Abstract
Twiddler’s syndrome (TS) is a hardware-related complication of deep brain stimulation which has not been well documented and is probably underreported. The objective of this study is to comprehensively describe TS by systematically reviewing the related literature. The methods include selecting the eligible studies based on the inclusion and exclusion criteria. Data about studies and TS were collected. A descriptive statistical analysis of the extracted data was performed. We found 18 eligible studies describing 23 patients with TS. The mean age of the 23 patients was 61.4 ± 15.9 years (range, 16–79 years.). The percentage of TS in the female population was 91.3% (females: 21/23). The incidence of postoperative TS was 1.4% (6 out of 437) per patient and 1.1% (8 out of 709) per extension wire. The mean time to clinical presentation was 9.9 ± 10.3 months (range, 0.5–36 months). Nineteen of the twenty-three patients presented with a rebound of previous symptoms. Twelve of the twenty-three patients had high impedance at the postoperative checkup of the DBS system. A plain X-ray indicated twisted extension wires in almost all these patients. All patients meeting the definition of postoperative device-related TS underwent revision surgery. TS is more prevalent in females. Based on the typical clinical symptoms (rebound of the previous symptoms, high impedance, and X-ray demonstration), the differential diagnosis can often be straightforward. TS should thus be taken into consideration when attempting to explain or rule out hardware malfunction. The timely recognition and proper revision of TS can prevent further serious damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Twiddler’s syndrome (TS) was first reported in patients fitted with a cardiac pacemaker in 1968 [1]. It is defined as a hardware malfunction caused by spontaneous rotation or intentional manipulation of the implanted pulse generator (IPG) in its subcutaneous pocket by the patient [1, 2]. TS was characterized as a rare yet dangerous complication.
To date, three variants of TS (reeling, ratchet, and coiling) have been described by cardiologists, based on the type of twisted pathogenesis. Reeling TS is caused by the rotation of the pacemaker around the Z-axis perpendicular to the horizontal plane of the generator, which eventually results in lead displacement and extraction [3, 4]. Ratchet TS results from excessive rotation of the pacemaker along the horizontal axis (X-axis) in a craniocaudal direction, where the oscillating movement gathers the pacer leads, rather than continuous rotational forces [5]. This kind of rotation and oscillating movement can result in the twisting of the leads and stepwise retraction of the leads toward the pacemaker [5]. Coiling TS is caused by the rotation of the pacemaker along the vertical axis of the pacer (Y-axis) in a lateral-medial direction [6]. The damage resulting from this kind of TS is similar to that of the reeling TS.
TS in patients with deep brain stimulation (DBS) surgery has only been described recently [7, 8]. However, it may be an underdiagnosed and underreported complication in DBS surgery. Given the growing number of DBS systems implanted for pathologies such as Parkinson’s disease (PD), dystonia, essential tremor (ET), epilepsy, and obsessive–compulsive disorder (OCD), TS as a cause of DBS hardware malfunction should be taken into account. Further damage to the intracranial electrode(s) can be prevented, and the adverse events (such as infections and intracranial hemorrhage) caused by removal and re-implantation of the electrode(s) can be avoided if TS is promptly identified and remedied [9, 10].
This systematic review describes and summarizes the characteristics, management, and preventive measures of TS which can contribute to better management of this complication in DBS patients.
Methods
Search strategy
A systematic review was conducted by searching PubMed (National Library of Medicine), EBSCO, Ovid-Medline, and Green Medical using the keywords deep brain stimulation (or deep brain stimulator) AND any of the following: Twiddler’s syndrome OR reel syndrome OR Ratchet syndrome OR coiling syndrome OR twist syndrome OR generator rotation OR twist of extension wire OR bowstringing OR wire tethering OR complications OR adverse events OR side effects. The search covered the period from the inception of each database to August 2020. A targeted search of the bibliographies and references to relevant articles was also performed to identify additional studies.
Study selection
Two assessors (XW. L and YY. X) screened each paper and a consensus was required for an article to be included. The inclusion criteria were as follows: (1) the selected articles were required to have the keywords (used for search) within the title and/or abstract; (2) the original full-text articles were published in English; (3) the study with the most complete and recent data was chosen, in case of partly overlapping populations reported by the same group. The exclusion criteria were as follows: (1) the articles were about a non-human study; (2) the studies did not report TS or complications caused by generator rotation or twisted extension wires; (3) the study did not provide detailed original data on patients with TS; (4) the full text was not available.
Data extraction
The data for eligible studies were independently extracted by two researchers (XW. L and YY. X). Then, the data on study characteristics, the epidemiological characteristics of patients, the parameters of the initial DBS treatment, and information about TS were collected.
Data analysis
A descriptive statistical analysis of the extracted data was performed with Spss23.
Results
Search results
The systematic search identified 3267 articles. After a review of the titles and abstracts, 20 articles [2, 7, 9, 11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27] were retained for full-text screening. Of these 20 studies, four studies were excluded because they met the exclusion criteria or were not in line with the inclusion criteria [13, 20, 22, 26]. Another 2 articles [8, 10] were included after the references for the 20 full-text reports were searched and assessed. Therefore, 18 studies were included in the final database for the systematic review [2, 7,8,9,10,11,12, 14,15,16,17,18,19, 21, 23,24,25, 27] (Fig. 1).
Study characteristics
The characteristics of the included studies and cases are summarized in Table 1. Of these studies, sixteen were case reports [7,8,9,10,11, 14,15,16,17,18,19, 21, 23,24,25, 27] and two were retrospective studies [2, 12]. The prevalence rate of TS in the DBS population was only reported in two articles [2, 12]. The first study on TS related to DBS was published in 2005 [8]. The disorders investigated were PD (12/23, 52.3%), ET (6/23, 26.1%), dystonia (2/23, 8.7%), epilepsy (1/23, 4.3%), OCD (1/23, 4.3%), and Tourette’s syndrome (1/23, 4.3%).
Patient characteristics
TS occurred in 23 patients and in 44 DBS extension wires or leads. There was 1 (4.4%) pediatric patient (< 18 years old), 5 (21.7%) middle-aged patients (aged 18–60), and 17 (73.9%) elderly patients (≥ 60 years old). The mean (± standard deviation [SD]) age of all patients was 61.4 ± 15.9 years (range, 16–79 yrs.). The mean (± SD) time to clinical presentation was 9.9 ± 10.3 months (range, 0.5–36 months). The prevalence of TS was higher in females (females: 21/23, 91.3%). Four (17.4%) patients were obese. The details on the initial DBS surgery are provided in Table 1.
TS: incidence and device characteristics
The incidence of postoperative TS was 1.4% (6/437) per patient. The risk of TS per extension wire was determined by assuming that a TS to a single-channel IPG or to a single lead extender was associated with one extension wire, whereas a TS associated with a dual-channel IPG or with two contiguous lead extenders was associated with two extension wires. For patients with recurring TS, the total number of extension wires was equal to the sum of the number of extension wires involved at the time of TS. Thus, the risk rate was 1.1% (8/709) per extension wire. Details are described in Table 2, based on the studies [2, 12].
TS: clinical presentation
Patients could have more than one presenting symptom. As shown in Table 3, 19 patients presented with a rebound of previous symptoms; 8 patients reported discomfort (such as tightening or a bowstringing sensation) and 5 patients felt pain at the device implantation site (such as the chest, neck, or retroauricular region); IPGs rotating in the subcutaneous pocket and difficulties in recharging the IPGs were reported in 3 and 1 patients, respectively.
In physical examinations, the abnormal signs included thickening or knobby structures of the extension cable and excessive movement of IPG in the subcutaneous pocket (Table 3). The examination of the DBS system revealed high impedance in 12 (12/23) patients and normal impedance in 5 (5/23) patients (Table 3). The plain X-ray exams indicated that almost all of the patients had twisted extension wires, and that some patients also had fractured extension cables or leads, or migrated IPG, connectors, or intracranial electrodes (Table 3).
Possible causes of TS
Six patients admitted that they intentionally manipulated the IPG, while sixteen patients denied any manipulation (Table 3). One elderly patient whose BMI (body mass index) was 46.7 occasionally felt as though the IPG were positioned perpendicularly within the pocket. He manipulated the IPG to relieve these sensations[12].
Management of TS
All patients meeting the definition of postoperative device-related TS underwent revision surgery (Table 3). Most patients with obvious hardware malfunctions were managed by replacing the dysfunctional devices, reconnecting the disconnected part, fixing the migrating devices, and/or reducing the subcutaneous pocket. Two patients were managed by repositioning the intracranial electrodes and replacing the extension wires, due to the displaced intracranial electrodes. Four patients who had normal impedance were simply managed by untwisting and (or) fixation of the extension wires and IPG, and/or by reduction of the subcutaneous pocket.
Nineteen patients recovered within follow-up period, whereas four patients with recurrent TS required further treatment before resolution. Two patients developed hardware-related infections after the revision operation.
Discussion
TS was first described in 1968 as one of the reasons for hardware failure of cardiac pacemakers, which could be life-threatening for patients who were dependent on this device [1]. Reports of TS complications related to DBS implantation have begun to accumulate with the concomitant increase in such surgeries for movement and certain mental disorders.
The incidence of TS in patients with DBS
TS, although an uncommon complication of DBS surgery, was reported in 1.4% of the patients and in 1.1% of the extension wires (Table 2). This complication may be underreported, because of underdiagnosis. For example, certain authors suggested that the lead fracture was caused by trichotillomania and hence did not label the occurrence as TS [8]. In actuality, the patient indeed compulsively manipulated her left IPG during the postoperative period and an X-ray revealed severe coiling of the left lead around the connector. Therefore, this patient should have been diagnosed as TS. In addition, TS may have occurred more frequently with older DBS systems and surgical techniques. For instance, some studies have speculated that compared to dual anchoring IPGs, IPGs with a single anchoring hole have a higher risk of TS [2]. However, no reports were found on TS before 2007. The third potential explanation is that DBS patients who feel discomfort may be ignored, especially when checkups indicate that the DBS system exhibits normal impedance. In fact, the twisted extension wires or the flipped IPG may have already been present in these patients [11, 12, 16,17,18]. Therefore, further study with larger samples is needed to confirm the exact incidence of TS.
The clinical presentation
The typical clinical symptom of TS is a sudden loss of efficacy of the DBS, with a rebound of the preexisting symptomatology. The characteristic symptom may at times be accompanied by problems in recharging the IPG or by a local abnormal sensation such as tightening (Table 3). One patient in the cohort reviewed here with TS after DBS only complained of the lead pulling in her left neck [16]. In some cases, physicians may be able to palpate the thickening or knobby structures of the extension cable, and identify excessive movement of the IPG in the subcutaneous pocket [2, 17, 18].
Regardless of the circumstances, when a hardware issue is suspected, the DBS programming should be used to interrogate the impedance for each of the electronic contacts, and a radiological study should also be conducted. The impedance check serves to verify the physical integrity of the DBS system. Retesting the impedance in revision surgery can precisely identify the location of the system malfunction. This procedure may help avoid the replacement of the intracranial electrode in certain cases [28]. High impedance may correspond to a fracture or dislodgement of the implanted hardware, which frequently occurs in TS patients [2, 8, 11, 12, 14, 15, 19, 21, 24, 25]. However, normal impedance values can also present in TS patients [11, 12, 16,17,18]. A simple X-ray of the neck and chest is usually sufficient to confirm or rule out a diagnosis of TS. This will typically show a double-helix or braided pattern of the extension wire(s) or the DBS lead(s), or even a flipped IPG (Fig. 2 and Fig. 3), which was found in almost every case of TS reported here [2, 7,8,9,10,11,12, 14,15,16,17,18,19, 21, 23,24,25, 27]. For the severe cases that may result in intracranial electrode migration or lead fracture, a brain X-ray or MRI should also be envisaged.
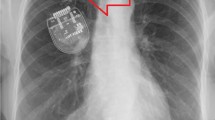
Extensive twisting of the extension leads and the IPG flipping around Y-axis. Tymchak Z, Vitali A (2017) What's the twist? Twiddler’s syndrome in deep brain stimulation. Can J Neurol Sci 44(6): 726–727. https://doi.org/10.1017/cjn.2017.230. Reproduced by permission # 5,106,920,061,509
Extremely coiled connection cable mainly above the IPG which rotated along the Z-axis. Sobstyl M, Ząbek M, Brzuszkiewicz-Kuźmicka G, Pasterski T (2017) Dual anchor internal pulse generator technique may lower risk of Twiddler’s syndrome: a case series and literature review. Neuromodulation 20(6): 606–612. https://doi.org/10.1111/ner.12581. Reproduced by permission # 5,106,920,645,742
Differential diagnosis of TS
The sudden recurrence of symptoms after DBS implantation can also be caused by other hardware failures or battery depletion, which should be distinguished from TS [21, 29]. Local discomfort in TS patients should be differentiated from pure wire tethering or ‘bowstringing’ which arises from the formation of scar tissue adhesions around the DBS extension wire(s) [30, 31]. In these patients without hardware rotation, the typical imaging of the extension wires and leads shows two radio-opaque metal cables extending side by side (Fig. 4) [7, 12].
The different imaging of normal lead and twisted lead. Geissinger G, Neal J (2007) Spontaneous Twiddler's syndrome in a patient with a deep brain stimulator. Surg Neurol 68(4): 454–456; discussion 456. https://doi.org/10.1016/j.surneu.2006.10.062. Reproduced by permission # 5,106,920,645,742
Causes of TS
Certain causative factors can be deduced from a detailed analysis of the postoperative history of patients’ complaints. One of the key culprits is intentional manipulation of DBS hardware by its bearer [2, 8, 9, 14, 19]. However, many patients state that they have not manipulated the device [2, 7, 10, 11, 15,16,17,18, 21, 23,24,25, 27]. In this case, one etiology may be spontaneous rotation of the IPG. Unconscious or subconscious manipulation is nevertheless very probable since extensive coiling is not likely to occur spontaneously. Sometimes, spontaneous rotation and manipulation may contribute to each other [12].
Risk factors of TS
Several factors may trigger spontaneous rotation of the IPG, or induce patients to intentionally or unintentionally manipulate the DBS hardware. TS is more likely to occur in female patients (91.3%). One possible reason is anatomical in that women tend to have loose tissue which facilitates the rotation of the IPG. Advanced age, obesity, and/or sudden weight loss may be physical risk factors for TS [7,8,9, 11, 12, 14, 15, 23, 25], but this supposition also requires larger sample sizes before drawing a definitive conclusion. In our systematic review, the percentages of TS patients who are above 60 and obese are 73.9% and 17.4%, respectively. Out of the 23 TS patients, 12 (52.3%) patients have PD. The possibility that PD tends to occur in elderly patients thus cannot be excluded. The frequency of TS may increase if patients engage in more physical activity too soon after DBS surgery, such as exercising, resuming household chores, or having a high incidence of epilepsy or tremor of the upper limbs [7, 10, 19, 23].
Pre- or postoperative psychiatric disorders can lead to TS [8, 12, 14, 19, 24]. Patients who have dementia, dysgnosia, or confusion are at risk of TS because they have difficulty remembering or complying with instructions to leave the implanted DBS devices alone [11, 12]. Patients with trichotillomania, Tourette’s syndrome, or OCD are prone to TS because disinhibition or impulsiveness can contribute to manipulation of the newly implanted DBS device [8, 11, 12, 14, 24]. Postoperative feelings of tightening, itching, or any other abnormal sensation in the region of the surgery can trigger an automatic reflex of manipulating the DBS hardware in an effort to relieve discomfort [7, 10,11,12, 15,16,17,18, 25, 27]. The shape and the construction of the IPG itself is also a predisposing factor: the bulky IPG with a single anchoring hole may have a higher risk of prompting twiddling than a dual anchor flat one [2]. Serous collection of fluid around IPG is another likely risk factor, since it can dissolve or weaken the nylon sutures used to fix the IPG [10]. Some surgical techniques may accelerate the development of TS such as inadequate fixation of the IPG, excessive enlargement of the subcutaneous pocket, abdominal pockets, looping of the extension cables outside the pocket, and extension wires exiting the IPG at a right angle [23].
Based on the literature review, there were 3 patients [11, 14, 17] for whom detailed information was provided about the replacement of IPG: non-rechargeable battery was replaced by a rechargeable one in 1 patient, 1 patient continued using the non-rechargeable IPG after revision surgery, and 1 patient switched from a non-rechargeable to a rechargeable battery but then switched back to a non-chargeable one, because of the repetitive occurrence of TS. The first two patients experienced no TS after the first replacement of the IPG. This makes it problematic to determine whether the switch from the non-chargeable to the chargeable battery led to the occurrence of TS or not, because of the limited data.
Table 3 shows that of the 23 patients, 19 experienced TS once, 2 patients experienced TS twice, 1 patient experienced TS three times, and 1 patient four times. However, one of the 19 patients had TS after re-implantation of the DBS system [27]. Previously, she had been implanted with a DBS system, but this was removed due to infection. Therefore, 22 patients had TS after the first IPG implantation, and 5 patients had TS after the corrective operation, which however does not constitute strong evidence that a repeat operation does not lead to the re-occurrence of TS. One possible explanation is that during the revision surgery, the IPGs are correctly attached, the size of the subcutaneous pocket is made smaller, and so on.
Management of TS
Typically, the treatment for TS involves appropriate fixation of the IPG, reduction of the volume of the subcutaneous pocket, replacement of the twisted and malfunctioning extension wire(s), or reconnection of the disconnected parts [2, 7,8,9,10,11,12, 14, 16,17,18, 21, 23,24,25, 27]. The anchoring of IPG should utilize non-absorbable sutures or artificial pouches [17, 25]. The sutures fix the IPG to strong tissue, whereas the artificial pouch fits snugly around the IPG and incites fibrous scarring within the subcutaneous pocket, thereby reducing its mobility. A tight-fitting pocket or an artificial pocket is especially important in obese patients [7, 23]. However, damage to the lead(s) or even migration of the intracranial electrode(s) from the brain parenchyma may be observed in some cases, because the connector transfers the twist momentum to the proximal part. These patients need to undergo the removal of the faulty or mal-located DBS electrode(s) and re-implantation of a new one in addition to the above treatment options. This is more complicated and carries a higher risk of intracranial hemorrhage than the first implantation [32]. One study suggested replacing the IPG in a deeper position, such as a submuscular pocket, due to the repetitive external manipulation of the pulse generator by the patient [19]. The advantages of submuscular positioning are obvious, including a more stably placed IPG, improved esthetic results, and better protection from external trauma. The disadvantages of this therapy comprise a more invasive procedure and more difficulty replacing, reloading, or reprogramming the IPG, especially in overweight patients. One study reported that the implantation of IPG in the suprascapular position was finally optioned for a patient with repetitive recurrence of TS, which proved to be effective [15]. However, it is awkward for patients to access when recharging or interrogating the IPG on the upper back.
Preventive measures for TS
Preventing the development of TS is a better solution than having to manage this complication. Greater attention should be paid to surgical techniques which may be the best way to prevent the complication. Specifically, when creating the subcutaneous pocket, the volume of the pocket should be tailored to the IPG dimensions and the pocket should be in a well-defined plane, either superficial or deep, to the pectoralis fascia [9, 17]. In addition, an artificial pouch is essential in patients with loose subcutaneous tissue [7, 23]. Dual anchor IPG may reduce the frequency of TS [2, 17]. Fixing the connector to the occipitalis fascia prevents transmitting the rotatory forces to the extracranial lead(s) or to the intracranial electrode(s), so that coiling of the proximal part can be avoided [12, 17, 25]. Non-absorbable silk sutures should be used for fixation rather than absorbable ones [17, 25]. Meticulous hemostasis of the subcutaneous pocket can help prevent postoperative seroma or infection[17]. Passing the extension leads along a strict subcutaneous and supra-fascial plane from the pectoralis muscle to the occipitalis muscle may prevent the extension wires from becoming stuck in scar tissue, so patients will not manipulate the implanted DBS hardware to relieve the uncomfortable sensation[17, 19]. Any ‘spare’ loop of extension cables in the generator pocket should not be tucked in tightly or placed entirely behind an anchored IPG. Rather, the surgeon should leave some slack in the same plane rostral to the IPG [17, 19]. Placing IPGs in a sub-fascial position or in the upper back can effectively curtail the occurrence of TS or its repetitive recurrence [15, 19].
However, yet other comprehensive factors should be considered to optimally avoid revision surgery. All patients should undergo meticulous psychiatric pre- and post-operation evaluations, and especially the sub-population of patients with a history of psychiatric disorders [7, 8, 11, 12, 14, 23,24,25]. Psychiatric consultations and therapies should be taken into consideration in these patients to make sure they are aware of the benefits of DBS and are motivated to avoid further manipulation of the IPG [8, 12, 14]. All patients should be educated to see their doctor whenever they feel any discomfort, and should be given a specific warning about the risks of massage of the region [7, 10,11,12, 15,16,17,18, 25, 27]. Patients, especially younger ones, should also be instructed to curtail physical activities for a few months after DBS surgery to reduce physical stress on the system before it has an opportunity to fully heal and scar [7, 10, 19, 23].
Conclusion
TS must be taken into consideration when attempting to explain or rule out hardware malfunctions. DBS multidisciplinary team members should be able to timely recognize and properly resolve TS to prevent further damage, such as migration or fracture of the electrode(s). Neurosurgeons should be familiarized with the risk factors and the preventative measures, in order to reduce the prevalence of this complication in the future. Studies with larger samples should be conducted, since to date most data are derived from case reports without strong quantitative evidence, which constitutes one of the key limitations of this systematic review.
Data availability
Not applicable.
Code availability
Not applicable.
References
Bayliss C, Beanlands D, Baird R (1968) The pacemaker-twiddler’s syndrome: a new complication of implantable transvenous pacemakers. Can Med Assoc J 99(8):371–373
Sobstyl M, Ząbek M, Brzuszkiewicz-Kuźmicka G, Pasterski T (2017) Dual anchor internal pulse generator technique may lower risk of Twiddler’s syndrome: a case series and literature review. Neuromodulation 20(6):606–612. https://doi.org/10.1111/ner.12581
Khalilullah M, Khanna SK, Gupta U, Padmavati S (1979) Pacemaker twiddler’s syndrome: a note on its mechanism. J Cardiovasc Surg (Torino) 20(1):95–100
Mohammad R, Pervaiz A et al (2018) Reel syndrome: an atypical cause for inappropriate shocks in a patient with automated implantable cardioverter defibrillator (AICD). Cureus 10(2):e2237. https://doi.org/10.7759/cureus.2237
Iftikhar H, Saleem M, Nadeem M, Caplan J, Kaji A (2019) A presentation of Twiddler’s syndrome with underlying ratchet mechanism. Cureus 11(2):e4060. https://doi.org/10.7759/cureus.4060
Carrizo AG, Hong PS, Amit G, Healey JS (2016) Double twiddle trouble, a new variant of twiddler syndrome. J Arrhythm 32(3):236–237. https://doi.org/10.1016/j.joa.2016.02.008
Geissinger G, Neal J (2007) Spontaneous twiddler’s syndrome in a patient with a deep brain stimulator. Surg Neurol 68(4):454–456. https://doi.org/10.1016/j.surneu.2006.10.062 (discussion 456)
Machado A, Hiremath G, Salazar F, Rezai A (2005) Fracture of subthalamic nucleus deep brain stimulation hardware as a result of compulsive manipulation: case report. Neurosurgery 57(6):E1318–E1318. https://doi.org/10.1227/01.neu.0000187566.01731.51
Gelabert-Gonzalez M, Relova-Quinteiro J, Castro-García A (2010) “Twiddler syndrome” in two patients with deep brain stimulation. Acta Neurochir (Wien) 152(3):489–491. https://doi.org/10.1007/s00701-009-0366-6
Goyal V, Vaishya S, Shukla G, Singh S, Behari M (2009) Unusual complication of deep brain stimulation in Parkinson’s disease. Mov Disord 24(8):1251–1252. https://doi.org/10.1002/mds.21876
Astradsson A, Schweder P, Joint C, Green A, Aziz T (2011) Twiddler’s syndrome in a patient with a deep brain stimulation device for generalized dystonia. J Clin Neurosci 18(7):970–972. https://doi.org/10.1016/j.jocn.2010.11.012
Burdick A, Okun M et al (2010) Prevalence of Twiddler’s syndrome as a cause of deep brain stimulation hardware failure. Stereotact Funct Neurosurg 88(6):353–359. https://doi.org/10.1159/000319039
Dobson R (2007) Neurosurgeons told to watch for signs of “twiddler’s syndrome.” BMJ 335(7611):114–115. https://doi.org/10.1136/bmj.39279.395787.BE
Franzini A, Ranieri R, Gambini O, Messina G (2018) Manipulating an internal pulse generator until twiddler’s syndrome in a patient treated with deep brain stimulation for obsessive-compulsive disorder. Acta Neurochir (Wien) 160(2):389–392. https://doi.org/10.1007/s00701-017-3412-9
Garg A, Mohan A, Garell P (2010) Placement of the internal pulse generator for deep brain stimulation in the upper back to prevent fracture of the extension wire due to generator rotation: case report. Parkinsons Dis. https://doi.org/10.4061/2010/189371
Ghanchi H, Taka T, Bernstein J, Kashyap S, Ananda A (2020) The unsuccessful twiddler: a case of twiddler’s syndrome without deep brain stimulator lead breakage. Cureus 12(4):e7786. https://doi.org/10.7759/cureus.7786
Israel Z, Spivak A (2008) A tremulous twiddler. Stereotact Funct Neurosurg 86(5):297–299. https://doi.org/10.1159/000155231
Jackowiak E, Patil P, Chou K (2019) The deep brain stimulation “Twiddler syndrome.” JAMA Neurol 76(5):620. https://doi.org/10.1001/jamaneurol.2019.0691
Menghetti C, Zekaj E, Saleh C, Porta M, Servello D (2014) How to avoid Twiddler’s syndrome in deep brain stimulation for dystonia? Neuromodulation 17(2):198–199. https://doi.org/10.1111/ner.12067
Moliz N, Katati M et al (2015) Twiddler’s syndrome in a patient with obsessive-compulsive disorder treated with deep brain stimulation. Neurocirugia 26(4):196–199. https://doi.org/10.1016/j.neucir.2014.11.001
Morishita T, Foote K et al (2010) Identification and management of deep brain stimulation intra- and postoperative urgencies and emergencies. Parkinsonism Relat Disord 16(3):153–162. https://doi.org/10.1016/j.parkreldis.2009.10.003
Morishita T, Hilliard J et al (2017) Postoperative lead migration in deep brain stimulation surgery: Incidence, risk factors, and clinical impact. PLoS ONE 12(9):e0183711. https://doi.org/10.1371/journal.pone.0183711
Penn D, Wu C, Skidmore C, Sperling M, Sharan A (2012) Twiddler’s syndrome in a patient with epilepsy treated with deep brain stimulation. Epilepsia 53(7):e119-121. https://doi.org/10.1111/j.1528-1167.2012.03489.x
Pourfar M, Budman C, Mogilner A (2015) A case of deep brain stimulation for Tourette’s complicated by Twiddler’s syndrome. Mov Disord Clin Pract 2(2):192–193. https://doi.org/10.1002/mdc3.12132
Silva P, Chamadoira C et al (2014) Twiddler (or Not) Syndrome: Questioning etiology for an uncommon form of hardware malfunction in deep brain stimulation. Surg Neurol Int 5(Suppl 8):S410–S412. https://doi.org/10.4103/2152-7806.140201
Sobstyl M, Ząbek M, Górecki W, Brzuszkiewicz-Kuźmicka G (2015) Twiddler syndrome in a patient with tremor dominant Parkinson's disease. A case report and literature review. Neurologia neurochirurgia polska 49(6): 467–471. https://doi.org/10.1016/j.pjnns.2015.10.004
Tymchak Z, Vitali A (2017) What’s the twist? Twiddler’s syndrome in deep brain stimulation. Can J Neurol Sci 44(6):726–727. https://doi.org/10.1017/cjn.2017.230
Okun M, Rodriguez R et al (2008) A case-based review of troubleshooting deep brain stimulator issues in movement and neuropsychiatric disorders. Parkinsonism Relat Disord 14(7):532–538. https://doi.org/10.1016/j.parkreldis.2008.01.001
Jitkritsadakul O, Bhidayasiri R et al (2017) Systematic review of hardware-related complications of deep brain stimulation: do new indications pose an increased risk? Brain Stimul 10(5):967–976. https://doi.org/10.1016/j.brs.2017.07.003
Herschman Y, Fellig Y, Israel Z (2019) Deep brain stimulation and bowstringing: case report and pathological correlation. Interdisciplinary Neurosurgery 17:104–106. https://doi.org/10.1016/j.inat.2019.04.003
Miller PM, Gross RE (2009) Wire tethering or “bowstringing” as a long-term hardware-related complication of deep brain stimulation. Stereotact Funct Neurosurg 87(6):353–359. https://doi.org/10.1159/000236369
Baizabal Carvallo JF, Simpson R, Jankovic J (2011) Diagnosis and treatment of complications related to deep brain stimulation hardware. Mov Disord 26(8):1398–1406. https://doi.org/10.1002/mds.23800
Author information
Authors and Affiliations
Contributions
Wei wang had the idea for the article; Xiaowei Liu and Siyu Li performed the literature search; Xiaowei Liu and Yangyang Xu performed the literature screening and data extraction; Xiaowei Liu analyzed data and drafted the work; Hagai Bergman critically revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, X., Xu, Y., Bergman, H. et al. A systematic review of Twiddler’s syndrome: a hardware-related complication of deep brain stimulation. Neurosurg Rev 45, 951–963 (2022). https://doi.org/10.1007/s10143-021-01636-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-021-01636-9