Abstract
Although the central canal is an integral component of the cerebral ventricular system, central canal dilation has not been examined adequately during the progression of subarachnoid hemorrhage–related hydrocephalus (SAH-H). Central canal dilation–associated ependymal cell desquamation or subependymal membrane rupture has been rarely reported. Herein, we try to describe possible mechanisms of central canal dilation “Hydromyelia,” developing after SAH. A total of 25 New Zealand hybrid female rabbits were recruited. Five served as controls, and five received sham operations. In the remaining animals (n = 15), 0.5 mL/kg of autologous blood was injected into the cisterna magna twice on 0 and 2nd days. Five of these animals died within a few days. A total of 10 survivor animals decapitated 3 weeks later, and the brains and cervical spinal cords were histologically examined. Central canal volumes, ependymal cell numbers on the canal surfaces, and the Evans’ indices of the ventricles were compared. On histological examination, central canal occlusion with desquamated ependymal cells and basement membrane rupture were evident. The mean Evans’ index of the brain ventricles was 0.31, the mean central canal volume was 1.054 mm3, and the normal ependymal cell density was 4.210/mm2 in control animals; the respective values were 0.34, 1.287 mm3, and 3.602/mm2 for sham-operated animals, and 0.41, 1.776 mm3, and 2.923/mm2 in the study group. The differences were statistically significant (p < 0.05). Hydromyelia, an ignored complication of SAH-H, features ependymal cell desquamation, subependymal basement membrane destruction, blood cell accumulation on the subependymal cell basement membrane, and increased CSF pressure. Hydromyelia may be a significant complication following SAH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrocephalus is a complicated serious clinicopathologic entity that may have occurred in the patients as a complication of subarachnoid hemorrhage (SAH) caused by obstruction of the cerebral aqueduct [1, 20]. Although the etiology of the secondary hydrocephalus following SAH is likely to be multifactorial, the obstruction of the cerebral aqueduct reported being one of the major factors that play an important role in the pathophysiology of SAH-related hydrocephalus (SAH-H) [17, 20]. The prevalence of SAH-H ranged from 15 to 20% [1, 20]. Even though more than half of SAH patients with acute SAH-H reported to be spontaneously improved, several studies reported that SAH patients with acute hydrocephalus had a poor prognosis [20]. Therefore, a complete understanding of the pathophysiology of SAH-H can help to improve the surgical outcomes of the treatment of the patients with aneurysmal SAH.
Hydromyelia is the concept usually ought to use to refer to an abnormal widening of the central canal of the spinal cord that can create a cavity in which the cerebrospinal fluid (CSF) accumulates. As CSF builds up, it may put abnormal excess pressure on the neural tissues surrounded the central canal of the spinal cord and damage nerve cells and their connections. When hydromyelia accumulated chronically and form cavities, it is called as syringomyelia. Syringomyelia features a closed cavity and refers to chronic accumulation of CSF in the central canal, in which we can find in Chiari malformations or traumatic spinal injuries [21]. Syringomyelitic cavities extend over several segments of the spinal cord. Syringomyelia causes dilation of the central canal that communicates with the fourth ventricle, non-communicating dilation of the central canal, and extracanalicular syringes in the spinal cord parenchyma. Communicating central canal syringes have been noted in patients with hydrocephalus [15].
Early published studies tried to interpret the pathophysiology of SAH-H. Liszczak et al. showed in their experimental study that several major morphological changes can be observed after SAH such as ventricular changes include dilation of the lateral ventricles, destruction of ciliated ependymal cells, and deposition of small amounts of blood throughout the ventricular system [13]. Black et al.’s study found that the absorption and formation rates of the CSF were unchanged in SAH-related hydrocephalic animals [2]. Minami et al demonstrated in their study that ependymal and arachnoid cells showed ischemic damage in SAH-H [16]. Cardell et al. suggested that vasoactive factors greatly affect the vascular tone of the cerebral circulation [3]. Fukumizu et al. supposed that fetal or neonatal hydrocephalus is associated with major periventricular tissue damage [7]. Nevertheless, further investigation, identification, and explanation of the pathophysiological changes in the central nervous system regarding SAH-H are necessary.
In the current study, the author demonstrated that multiple factors such as ependymal cell desquamation, subependymal basement membrane destruction, blood cell accumulation on the subependymal basement membrane, and increased CSF secretion can play an important role in the development of the hydromyelia following SAH.
Material and methods
We adhered to all animal care and experimental protocols approved by the Ethics Committee of the Ataturk University, Medical Faculty, under decision number 42190979-050.01.04-E.1700243019, and animal research: reporting in vivo experiments (ARRIVE) guidelines [11].
Animals
We employed 25 New Zealand hybrid female rabbits (1.5 years of age; 3.7 ± 0.3 kg) used in our study. The rabbits had free access to standard chow and tap water in a temperature-controlled circumstance at 24 °C with a cycle of 12 h light/dark. The animals were assigned into three groups randomly; five served as controls, and five received sham operations (sham-injected). SAH group comprised the remaining animals (n = 15), 0.5 mL/Kg of autologous blood was injected into the cisterna magna twice, with an interval of 48 h between both injections. Five of these animals died within a few days (1–3 days). We think that the animals died related to increased intracranial pressure, brainstem herniation–induced cardiorespiratory arrest (n = 3, immediately died after the second injection). A total of 2 rabbits experienced severe postoperative deficits after the second blood injection. Therefore, they were early sacrificed on the 2nd and 3rd days. A total of 10 survivor animals decapitated 3 weeks later, and the brains and cervical spinal cords were histologically examined. Central canal volumes, ependymal cell numbers on the canal surfaces, and the Evans’ indices of the ventricles were compared. Power analysis was applied to the study results and it was concluded that the number of animals included in the study formed a sufficient sample with a power of 0.80.
Experimental protocol
To reduce pain and mortality, a balanced injectable anesthetic solution was given. After anesthesia was induced with isoflurane given via a face mask, 0.2 mL/kg of an anesthetic combination (ketamine HCL, 150 mg/1.5 mL; xylazine HCL, 30 mg/1.5 mL; and distilled water, 1 mL) was subcutaneously injected. During each procedure, 0.1 mL/Kg amounts of the anesthetic solution were injected when required. The rabbits in all groups were laterally positioned during spontaneous breathing. After sterilizing the skin, a midline skin and galea incision was achieved before insertion of a small surgical retractor. After stereotactic determining the appropriate coordinates for burr hole placement to monitor intracranial pressure (ICP). The points in the mid-pupillary lines, 1–2 mm from the midsagittal line, and intracerebral laser Doppler probes 4–5 mm anterolateral to the bregma point. Three osteotomies with a diameter of 2 mm were made using a high-speed micro-drill in the frontal part. The tip of the ICP monitor (Codman Disposable ICP kit, Spreitenbach, Switzerland) was inserted into the right olfactory bulb to a depth of 2 mm. In the corresponding burr holes in the right and the left frontal lateral to ventricles, 2 fine needle probes of the laser Doppler flowmetry were inserted to a depth of 2.5 mm using an external clamp. Thereafter, the atlantooccipital membrane was stereotactically pierced with a 22-G needle inserted into the cisterna magna to extract 0.5 mL/kg (1.5–2 mL) of CSF (the aim of this step to minimize the severity of neurological deficits can occur during blood injection step). Subsequently, an equal volume of autologous blood obtained from the auricular arteries was injected into the cisterna magna using the same needle for 1 min via the occipital horns of animals in the SAH group. To facilitate the injected blood spreading through the subarachnoid space to reach anterior circulation, the rabbits were kept titled in a 30° angle position for 2–3 min. The extraction and injection procedures were achieved twice with an interval of 48 h. Five of these animals died within a few days (1–3 days). A total of 10 survivor animals were included in our study. Considering the adverse effects may occur throughout the surgical procedure, in the sham-operated group, the equal volume of extracted CSF of physiological serum was injected into the cisterna magna. Control animals were not subjected to any procedure. To measure PaO2 and PaCO2, arterial blood was analyzed within 2 min after injection using the ABL90 FLEX PLUS blood gas analyzer. The blood pressure values were obtained from the femoral artery using (Siemens SC-7000 ASA model no: 5202994-Electromedical Group-USA). All survivor operated animals were followed up for 21 days without any medical treatment and then sacrificed. After cleaning, the whole bodies were stored in 10% formalin solutions before the histological examination.
Histological examinations
Brains were coronally sectioned at the level of greatest enlargement of the biparietal diameters. To estimate aqueduct volumes and ependymal cell numbers, longitudinal brainstem sections were created and embedded in paraffin blocks; this allowed us to observe all brainstem roots during histological examinations performed after staining with hematoxylin and eosin (H&E) and for glial fibrillary acidic protein (GFAP). Consecutive slices of 5 μm were obtained (n = 20–50 for each brain). We estimated ependymal cell densities in aqueductal spaces using the Cavalieri method, which is simple and accurate, and makes no assumptions in terms of particle shape, size, or orientation. Figure 1 shows the histological features of the aqueductal surfaces, choroid plexuses, and ventricles.
Histologic appearance of the spinal cord, central canal, and ependymal cells in a normal animal staining with H&E. (A) Light microscopy, hematoxylin and eosin, × 4. (B) Light microscopy, hematoxylin and eosin, ×20. Central canal surface estimation formula S = πr2; E, ependymal cells; r, radius of the central canal. Note the normal cells’ number and sequence of spinal cord motor neurons
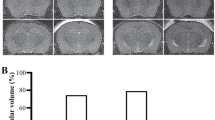
We also embedded 20–50 consecutive, horizontal 5-μm-thick sections from each brain in paraffin blocks to observe all sectioned ventricles and aqueducts; we again used the Cavalieri method to estimate aqueduct volumes. The consecutive sections were arranged to show both sides of the aqueducts, which were considered cylindrical with volumes of V = πr2h, with h = 50 μm and r = 25 μm (see scale bar of Fig. 2). To calculate Evans’ indices, the slides were photographed and the bifrontal indices were estimated by the superimposition of the photographs onto paper bearing mini squares (Fig. 2). The density of ependymal cells/mm2, which presented as tiles (Fig. 3), comprising the aqueductal surface, calculated as 2 πrh, was 3.925/μm2. As the length of each ependymal cell was 6 μm, the height of our model cylinder was estimated to be equal to the lengths of 8.5 ependymal cell heights, and the area of the bottom circle was estimated to be equal to 2 μr/6 = 150/8 = 18.75 ependymal cells (Fig. 3). Thus, each cylinder included 18.75 × 8.5 = 159.375 cells.
Histologic appearance of the spinal cord and partially dilated central canal in a sham-operated animal staining with H&E. (A) Light microscopy, hematoxylin and eosin, × 4, (B) Light microscopy, hematoxylin and Eosin, × 20. DE, degenerated/desquamated ependymal cells of the central canal. Note that the number of normal motor neurons in Fig. 1 is decreased, note degenerated motor neurons of the spinal cord
Statistical analysis
All data were expressed as the median or mean ± standard deviation with the range shown in parentheses. All data were analyzed using a commercial software package (SPSS® for Windows ver. 25.0; SPSS, Chicago, IL, USA). We performed one-way analysis of variance (ANOVA) with the post hoc Tukey test. A p value < 0.05 was considered to reflect significance. All tests were two-tailed. Power analysis was applied to the study results using G-power 3.1.9.4 software.
Results
Pathophysiological parameters of three groups at baseline characteristics are given in Table 1. The SAH group did not differ significantly in body weight, pH, PaO2, PaCO2, heart rate, and mean arterial blood pressure. The differences were not statistically significant (p > 0.05). However, during blood injection, all animals in the SAH group showed a significantly marked ICP increase with marked CPP decrease to peak values and returned to a steady-state after 5–10 min. The values recorded after 15 min were significantly different (p < 0.001). Gross examination revealed brain swelling, edema, pia-arachnoid adhesions, and ventricular enlargement; spinal cord swelling, edema, and arachnoiditis; and central canal hemorrhage, occlusions, and dilation. Massive cisterna magna and fourth ventricular SAH was observed in animals of the SAH group, with meningeal irritation, brain edema, stiffness, enhanced leptomeningeal thickness, and increased brain weight. Two animals also evidenced brain lacerations. Although no degenerative changes in the choroid plexus were noted in animals that were decapitated early (n = 5), those that were decapitated later exhibited degenerative changes including villus desquamation, choroidal cell shrinkage, angulation, cytoplasmic condensation, and cellular loss.
Histological results
Our histological results were given in five figures as follows:
Figure 1 shows the histological appearance of the spinal cord, central canal, and ependymal cells in a normal animal staining with H&E; note the normal number of spinal cord motor neurons in a normal animal; note the normal number and sequence of spinal cord motor neurons.
Figure 3 shows the histological appearance of the spinal cord and the partially dilated central canal. The figure shows mild degenerated/desquamated ependymal cells of the central canal and degenerated motor neurons of the spinal cord in a sham-operated animal staining with H&E; note the reduced number of cells and impaired sequence of the spinal cord neurons.
Figure 4 shows the histological appearance of the spinal cord and dilated central canal. The figure shows degenerated/desquamated ependymal cells of the central canal and degenerated motor neurons of the spinal cord in an animal with a SAH staining with H&E; note the reduced number of cells and impaired sequence of the spinal cord neurons.
Histological appearance of the spinal cord and dilated central canal in an animal with a SAH staining with H&E. (A) Light microscopy, hematoxylin and eosin, × 4, (B) Light microscopy, hematoxylin and Eosin, × 20. DE, degenerated/desquamated ependymal cells of the central canal. Note that the number of normal motor neurons in Fig. 1 is decreased, impaired sequence of the spinal cord neurons
Figure 5 shows the histological appearance of the spinal cord, central canal, and ependymal cells in a normal animal staining with GFAP; note the normal number and sequence of spinal cord motor neurons.
Figure 6 shows the histological appearance of the spinal cord and dilated central canal. Degenerated/desquamated ependymal cells of the central canal and degenerated motor neurons of the spinal cord in an animal with a SAH staining with GFAP; note the reduced number of cells and impaired sequence of the spinal cord neurons.
Histological appearance of the spinal cord and dilated central canal in an animal with a SAH staining with GFAP. (A) Light microscopy, GFAP, × 4. (B) Light microscopy, GFAP, × 20. DE, degenerated/desquamated ependymal cells of the central canal. Note that the number of normal motor neurons in Fig. 5 is decreased, impaired sequence of the spinal cord neurons
Numerical results
Table 2 lists the Evans’ indices of the brain ventricles, the mean volumes of the central canals, and the ependymal cell densities. The Evans’ indices differed significantly among the three groups (Table 3); the index of the SAH group was higher than those of the sham-operated and control groups. The mean central canal volumes differed significantly among the three groups (Table 4); the mean volume of the SAH group was higher than those of the sham-operated and control groups. The ependymal cell density differed among the three groups (Table 5); the density of the SAH group was higher than those of the sham-operated and control groups.
Discussion
Several experimental models of SAH were used for different investigations of its influences on neurological functions. The advantages of the cisterna magna injection (CMI) model are the low mortality rate, reproducibility, and suitability for investigating blood-brain barrier permeability. Therefore, the author selected the CMI model rather than endovascular perforation or prechiasmatic cistern injection models. Even though it is preferred to investigate initial phase of vasospasm, endovascular perforation model has several disadvantages such as high early mortality rate after 24 h (ranges from 37.5 to 50%) [12, 14], uncontrollable blood spreading through the subarachnoid space, high intracerebral hemorrhage complication rate (up to 11%), and the high failure rate (up to 12%) [14]. Prechiasmatic cistern injection model preferred to study vasospasm, however, it has major disadvantages such as causing severe hemorrhagic insulting that restricts follow-up animals for 3 weeks, and limitation of spreading blood through subarachnoid space that restricts investigating the influences of SAH on the central canal in the spine. In the CMI model, an increase of ICP may lead the blood to enter the central canal in the spinal cord [12, 14].
Spinal SAH increases the intraspinal epidural pressure to 20 or even 30 mmHg and triggers hydrocephalus [8]. In adults, spinal SAH can associate with normal pressure hydrocephalus that characterized by a combination of gait disturbance, varying extents of cognitive decline, urinary incontinence, ventricular enlargement, and an increase in the intracranial pressure [9]. A spinal subdural hematoma was developed after a SAH triggered hydromyelia [22]. Thus, the central canal acts as a complementary part of the ventricular system. Any changes in one will reflect in other’s compartments. This may be the main cause of shunt-requiring hydrocephalus in patients with SAH.
In patients with hydrocephalus, the ependymal cells of the central spinal canal exhibit abnormalities that vary by the etiology of ventricular dilation [17, 22]. Although central canal dilation is an integral feature of hydrocephalus, the progression of such dilation has not been sufficiently discussed. Neither dilation-associated ependymal cell desquamation nor subependymal membrane rupture has been sufficiently reported. Not all developed hydrocephalus after SAH requiring treatment. Therefore, there are several factors and mechanisms that may influence the permanent SAH-H.
Central canal and ependymal cell status after SAH
The findings of periventricular leukomalacia, pontosubicular necrosis, and Purkinje cell loss are previously reported findings in older infants with fetal/neonatal posthemorrhagic hydrocephalus. The levels of ferritin/GFAP-positive glia increase in the molecular basement as well as in granular layers and the white matter. Neonates exhibit more severe brain lesions, accompanied by hemosiderin deposits, nodular gliosis, ependymal cell loss, and subependymal rosette formation [7]. Changes in the choroid plexus include the development of electron-dense cytoplasmic inclusions and dilation of the lateral and subcellular spaces. The ventricular surface and the choroid plexus are both affected by intracisternal blood injection. SAH-H may be exacerbated by ependymal disruption, loss of ventricular ciliary activity, and changes in the choroid plexus [13].
The experimental study of Olopade et al. showed that the histological examination of hydrocephalic rats revealed the peeling of the ependymal layer from the subependymal tissue, edema of periventricular tissue, perivascular gliofibrosis, perivascular hemorrhage, plasma exsanguination, venous stasis of the ventricular wall, and a concave cerebral cortex. Both the extracellular space of subependymal tissue and the space between the pia and glial membrane were remarkable. Thus, it is considered that two factors might contribute to ventricular enlargement, i.e., hydrodynamic CSF disturbance caused by CSF malabsorption by the superior sagittal sinus via the arachnoid villi and disturbance of the venous circulation caused by sinus occlusion [18].
Syringomyelitic cavities extend over several segments of the spinal cord, triggering arachnoid scarring of the basal cisterna, segmentation abnormalities of the superior cervical vertebrae, and hydrocephalus or an arachnoid cyst of the posterior fossa. Fluid travels to the syrinx along the embryologically natural route down the central canal. During each arterial pulse, CSF flows out of the fourth ventricle to the syrinx via the central canal. Most patients exhibit patent fourth ventricle foramina; communication between the ventricle and the syrinx is rare. Williams proposed a “craniospinal pressure dissociation theory,” which posited that significant pressure differentials were develop during daily activities, such as sneezing and coughing; these increase intrathoracic pressure, which is transmitted to the spinal fluid via the epidural spinal veins [21]. CSF flow from the cranial to the spinal subarachnoid space reflects expansile brain motion during the cardiac cycle. Cavity progression reflects the pressure on the cord surface, and does not require communication between the fourth ventricle and the central canal or syrinx. The perivascular spaces and the dorsal root entry zone affect communication between the perimedullary CSF, the extracellular spaces of the spinal cord, and the central canal. Arachnoid scarring is often associated with spinal trauma, developing after spinal meningitis, intradural spinal surgery, peridural anesthesia, and SAH. Rarely, extramedullary compression occurs. The mechanism involves changes in CSF flow at the spinal level [10].
Extracanalicular (parenchymal) syringes typically occur in the cord watershed in association with injuries to cord tissue caused by trauma, infarction, and hemorrhage. Such cavitation is frequently associated with myelomalacia. Both the extracanalicular syringes and paracentral dissections of the central canal syringes are lined with glial or fibroglial tissue that frequently ruptures into the subarachnoid spinal space, characterized by central chromatolysis, neuronophagia, and Wallerian degeneration. Although the clinical data are incomplete, simple dilations of the central canal tend to produce nonspecific neurological findings such as spastic paraparesis, whereas deficits associated with extracanalicular syringes and paracentral dissections of central canal syringes include segmental signs referable to the affected nuclei and tracts. Syringomyelia exhibits several distinct cavitary patterns associated with different pathogeneses; these determine the clinical features [15].
Expansion of the central spinal canal
Lumbar intraspinal epidural pressure is a reliable index of intracranial pressure after SAH or SAH-H [6]. This reflects the pressure in the spinal canal, lateral ventricles, subarachnoid spaces, operative cavity, and other compartments [22]. A spinal subdural hematoma was developed after SAH triggered hydromyelia [4]. Chronic hydromyelia/syringomyelia can cause an insidious decline in motor and sensory function even years later, accompanied by slow progressive destruction of the cytoarchitecture [5]. Expansion of the central canal lumen beyond a critical diameter triggers the loss of ependymal ciliary cells, empirically predictable thinning of the ependymal region, and a reduction in cell proliferation. Large dilations of the central canal were accompanied by disruptions to the ependymal layer, periependymal edema, gliosis, and destruction of adjacent neuropils. Cells of the ependymal region play important roles in CSF homeostasis, cellular signaling, and repair of spinal cord wounds [19]. We tried in this study to understand the basis of the SAH-H requiring treatment. The findings of this study suggest that central canal dilation and disruption of the ependymal region are steps in the pathogenesis of SAH-H. Since 2002, in our clinical practice in patients with SAH, we ought to open terminal lamina or drain CSF via lumbar drainage preoperatively to reduce the possibility of SAH-H. Even though we failed to prevent developing SAH-H with our findings in this study, we identified novel targets for therapeutic interventions. Thus, we believe if we can prevent large dilation and degeneration of ependymal cells in the central canal, maybe we can avoid developing SAH-H.
The current study has three limitations; the first, the model of SAH represented the anterior circulation SAH. However, further animal trials with better representative models included posterior circulation SAH model that must be conducted to get better recognizing the real relationship between hydromyelia and the SAH requiring treatment. Second, we did not perform neuroimaging scans to prove hydrocephalus radiologically. Third, even though we evaluated neurological functions for animals to evaluate the severe postoperative deficits, we did not perform a comparison regarding neurological functions among the three groups.
Conclusion
Although central canal dilation is an integral feature of hydrocephalus, such dilation has been ignored in the context of SAH-related hydrocephalus progression. As CSF is transported from the cranial subarachnoid spaces to the spinal cord through this canal, dilation thereof may increase intraspinal pressure. Central canal dilation–associated ependymal cell desquamation and subependymal membrane rupture have not been sufficiently reported. Hydrocephalus, ependymal cell desquamation, subependymal basement membrane destruction, blood cell accumulation on the subependymal basement membrane, and increased CSF secretion may all contribute to the development of hydromyelia following SAH. When these changes are apparent for a long time, it can result in SAH-related hydrocephalus. We postulate that preventing long time hydromyelia after SAH may reduce the high rate of SAH-H; further prospective studies in SAH patients with large size are needed to support our results.
Change history
13 November 2021
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s10143-021-01694-z
References
Asiltürk M, Abdallah A (2018) Clinical outcomes of multiple aneurysms microsurgical clipping: evaluation of 90 patients. Neurol Neurochir Pol 52(1):15–24
Black PM, Tzouras A, Foley L (1985) Cerebrospinal fluid dynamics and hydrocephalus after experimental subarachnoid hemorrhage. Neurosurgery 17:57–62
Cardell LO, Uddman R, Edvinsson L (1994) Endothelins: a role in cerebrovascular disease? Cephalalgia. 14(4):259–265
Collice M, Porta M (1975) Monitoring of intracranial pressure. Minerva Chir 30(19):1003–1010
Emel E, Abdallah A (2016) Spinal tümörler ve siringomiyeli; In Özer AF (ed), Siringomiyeli. Usakademi; 81-99 (Chapter in Turkish).
Fujioka S, Kaku M, Hamada J, Yokota A, Ushio Y (1989) The usefulness of lumbar epidural pressure as an index of intracranial pressure. Neurol Med Chir (Tokyo) 29(6):484–489
Fukumizu M, Takashima S, Becker LE (1995) Neonatal posthemorrhagic hydrocephalus: neuropathologic and immunohistochemical studies. Pediatr Neurol 13(3):230–234
Hamada J, Fujioka S, Ushio Y (1993) Experimental investigation of lumbar epidural pressure measurement. Neurosurgery. 32(5):817–821
Hamlat A, Sid-Ahmed S, Adn M, Askar B, Pasqualini E (2006) Idiopathic normal pressure hydrocephalus: theoretical concept of a spinal etiology. Med Hypotheses 67(1):110–114
Heiss JD, Snyder K, Peterson MM, Patronas NJ, Butman JA, Smith RK, Devroom HL, Sansur CA, Eskioglu E, Kammerer WA, Oldfield EH (2012) Pathophysiology of primary spinal syringomyelia. J Neurosurg Spine 17(5):367–380
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2010) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8(6):e1000412
Leclerc JL, Gracia JM, Diller MA, Carpenter AM, Kamat PK, Hoh BL, Dore S (2018) A comparison of pathophysiology in human and rodent models of subarachnoid hemorrhage. Front Mol Neurosci 11:71
Liszczak TM, Black PM, Tzouras A, Foley L, Zervas NT (1984) Morphological changes of the basilar artery, ventricles, and choroid plexus after experimental SAH. J Neurosurg 61(3):486–493
Marbacher S, Grüter B, Schöpf S, Croci D, Nevzati E, D’Alonzo D, Lattmann J, Roth T, Bircher B, Wolfert C, Muroi C, Dutilh G, Widmer HR, Fandino J (2018) Systematic review of ın vivo animal models of subarachnoid hemorrhage: species, standard parameters, and outcomes. Transl Stroke Res 10:250–258. https://doi.org/10.1007/s12975-018-0657-4
Milhorat TH, Capocelli AL Jr, Anzil AP, Kotzen RM, Milhorat RH (1995) Pathological basis of spinal cord cavitation in syringomyelia: analysis of 105 autopsy cases. J Neurosurg 82(5):802–812
Minami N, Tani E, Yokota M, Maeda Y, Yamaura I (1991) Immunohistochemistry of leukotriene C4 in experimental cerebral vasospasm. Acta Neuropathol 81(4):401–407
Mise B, Klarica M, Seiwerth S, Bulat M (1996) Experimental hydrocephalus and hydromyelia: a new insight in mechanism of their development. Acta Neurochir 138(7):862–868
Olopade FE, Shokunbi MT, Sirén AL (2012) The relationship between ventricular dilatation, neuropathological and neurobehavioural changes in hydrocephalic rats. Fluids Barriers CNS 9(1):19
Radojicic M, Nistor G, Keirstead HS (2007) Ascending central canal dilation and progressive ependymal disruption in a contusion model of rodent chronic spinal cord injury. BMC Neurol 7:30
Shah AH, Komotar RJ (2013) Pathophysiology of acute hydrocephalus after subarachnoid hemorrhage. World Neurosurg 80:304–306
Williams HF (2017) The central nervous system pressure histogram in hydrocephalus and hydromyelia. Med Hypotheses 108:117–123
Yamaguchi S, Hida K, Akino M, Yano S, Iwasaki Y (2003) Spinal subdural hematoma: a sequela of a ruptured intracranial aneurysm? Surg Neurol 59(5):408–412
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This study was approved under decision number 42190979-050.01.04-E.1700243019 by the medical ethics committee of the Medical Faculty of Ataturk University in Erzurum-Turkey.
Informed consent
Informed consent was not obtained (it is an animal experiment).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1007/s10143-021-01694-z
About this article
Cite this article
Abdallah, A. RETRACTED ARTICLE:Correlation of hydromyelia with subarachnoid hemorrhage–related hydrocephalus: an experimental study. Neurosurg Rev 44, 1437–1445 (2021). https://doi.org/10.1007/s10143-020-01330-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-020-01330-2