Abstract
Early brain injury and hydrocephalus (HCP) are important mediators of poor outcome in subarachnoid hemorrhage (SAH) patients. We aim to understand the development of HCP and subependymal cellular injury after intraventricular injection of noncellular human SAH cerebrospinal fluid (CSF) into rat ventricles. Two-hundred microliters of noncellular CSF from SAH patients or normal controls were injected into the right lateral ventricle of seven adult male Sprague-Dawley rats. Propidium iodide (PI) was simultaneously injected to detect necrotic cellular death. Rats were then sacrificed 24 h after surgery and the brain specimens were cut and stained for heme oxygenase 1 (HO-1), an oxidative stress marker. We found that the ventricular area at the bregma level in the CSF injection group was significantly larger than that in the control group (p < 0.05). The periventricular tissue in the CSF injection group had significantly more necrotic cell death as well as HO-1 expression as compared with the control group (p < 0.05). In conclusion, injection of SAH patients’ CSF into the rat ventricle leads to HCP as well as subependymal injury compared with injection of control CSF.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Aneurysmal subarachnoid hemorrhage (SAH) is a life-threatening condition leading to significant morbidity and mortality and affecting nearly 35,000 patients on a yearly basis [1, 2]. SAH patients can present with acute hydrocephalus (HCP) leading to increase in intracranial pressure (ICP) and thus reduced cerebral perfusion pressure [3], leading to significant secondary early brain injury (EBI) [4]. Acute HCP development post SAH could be explained by several mechanisms: cerebrospinal fluid (CSF) flow obstruction secondary to blood products, CSF dynamic changes, and CSF absorption deficit [5]. Interestingly, one study has shown that intraventricular fibrinolysis aimed at lysing intraventricular blood neither reduced permanent shunt dependency nor influenced functional outcome [6]. Our previous animal studies revealed that not only lysed red blood cell and iron could induce HCP but that HCP could also be induced by intraventricular injection of thrombin [7, 8]. Thus, the metabolic products of blood seem to play a role in the development of HCP after hemorrhage. Nonetheless, the mechanisms of developing acute HCP and EBI post SAH are not well understood. We aimed to document the development of HCP and subependymal injury after injecting CSF from human SAH patients into rat ventricles with the goal of determining the mechanism of such injuries.
Materials and Methods
Animal use protocols were approved by the University of Michigan Committee on the Use and Care of Animals. A total of seven male Sprague-Dawley rats (3 months old, Charles River Laboratories, Portage, MI, USA), weighing 250–300 g, were used in this study and randomly enrolled in the CSF injection group (n = 4) or control group (n = 3). Informed consents were obtained from SAH patients whose CSF was collected for this study.
Model Establishment
Rats were anesthetized with pentobarbital (50 mg/kg, intraperitoneally) and the right femoral artery was catheterized to monitor arterial blood pressure, blood pH, PaO2, PaCO2, hematocrit, and glucose levels. Core body temperature was maintained at 37.5 °C with a feedback-controlled heating pad. Rats were positioned in a stereotaxic frame (Kopf Instruments, Tujunga, CA, USA). A cranial burr hole (1 mm) was drilled and a 26-gauge needle was inserted stereotaxically into the right lateral ventricle (coordinates: 0.6 mm posterior, 4.5 mm ventral, and 1.6 mm lateral to the bregma). Two-hundred microliters of SAH patient’s noncellular CSF or control CSF was injected with 0.04 mg/mL propidium iodide (PI), an indicator of necrotic cell death, using a micro-infusion pump (World Precision Instruments, Sarasota, FL, USA). After injection, the needle was removed, the burr hole was filled with bone wax, and the skin incision was closed with sutures. The rats were kept on a warm pad separately until fully awake. Rats that had intraventricular injection with patient SAH CSF were called the experimental group as compared with the rats injected with control patient non-SAH CSF, denoted as the control group.
Ventricle Area Measurement and PI-Positive Cell Density
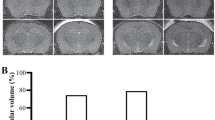
Brain sections were dehydrated with ethanol at graded concentrations of 50–100 %(v/v) and then stained with hematoxylin and eosin (H&E staining). Three brain sections (50 μm anterior and posterior to the bregma level) were scanned and the ventricle was outlined on a computer by a blinded observer. The ventricular areas were measured using NIH Image J software (Image Version 1.63, National Institutes of Health). The mean of the three measurements was regarded as the ventricular area at the bregma level. PI-positive cell density was meanwhile analyzed on the same section.
Immunohistochemistry
Rats were anesthetized with pentobarbital (100 mg/kg, intraperitoneally) and perfused with 4 % paraformaldehyde in 0.1 mol/L phosphate-buffered saline (pH 7.4). The brains were removed and kept in 4 % paraformaldehyde for 24 h and then immersed in 30 % sucrose for 2–3 days at 4 °C. Brains were embedded in optimal cutting temperature compound (Sakura Finetek, Torrance, CA, USA) and 18-mm-thick slices cut using a cryostat. Immunohistochemical studies were performed using the avidin–biotin complex technique as previously described [9]. The primary antibody was rabbit anti-HO-1 (1:400 dilution; Abcam, Cambridge, MA, USA). The numbers of HO-1–positive cells in each tissue sample were counted in three fields of view and expressed as the mean of the positive cells per square millimeter.
Statistical Analysis
All data in this study are presented as mean ± standard deviation (SD). Data from different animal groups were analyzed using analysis of variance with a Student t-test. Differences were considered significant at probability values less than 0.05.
Results
Physiologic parameters, including mean arterial blood pressure, arterial blood gases, pH, hematocrit, and glucose, were within normal range. All rats within the study survived.
There was overall enlargement of the lateral ventricles in the rats that had patient SAH CSF injection as compared with control patient CSF injection. This was objectively appreciated as rats with patient SAH CSF injection had much larger ventricles as compared with rats with control patient CSF injection (3.1 ± 0.3 mm2 vs 1.9 ± 0.2, p < 0.05, Fig. 1). More severe periventricular injury was documented in the experimental rats as compared with control rats as evident by the greater periventricular PI staining within the experimental group (0.192 ± 0.083 vs 0.008 ± 0.002, p < 0.05, Fig. 2). Increasing periventricular injury was also appreciated in the experimental group as compared with the control group as evident by the greater oxidative injury based on elevated HO-1 expression (HO-1–positive cell intensity, 0.589 ± 0.170 vs 0.044 ± 0.018, p < 0.05, Fig. 3).
Discussion
We aim to understand the role of human SAH noncellular CSF in causing hydrocephalus and periventricular cellular injury toward the goal of understanding the underlying mechanisms. We were able to show that the experimental group of rats, intraventricular injection of SAH CSF, had larger ventricles as compared with control rats, intraventricular injection of control patient CSF. Although this outcome could be related to immune or inflammatory reactions related to cross-species reaction, we controlled for this by having the control group also have intraventricular injection with human CSF. Thus, any inflammatory changes should also be present within the control rats.
Our previous studies [7, 8, 10] successfully proved that different solutions, such as iron, thrombin, and lysed red blood cells, have the possibility to induce a hydrocephalus regardless of the real concentration of these content in CSF. Shim et al. [11] analyzed the concentration of vascular endothelial growth factor (VEGF) in the CSF from patients with hydrocephalus and injected a VEGF solution with similar concentration into the lateral ventricle of rats and also succeeded in establishing a hydrocephalus model. Nonetheless, numerous biochemical components are present within the SAH CSF and, most likely, have a complex interplay in causing hydrocephalus. We aim to evaluate this mechanism by mixing inhibitors of certain pathways to understand whether SAH CSF injection will continue to cause hydrocephalus.
Propidium iodide is an intercalating and fluorescent agent, which is impermeable to membrane and excluded from viable cells. However, PI is able to penetrate necrotic cells and is incorporated into the nucleic acid [12]. The experimental group of rats had increased PI and HO-1 staining within the periventricular region, thus representing greater cellular death as well as oxidative injury in rats injected with SAH CSF.
Our study is limited by the fact that human CSF was injected within rats, thus immune reactions are to be expected and could be contributing to the changes observed. In addition, our current study represents rats that were only injected with one patient’s SAH CSF and thus injection with more patients’ SAH CSF will increase the power and reliability of the results.
References
Van Gijn J, Kerr RS, Rinkel GJ (2007) Subarachnoid haemorrhage. Lancet 369:306–318
Ferro JM, Canhao P, Peralta R (2008) Update on subarachnoid haemorrhage. J Neurol 255:465–479
Van Asch CJ, van der Schaaf IC, Rinkel GJ (2010) Acute hydrocephalus and cerebral perfusion after aneurysmal subarachnoid hemorrhage. AJNR Am J Neuroradiol 31:67–70
Milhorat TH (1987) Acute hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery 20:15–20
Dorai Z, Hynan LS, Kopitnik TA, Samson D (2003) Factors related to hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery 52:763–769; discussion 769–71
Gerner ST, Kuramatsu JB, Abel H (2014) Intraventricular fibrinolysis has no effects on shunt dependency and functional outcome in endovascular-treated aneurysmal SAH. Neurocrit Care 21:435–443
Gao C, Du H, Hua Y, Keep RF, Strahle J, Xi G (2014) Role of red blood cell lysis and iron in hydrocephalus after intraventricular hemorrhage. J Cereb Blood Flow Metab 34:1070–1075
Gao F, Liu F, Chen Z, Hua Y, Keep RF, Xi G (2014) Hydrocephalus after intraventricular hemorrhage: the role of thrombin. J Cereb Blood Flow Metab 34:489–494
Wu J, Hua Y, Keep RF, Nakamura T, Hoff JT, Xi G (2003) Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke 34:2964–2969
Okubo S, Strahle J, Keep RF, Hua Y, Xi G (2013) Subarachnoid hemorrhage-induced hydrocephalus in rats. Stroke 44:547–550
Shim JW, Sandlund J, Han CH (2013) VEGF, which is elevated in the CSF of patients with hydrocephalus, causes ventriculomegaly and ependymal changes in rats. Exp Neurol 247:703–709
Jin H, Xi G, Keep RF, Wu J, Hua Y (2013) DARPP-32 to quantify intracerebral hemorrhage-induced neuronal death in basal ganglia. Transl Stroke Res 4:130–134
Acknowledgment
This study was supported by grants NS073959, NS079157, and NS084049 from the National Institutes of Health and 973 Program-2014CB541600.
Conflict of Interest Statement
None of the authors have any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Li, P. et al. (2016). Intraventricular Injection of Noncellular Cerebrospinal Fluid from Subarachnoid Hemorrhage Patient into Rat Ventricles Leads to Ventricular Enlargement and Periventricular Injury. In: Applegate, R., Chen, G., Feng, H., Zhang, J. (eds) Brain Edema XVI. Acta Neurochirurgica Supplement, vol 121. Springer, Cham. https://doi.org/10.1007/978-3-319-18497-5_57
Download citation
DOI: https://doi.org/10.1007/978-3-319-18497-5_57
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-18496-8
Online ISBN: 978-3-319-18497-5
eBook Packages: MedicineMedicine (R0)