasion and may only affect meningioma tumors graded as WHO-1 in the 2007 edition. This only affected a fraction of the compiled cohort, and re-classification according to the 2016 edition would unlikely affect the main findings. The 2021 WHO classification of CNS tumors is expected to provide better prognostication of meningioma patients, as it will include TERT promoter mutations and CDKN2A/B homozygous deletions as biomarkers for aggressive phenotypes [35].
Regarding toxicities, it is accepted that including more than 60 patients in phase I trials does not improve detection of clinically relevant toxicities in later large-volume trials [25]. In this context, we consider the 133 included patients feasible for assessment of clinically relevant toxicities in meningioma patients receiving SSA treatment.
A major weakness to all studies that deal with second and third tier therapies are the increasingly heterogenous populations that are offered these therapies. It is generally stated that patients have undergone surgery and have been treated with the therapy in question for MRI confirmed progressive recurrences. The number of surgeries and other previous adjuvant therapies are frequently not described. The quality “intractable” typically reflects professional assessment of a treating physician. We assessed the lack of pretreatment data for five of Schultz’ [53] patients with a sensitivity analysis that did not suggest bias by inclusion of those patients.
Prospective randomization with objective prognostic and response criteria would be necessary to handle heterogeneity and improve traceability in future trials. Still, for the objectives described herein, the presented data compilation of most meningioma patients previously treated with SSA yielded a more reliable effect estimate than obtained by the nine studies individually. We propose a randomized trial of SSA applied to a more homogenous cohort comprising less aggressive meningioma patients, with same radiological protocols, WHO classifications and with sufficient follow-up time to account for variation in meningioma growth kinetics across different WHO grades and over time [21, 36].
Conclusions
We conclude that quality of available evidence was very low. Limitations of present literature complicated exact quantification of SSA treatment-efficacy, and studies were limited to meningioma patients with advanced disease. Still, approximately half of the patients obtained disease control. SSA was associated with transient and manageable toxicities in most cases, which positively support the clinical utility. We conclude that available evidence was insufficient either to discard SSA treatment for meningiomas or implement it for wide clinical use, while our review taken together with established applications for other SSTR-expressing tumors justify a well-designed prospective trial.
Data availability
The manuscript has associated data in data repository.
Code availability
Not applicable.
References
Arena S, Barbieri F, Thellung S, Pirani P, Corsaro A, Villa V, Dadati P, Dorcaratto A, Lapertosa G, Ravetti JL, Spaziante R, Schettini G, Florio T (2004) Expression of somatostatin receptor mRNA in human meningiomas and their implication in in vitro antiproliferative activity. J Neurooncol. https://doi.org/10.1023/B:NEON.0000013498.19981.55
Behling F, Fodi C, Skardelly M et al (2022) Differences in the expression of SSTR1–5 in meningiomas and its therapeutic potential. Neurosurg Rev 45:467–478. https://doi.org/10.1007/s10143-021-01552-y
Behling F, Fodi C, Skardelly M, Renovanz M, Castaneda S, Tabatabai G, Honegger J, Tatagiba M, Schittenhelm J (2021) Differences in the expression of SSTR1-5 in meningiomas and its therapeutic potential. Neurosurg Rev. https://doi.org/10.1007/s10143-021-01552-y
Bi WL, Greenwald NF, Abedalthagafi M, Wala J, Gibson WJ, Agarwalla PK, Horowitz P, Schumacher SE, Esaulova E, Mei Y, Chevalier A, A Ducar M, Thorner AR, van Hummelen P, O Stemmer-Rachamimov A, Artyomov M, Al-Mefty O, Dunn GP, Santagata S, Dunn IF, Beroukhim R (2017) Erratum: genomic landscape of high-grade meningiomas. NPJ Genom Med 2:26. https://doi.org/10.1038/s41525-017-0014-7
Cardona AF, Ruiz-Patiño A, Zatarain-Barrón ZL, Hakim F, Jiménez E, Mejía JA, Ramón JF, Useche N, Bermúdez S, Pineda D, Cifuentes H, Rojas L, Ricaurte L, Pino LE, Balaña C, Arrieta O (2019) Systemic management of malignant meningiomas: a comparative survival and molecular marker analysis between Octreotide in combination with Everolimus and Sunitinib. PLoS One 14:1–13. https://doi.org/10.1371/journal.pone.0217340
Chamberlain M, Glantz MJ, Fadul CE (2007) Recurrent meningioma—salvage therapy with long-acting somatostain analogue. Neurology 69:969–973
Clark VE, Erson-Omay EZ, Serin A, Yin J, Cotney J, Ozduman K, Avşar T, Li J, Murray PB, Henegariu O, Yilmaz S, Günel JM, Carrión-Grant G, Yilmaz B, Grady C, Tanrikulu B, Bakircioğlu M, Kaymakçalan H, Caglayan AO, Sencar L, Ceyhun E, Atik AF, Bayri Y, Bai H, Kolb LE, Hebert RM, Omay SB, Mishra-Gorur K, Choi M, Overton JD, Holland EC, Mane S, State MW, Bilgüvar K, Baehring JM, Gutin PH, Piepmeier JM, Vortmeyer A, Brennan CW, Pamir MN, Kiliç T, Lifton RP, Noonan JP, Yasuno K, Günel M (2013) Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science 339:1077–1080. https://doi.org/10.1126/science.1233009
Ferjoux G, Lopez F, Esteve JP, Ferrand A, Vivier E, Vely F, Saint-Laurent N, Pradayrol L, Buscail L, Susini C (2003) Critical role of Src and SHP-2 in sst2 somatostatin receptor-mediated activation of SHP-1 and inhibition of cell proliferation. Mol Biol Cell. https://doi.org/10.1091/mbc.E03-02-0069
Florio T (2008) Somatostatin/somatostatin receptor signalling: phosphotyrosine phosphatases. Mol Cell Endocrinol 286:40–48. https://doi.org/10.1016/j.mce.2007.08.012
Furtner J, Schöpf V, Seystahl K, Le Rhun E, Rudà R, Roelcke U, Koeppen S, Berghoff AS, Marosi C, Clement P, Faedi M, Watts C, Wick W, Soffietti R, Weller M, Preusser M, Seystahl K, Le RE, Ruda R, Scho V, Koeppen S, Berghoff AS, Marosi C, Clement P, Faedi M, Watts C, Wick W, Soffietti R, Weller M, Preusser M (2016) Kinetics of tumor size and peritumoral brain edema before, during, and after systemic therapy in recurrent WHO grade II or III meningioma. Neuro Oncol 18:401–407. https://doi.org/10.1093/neuonc/nov183
García-Luna, P.P., Relimpio, F., Pumar, A., Pereira, J.L., Leal-Cerro, A., Trujillo, F., Cortes, A., & Astorga, R. (1993). Clinical use of octreotide in unresectable meningiomas. A report of three cases. J Neurol Sci 37(4):237–41
Goldbrunner R, Stavrinou P, Jenkinson MD, Sahm F, Mawrin C, Weber DC, Preusser M, Minniti G, Lund-Johansen M, Lefranc F, Houdart E, Sallabanda K, Le Rhun E, Nieuwenhuizen D, Tabatabai G, Soffietti R, Weller M (2021) EANO guideline on the diagnosis and management of meningiomas. Neuro Oncol. https://doi.org/10.1093/neuonc/noab150
Graillon T, Defilles C, Mohamed A, Lisbonis C, Germanetti AL, Chinot O, Figarella-Branger D, Roche PH, Adetchessi T, Fuentes S, Metellus P, Dufour H, Enjalbert A, Barlier A (2015) Combined treatment by octreotide and everolimus: octreotide enhances inhibitory effect of everolimus in aggressive meningiomas. J Neurooncol 124:33–43. https://doi.org/10.1007/s11060-015-1812-3
Graillon T, Romano D, Defilles C, Lisbonis C, Saveanu A, Figarella-Branger D, Roche P-H, Fuentes S, Chinot O, Dufour H, Barlier A (2017) Pasireotide is more effective than octreotide, alone or combined with everolimus on human meningioma <i>in vitro</i>. Oncotarget 8:55361–55373. https://doi.org/10.18632/oncotarget.19517
Graillon T, Romano D, Defilles C, Saveanu A, Mohamed A, Figarella-Branger D, Roche P-H, Fuentes S, Chinot O, Dufour H, Barlier A (2017) Octreotide therapy in meningiomas: in vitro study, clinical correlation, and literature review. J Neurosurg 127:660–669. https://doi.org/10.3171/2016.8.JNS16995
Graillon T, Sanson M, Campello C, Idbaih A, Peyre M, Peyriere H, Basset N, Autran D, Roche C, Kalamarides M, Roche PH, Fuentes S, Tabouret E, Barrie M, Cohen A, Honore S, Boucekine M, Baumstarck K, Figarella-Branger D, Barlier A, Dufour H, Chinot OL, Graillon T, Sanson M, Peyre M, Peyrière H, Autran D, Kalamarides M, Roche P-H, Fuentes S, Tabouret E, Barrie M, Campello C, Idbaih A, Boucekine M, Figarella-Branger D, Chinot OL (2020) Everolimus and octreotide for patients with recurrent meningioma: results from the phase II CEVOREM trial. Clin Cancer Res 26:552–557. https://doi.org/10.1158/1078-0432.CCR-19-2109
Guillermet-Guibert J, Saint-Laurent N, Davenne L, Rochaix P, Cuvillier O, Culler MD, Pradayrol L, Buscail L, Susini C, Bousquet C (2007) Novel synergistic mechanism for sst2 somatostatin and TNFalpha receptors to induce apoptosis: crosstalk between NF-kappaB and JNK pathways. Cell Death Differ 14:197–208. https://doi.org/10.1038/sj.cdd.4401939
Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso-Coello P, Falck-Ytter Y, Jaeschke R, Vist G, Akl EA, Post PN, Norris S, Meerpohl J, Shukla VK, Nasser M, Schünemann HJ (2011) GRADE guidelines: 8. Rating the quality of evidence—indirectness. J Clin Epidemiol 64:1303–1310. https://doi.org/10.1016/j.jclinepi.2011.04.014
Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, Montori V, Akl EA, Djulbegovic B, Falck-Ytter Y, Norris SL, Williams JW, Atkins D, Meerpohl J, Schünemann HJ (2011) GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol 64:407–415. https://doi.org/10.1016/j.jclinepi.2010.07.017
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336:924–926. https://doi.org/10.1136/bmj.39489.470347.AD
Haslund-Vinding J, Skjoth-Rasmussen J, Poulsgaard L, Fugleholm K, Mirian C, Maier AD, Santarius T, Rom Poulsen F, Meling T, Bartek JJ, Förander P, Larsen VA, Kristensen BW, Scheie D, Law I, Ziebell M, Mathiesen T (2021) Proposal of a new grading system for meningioma resection: the Copenhagen Protocol. Acta Neurochir (Wien). https://doi.org/10.1007/s00701-021-05025-5
Hofland LJ, Lamberts SW (1996) Somatostatin receptors and disease: role of receptor subtypes. Baillieres Clin Endocrinol Metab 10:163–176. https://doi.org/10.1016/s0950-351x(96)80362-4
Hrachova M, Nguyen ENT, Fu BD, Dandekar MJ, Kong XT, Cadena G, Hsu FPK, Billimek J, Taylor TH, Bota DA (2020) A retrospective interventional cohort study to assess the safety and efficacy of sandostatin LAR for treatment of recurrent and/or refractory meningiomas. Front Neurol 11:1–11. https://doi.org/10.3389/fneur.2020.00373
Jaffrain-Rea ML, Minniti G, Santoro A, Bastianello S, Tamburrano G, Gulino A, Cantore G (1998) Visual improvement during octreotide therapy in a case of episellar meningioma. Clin Neurol Neurosurg 100(1):40–3. https://doi.org/10.1016/S0303-8467(97)00110-8
Jardim DL, Hess KR, Lorusso P, Kurzrock R, Hong DS (2014) Predictive value of phase I trials for safety in later trials and final approved dose: analysis of 61 approved cancer drugs. Clin Cancer Res 20:281–288. https://doi.org/10.1158/1078-0432.CCR-13-2103
Johnson DR, Kimmel DW, Burch PA, Cascino TL, Giannini C, Wu W, Buckner JC (2011) Phase II study of subcutaneous octreotide in adults with recurrent or progressive meningioma and meningeal hemangiopericytoma. Neuro Oncol 13(5):530–5. https://doi.org/10.1093/neuonc/nor044
Lamszus K, Lengler U, Schmidt NO, Stavrou D, Ergün S, Westphal M (2000) Vascular endothelial growth factor, hepatocyte growth factor/scatter factor, basic fibroblast growth factor, and placenta growth factor in human meningiomas and their relation to angiogenesis and malignancy. Neurosurgery 46(4):938–47. https://doi.org/10.1097/00006123-200004000-00033
Kaley T, Barani I, Chamberlain M, McDermott M, Panageas K, Raizer J, Rogers L, Schiff D, Vogelbaum M, Weber D, Wen P (2014) Historical benchmarks for medical therapy trials in surgery- and radiation-refractory meningioma: a RANO review. Neuro Oncol 16:829–840. https://doi.org/10.1093/neuonc/not330
Kyriakakis N, Seejore K, Hanafy A, Murray RD (2020) Management of persistent acromegaly following primary therapy: The current landscape in the UK. Endocrinol Diabetes Metab 3(3)e00158. https://doi.org/10.1002/edm2.158
Lopez F, Estève JP, Buscail L, Delesque N, Saint-Laurent N, Théveniau M, Nahmias C, Vaysse N, Susini C (1997) The tyrosine phosphatase SHP-1 associates with the sst2 somatostatin receptor and is an essential component of sst2-mediated inhibitory growth signaling. J Biol Chem 272:24448–24454. https://doi.org/10.1074/jbc.272.39.24448
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23:1231–1251. https://doi.org/10.1093/neuonc/noab106
Maier AD, Stenman A, Svahn F, Mirian C, Bartek JJ, Juhler M, Zedenius J, Broholm H, Mathiesen T (2020) TERT promoter mutations in primary and secondary WHO grade III meningioma. Brain Pathol. https://doi.org/10.1111/bpa.12892
Mirian C, Duun-Henriksen AK, Juratli T, Sahm F, Spiegl-Kreinecker S, Peyre M, Biczok A, Tonn JC, Goutagny S, Bertero L, Maier AD, Møller Pedersen M, Law I, Broholm H, Cahill DP, Brastianos P, Poulsgaard L, Fugleholm K, Ziebell M, Munch T, Mathiesen T (2020) Poor prognosis associated with TERT gene alterations in meningioma is independent of the WHO classification: an individual patient data meta-analysis. J Neurol Neurosurg Psychiatry 91(4):378–387. https://doi.org/10.1136/jnnp-2019-322257
Mirian C, Duun-Henriksen AK, Maier AD, Pedersen MM, Jensen LR, Bashir A, Graillon T, Hrachova M, Bota D, van Essen M, Spanjol P, Kreis C, Law I, Broholm H, Poulsgaard L, Fugleholm K, Ziebell M, Munch T, Walter MA, Mathiesen T (2020) Somatostatin receptor-targeted radiopeptide therapy in treatment-refractory meningioma: an individual patient data meta-analysis. J Nucl Med. https://doi.org/10.2967/jnumed.120.249607
Mirian C, Grell K, Juratli TA, Sahm F, Spiegl-Kreinecker S, Peyre M, Biczok A, Tonn JC, Goutagny S, Bertero L, Maier AD, Jensen LR, Schackert G, Broholm H, Scheie D, Cahill DP, Brastianos PK, Skjøth-Rasmussen J, Fugleholm K, Ziebell M, Munch TN, Kristensen BW, Mathiesen T (2021) Implementation of TERT promoter mutations improve prognostication of the WHO classification in meningioma. Neuropathol Appl Neurobiol. https://doi.org/10.1111/nan.12773
Mirian C, Skyrman S, Bartek J, Jensen LR, Kihlström L, Förander P, Orrego A, Mathiesen T (2020) The Ki-67 proliferation index as a marker of time to recurrence in intracranial meningioma. Neurosurgery. https://doi.org/10.1093/neuros/nyaa226
Nassiri F, Liu J, Patil V, Mamatjan Y, Wang JZ, Hugh-White R, Macklin AM, Khan S, Singh O, Karimi S, Corona RI, Liu LY, Chen CY, Chakravarthy A, Wei Q, Mehani B, Suppiah S, Gao A, Workewych AM, Tabatabai G, Boutros PC, Bader GD, de Carvalho DD, Kislinger T, Aldape K, Zadeh G (2021) A clinically applicable integrative molecular classification of meningiomas. Nature 597:119–125. https://doi.org/10.1038/s41586-021-03850-3
National Cancer Institute (2006) Common terminology criteria for adverse events v3.0 (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf
National Cancer Institute (2009) Common terminology criteria for adverse events v4.0 (CTCAE) NIH Publ. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf
Norden AD, Ligon KL, Hammond SN, Muzikansky A, Reardon DA, Kaley TJ, Batchelor TT, Plotkin SR, Raizer JJ, Wong ET, Drappatz J, Lesser GJ, Haidar S, Beroukhim R, Lee EQ, Doherty L, Lafrankie D, Gaffey SC, Gerard M, Smith KH, McCluskey C, Phuphanich S, Wen PY (2015) Phase II study of monthly pasireotide LAR (SOM230C) for recurrent or progressive meningioma. Neurology 84:280–286. https://doi.org/10.1212/WNL.0000000000001153
Öberg K, Lamberts SWJ (2016) Somatostatin analogues in acromegaly and gastroenteropancreatic neuroendocrine tumours: past, present and future. Endocr Relat Cancer 23:R551–R566. https://doi.org/10.1530/ERC-16-0151
Ortolá Buigues A, Crespo Hernández I, Jorquera Moya M, Díaz Pérez JÁ (2016) Unresectable recurrent multiple meningioma: a case report with radiological response to somatostatin analogues. Case Rep Oncol 9:520–525. https://doi.org/10.1159/000448212
Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS (2015) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol 17:iv1–iv62. https://doi.org/10.1093/neuonc/nov189
Papa S, Bubici C, Zazzeroni F, Pham CG, Kuntzen C, Knabb JR, Dean K, Franzoso G (2006) The NF-kappaB-mediated control of the JNK cascade in the antagonism of programmed cell death in health and disease. Cell Death Differ 13:712–729. https://doi.org/10.1038/sj.cdd.4401865
Pyronnet S, Bousquet C, Najib S, Azar R, Laklai H, Susini C (2008) Antitumor effects of somatostatin. Mol Cell Endocrinol. https://doi.org/10.1016/j.mce.2008.02.002
Rammo R, Rock A, Transou A, Raghunathan A, Rock J (2016) Anaplastic meningioma: octreotide therapy for a case of recurrent and progressive intracranial disease. J Neurosurg 124:496–500. https://doi.org/10.3171/2015.1.JNS142260
Reuss DE, Piro RM, Jones DTW, Simon M, Ketter R, Kool M, Becker A, Sahm F, Pusch S, Meyer J, Hagenlocher C, Schweizer L, Capper D, Kickingereder P, Mucha J, Koelsche C, Jäger N, Santarius T, Tarpey PS, Stephens PJ, Andrew Futreal P, Wellenreuther R, Kraus J, Lenartz D, Herold-Mende C, Hartmann C, Mawrin C, Giese N, Eils R, Collins VP, König R, Wiestler OD, Pfister SM, von Deimling A (2013) Secretory meningiomas are defined by combined KLF4 K409Q and TRAF7 mutations. Acta Neuropathol 125:351–358. https://doi.org/10.1007/s00401-013-1093-x
Rinke A, Müller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, Mayer C, Aminossadati B, Pape UF, Bläker M, Harder J, Arnold C, Gress T, Arnold R (2009) Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID study group. J Clin Oncol. https://doi.org/10.1200/JCO.2009.22.8510
Rünzi MW, Jaspers C, Windeck R, Benker G, Mehdorn HM, Reinhardt V, Reinwein D (1989) Successful treatment of meningioma with octreotide. Lancet 333:1074. https://doi.org/10.1016/S0140-6736(89)92465-3
Sahm F, Schrimpf D, Stichel D, Jones DTW, Hielscher T, Schefzyk S, Okonechnikov K, Koelsche C, Reuss DE, Capper D, Sturm D, Wirsching H-G, Berghoff AS, Baumgarten P, Kratz A, Huang K, Wefers AK, Hovestadt V, Sill M, Ellis HP, Kurian KM, Okuducu AF, Jungk C, Drueschler K, Schick M, Bewerunge-Hudler M, Mawrin C, Seiz-Rosenhagen M, Ketter R, Simon M, Westphal M, Lamszus K, Becker A, Koch A, Schittenhelm J, Rushing EJ, Collins VP, Brehmer S, Chavez L, Platten M, Hanggi D, Unterberg A, Paulus W, Wick W, Pfister SM, Mittelbronn M, Preusser M, Herold-Mende C, Weller M, von Deimling A (2017) DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. Lancet Oncol 18:682–694. https://doi.org/10.1016/S1470-2045(17)30155-9
Schreglmann SR, Jelčić I, Taegtmeyer AB, Linnebank M, Weller M (2013) Multifocal CNS demyelination after octreotide treatment for metastatic meningioma. Clin Neurol Neurosurg 115:817–819. https://doi.org/10.1016/j.clineuro.2012.07.021
Schuler M, Green DR (2001) Mechanisms of p53-dependent apoptosis. Biochem Soc Trans 29:684–688. https://doi.org/10.1042/0300-5127:0290684
Schulz C, Ulm B, Kunz U, Mathieu R, Kunz U, Mauer UM (2011) Treatment of unresectable skull base meningiomas with somatostatin analogs. Neurosurg Focus 30:E11. https://doi.org/10.3171/2011.1.FOCUS111
Seystahl K, Stoecklein V, Schuller U, Rushing E, Nicolas G, Schafer N, Ilhan H, Pangalu A, Weller M, Tonn J-C, Sommerauer M, Albert NL (2016) Somatostatin receptor-targeted radionuclide therapy for progressive meningioma: benefit linked to 68Ga-DOTATATE/-TOC uptake. Neuro Oncol 18:1538–1547. https://doi.org/10.1093/neuonc/now060
Shimatsu A, Nakakuki T, Kimura T, Hagiwara H, Usui T, Nagata D, Tagami T, Naruse M, Tsukahara T (2006) Meningioma in an acromegalic patient treated with long-term octreotide therapy. Front Neuroendocrinol. https://doi.org/10.1016/j.yfrne.2006.03.194
Sievers P, Hielscher T, Schrimpf D, Stichel D, Reuss DE, Berghoff AS, Neidert MC, Wirsching H-G, Mawrin C, Ketter R, Paulus W, Reifenberger G, Lamszus K, Westphal M, Etminan N, Ratliff M, Herold-Mende C, Pfister SM, Jones DTW, Weller M, Harter PN, Wick W, Preusser M, von Deimling A, Sahm F (2020) CDKN2A/B homozygous deletion is associated with early recurrence in meningiomas. Acta Neuropathol 140:409–413. https://doi.org/10.1007/s00401-020-02188-w
Simó M, Argyriou AA, Macià M, Plans G, Majós C, Vidal N, Gil M, Bruna J (2014) Recurrent high-grade meningioma: a phase II trial with somatostatin analogue therapy. Cancer Chemother Pharmacol 73(5):919–23. https://doi.org/10.1007/s00280-014-2422-z
Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan A-W, Churchill R, Deeks JJ, Hrobjartsson A, Kirkham J, Juni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schunemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919. https://doi.org/10.1136/bmj.i4919
Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, Tierney JF (2015) Preferred Reporting Items for Systematic Review and Meta-analyses of Individual Participant Data: the PRISMA-IPD statement. JAMA 313:1657–1665. https://doi.org/10.1001/jama.2015.3656
Stueven AK, Kayser A, Wetz C, Amthauer H, Wree A, Tacke F, Wiedenmann B, Roderburg C, Jann H (2019) Somatostatin analogues in the treatment of neuroendocrine tumors: past, present and future. Int J Mol Sci 20(12):3049. https://doi.org/10.3390/ijms20123049
Thangaraju M, Sharma K, Leber B, Andrews DW, Shen SH, Srikant CB (1999) Regulation of acidification and apoptosis by SHP-1 and Bcl-2. J Biol Chem 274:29549–29557. https://doi.org/10.1074/jbc.274.41.29549
Villaume K, Blanc M, Gouysse G, Walter T, Couderc C, Nejjari M, Vercherat C, Cordier-Bussat M, Roche C, Scoazec JY (2010) VEGF secretion by neuroendocrine tumor cells is inhibited by octreotide and by inhibitors of the PI3K/AKT/mTOR pathway. Neuroendocrinology. https://doi.org/10.1159/000289569
Weckbecker G, Lewis I, Albert R, Schmid HA, Hoyer D, Bruns C (2003) Opportunities in somatostatin research: biological, chemical and therapeutic aspects. Nat Rev Drug Discov 2:999–1017. https://doi.org/10.1038/nrd1255
Youngblood MW, Miyagishima DF, Jin L, Gupte T, Li C, Duran D, Montejo JD, Zhao A, Sheth A, Tyrtova E, Özduman K, Iacoangeli F, Peyre M, Boetto J, Pease M, Avşar T, Huttner A, Bilguvar K, Kilic T, Pamir MN, Amankulor N, Kalamarides M, Erson-Omay EZ, Günel M, Moliterno J (2021) Associations of meningioma molecular subgroup and tumor recurrence. Neuro Oncol 23:783–794. https://doi.org/10.1093/neuonc/noaa226
Acknowledgements
We thank the Statistical Advisory Board at the University of Copenhagen for their supervision of this project
Funding
Unrelated to this work, Christian Mirian is funded by The Novo Nordisk Foundation Grant No. 0052813.
Unrelated to this work, Mathias Preusser has received honoraria for lectures, consultation or advisory board participation from the following for-profit companies: Bayer, Bristol-Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, BMJ Journals, MedMedia, Astra Zeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp & Dome, Tocagen, Adastra, and Gan & Lee Pharmaceuticals.
Author information
Authors and Affiliations
Contributions
Lasse Rehné Jensen, Tiit Mathiesen, and Christian Mirian conceived and designed the study. Lasse Rehné Jensen and Christian Mirian reviewed articles independently and extracted the data jointly. Tiit Mathiesen, Maya Hrachova, Daniela Bota, Alejandro Ruiz-Patiño, Oscar Arrieta, Andrés Felipe Cardona, Roberta Rudà, Julia Furtner, Ulrich Roeckle, Paul Clement, and Matthias Preusser provided data from original articles. Analyzed and interpreted: Lasse Rehné Jensen, Christian Mirian, and the Statistical Advisory Board at the University of Copenhagen analyzed and interpreted the data. Writing: Lasse Rehné Jensen, Tiit Mathiesen, and Christian Mirian. Critical revision: All authors. All authors agreed with the results and conclusions and approved the final draft.
Corresponding author
Ethics declarations
Ethics approval
Included data was untraceable, anonymized patient data that already has been published previously, thus not requiring Institutional Review Board approval by Danish law.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
PROSPERO: CRD42019119140.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jensen, L.R., Maier, A.D., Lomstein, A. et al. Somatostatin analogues in treatment-refractory meningioma: a systematic review with meta-analysis of individual patient data. Neurosurg Rev 45, 3067–3081 (2022). https://doi.org/10.1007/s10143-022-01849-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-022-01849-6