Abstract
Post-traumatic hydrocephalus (PTH) is a potentially morbid sequela of decompressive craniectomy for traumatic brain injury (TBI). Subdural hygromas are commonly identified following decompressive craniectomy, but the clinical relevance and predictive relationship with PTH in this patient cohort is not completely understood. Survey of seven electronic databases from inception to June 2019 was conducted following PRISMA guidelines. Articles were screened against pre-specified criteria. Multivariate hazard ratios (HRs) for PTH by the presence of subdural hygroma were extracted and pooled by meta-analysis of proportions with random effects modeling. We systematically identified nine pertinent studies describing outcomes of 1010 TBI patients managed by decompressive craniectomy. Of the overall cohort, there were 211 (21%) females and median age was 37.5 years (range 33–53). On presentation, median Glasgow Coma Scale was 7 (range, 5–8). In sum, PTH was reported in 228/840 (27%) cases, and subdural hygroma was reported in 449/1010 (44%) cases across all studies. Pooling multivariate-derived HRs indicated that subdural hygroma was a significant, independent predictor of PTH (HR, 7.1; 95% CI, 3.3–15.1). The certainty of this association was deemed low due to heterogeneity concerns. The presence of subdural hygroma is associated with increased risk of PTH after decompressive craniectomy among TBI patients based on the current literature and may mandate closer clinical surveillance when detected. Prospective studies, including those of intracranial hydrodynamics following decompressive craniectomy in the setting of TBI, will better validate the certainty of these findings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Raised intracranial pressure following traumatic brain injury (TBI) often mandates the need for neurosurgical intervention, particularly where pressure elevations are severe or refractory to non-operative treatments. Decompressive craniectomy is an effective procedure to rapidly reduce intracranial pressure in several randomized, controlled studies. [1,2,3,4] Of the two landmark randomized trials, the Rescue-ICP study [5] found comparable functional outcomes after surgical and conservative management. Yet, the DECRA (Decompressive Craniectomy in Patients with Severe Traumatic Brain Injury) study [6] reported that decompressive craniectomy led to worse functional outcomes. Therefore, although there remains debate as to the absolute benefit of surgically intervening in severe TBI outside the possible reduction in mortality risk, there should be high agreement in that the decision to pursue decompressive craniectomy must be well-informed and justified based on clinical indications. Correspondingly, any prognostic feature which can assist in further identifying the ideal candidate to benefit most from decompressive craniectomy is desirable.
An important and potentially morbid treatment sequela of TBI is the development of post-traumatic hydrocephalus (PTH)—typically defined as new neurologic symptoms consistent with hydrocephalus in the setting of radiographic ventriculomegaly and a history of TBI. [7,8,9,10] PTH often develops insidiously and is challenging to diagnose due to injury-related neurologic deficits. Furthermore, it is often difficult to treat given that it is associated with significantly increased morbidity and mortality, as compared to TBI patients who do not develop PTH. [11,12,13,14,15,16] Correspondingly, early diagnosis and treatment of PTH are an important translational goal with the potential to significantly improve patient outcomes.
Subdural hygromas are extra-axial cerebrospinal fluid (CSF) collections encountered in a variety of neurosurgical contexts, including after both elective and emergency operations. [17, 18] At present, a large volume of level III evidence describes the evolution of subdural hygroma following decompressive craniectomy for TBI, with indirect evidence suggesting that disruption of the CSF hydrodynamic equilibrium may precipitate subdural hygroma formation in the post-TBI setting. [8, 19,20,21] However, the pathophysiology is poorly understood, and reported incidence rates have varied markedly. Given that PTH is associated with abnormal CSF circulation, it has been hypothesized that post-craniectomy development of subdural hygroma predicts development of PTH, either as a clinical/radiographic marker or a pathophysiologic element in the chain of causality. [8, 18, 22, 23] However, the relationship between subdural hygroma and development of PTH remains poorly characterized, with data limited to case series or reports, with no higher-quality evidence available. Correspondingly, the goal of the current study was to critically assess and statistically pool systematically identified studies describing subdural hygroma and PTH in the setting of TBI managed by decompressive craniectomy.
Methods
Search strategy
Our search strategy was designed using the Population, Intervention, Comparison, Outcome, Study type (PICOS) question format: Do TBI patients treated with decompressive craniectomy for refractory intracranial pressure elevation (Population), in whom subdural hygroma is detected (Intervention), when compared to those patients in whom subdural hygroma is not detected (Comparator), differ in incidence of PTH (Outcome), based on studies reporting outcomes by multivariate regression (Study Type)? We conducted the review in compliance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines and recommendations. [24] Electronic searches were performed using Ovid Embase, PubMed, SCOPUS, Cochrane Central Register of Controlled Trials (CCTR), Cochrane Database of Systematic Reviews (CDSR), American College of Physicians (ACP) Journal Club and Database of Abstracts of Review of Effectiveness (DARE), from their dates-of-inception to June 2019. Database searches were completed using the following string: (hydrocephalus) AND (decompressive OR craniectomy) AND (hygroma). An example of MeSH translations of terms used in PubMed is provided (Supplementary Table 1).
Selection criteria
All retrieved articles were screened against predetermined selection criteria independently by two investigators (V.M.L. and R.D.) for identification of relevant studies, as per the PRISMA guidelines. Discrepancy was resolved by discussion. Inclusion criteria for all articles were (1) TBI patients managed by decompressive craniectomy, (2) directly reporting a comparative hazard ratio (HR) for PTH confirmed by radiography and symptoms, (3) accompanied by estimation of error (e.g., 95% confidence interval, CI), (4) measuring the effect of subdural hygroma presence, (5) derived from adjusted multivariate regression analysis, and (6) in patients aged ≥ 18 years. Definitions of PTH and subdural hygroma were study-specific; however, all included computed tomography (CT) imaging criteria. The definitions for PTH utilized in this study were the two prevailing definitions of PTH throughout the literature provided by two seminal works: Evans et al. [13] defined PTH as radiological evidence of progressive ventricular dilatation with trans-ependymal edema, together with (1) the presence of either clinical deterioration or failure to make neurological progress over time and (2) some evidence of clinical improvement after ventriculoperitoneal (VP) shunt insertion; Huh et al. [14] defined PTH radiographically as modified frontal horn index ≥ 0.33 based on CT imaging with accompanying symptoms and/or response. Consensus features of PTH included radiological evidence of progressive ventriculomegaly, trans-ependymal edema, and clinical deterioration or failure of neurologic improvement over time, with ambiguous diagnoses supported by clinical improvement after CSF diversion. [8] Subdural hygroma was defined as hypodense, localized, extra-axial regions of subdural CSF accumulation, without laterality restrictions. [7]
Exclusion criteria were (1) decompressive craniectomy for a non-traumatic indication and (2) no HR or comparable metric of effect size with error estimation reported. Where duplicate studies with overlapping cohorts were reported from individual institutions, only the most complete report was included. Studies were limited to English language publications; database studies, review articles, conference abstracts or presentations, and editorials or expert opinions were excluded.
Data extraction
All HR and covariate data from multivariate analyses were abstracted from article texts, tables, and figures. The HRs and their corresponding error estimates included in our analysis were obtained from results of multivariable Cox models directly reported by component studies only. The presence of subdural hygroma was the primary independent variable of interest, analyzed as a predictor of the dependent variable PTH, assessed using measures of effect size and precision.
Meta-analysis
For the outcome of interest, logarithmic HRs and their corresponding standard errors were pooled by meta-analysis of proportions using the generic inverse-variance method to provide the overall summary statistic. I2 was used to estimate heterogeneity across included studies. [25] A random-effects model was tested to account for clinical diversity and methodological variation. Statistical tests were two-sided, significance was defined using the alpha threshold of 0.05, and all analyses were conducted using STATA 14.1 (StataCorp, College Station, Texas).
Certainty, quality, and bias assessments
To evaluate the certainty of the pooled results based on the characteristics of included studies, the strength of evidence was evaluated using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) criteria. [26] To satisfy concerns regarding quality scoring in meta-analyses of observational studies, each included article was appraised using a modified set criteria deriving from the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) and Dutch Cochrane Group Strengthening the reporting of observational studies in epidemiology (STROBE) checklists, to determine the quality of the original study design to answer the PICOS question in light of possible intra-study bias. [27,28,29] If the number of variables pooled for an outcome was at least 10, publication bias was assessed through the generation of a funnel plot, and small study biases were assessed by Egger’s linear regression test and Begg’s correlation tests. [30, 31] A trim-and-fill method was prespecified for recalculation of pooled effect size if bias was suspected, irrespective of sample size. [32]
Results
Search results
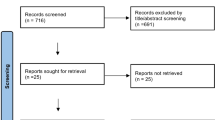
Following a primary search result of 127 articles and the removal of 48 duplicate citations, the titles and abstracts of 79 articles were evaluated against the selection criteria to screen out studies that did not evaluate the hazard of PTH after decompressive craniectomy with mention of subdural hygroma (Fig. 1). Full-text analysis was performed for 19 articles, of which 9 retrospective cohort studies [7,8,9, 18, 22, 23, 33,34,35] published between 2010 and 2019 satisfied all selection criteria (Table 1). All included studies were considered good quality based on a modified set of criteria based on MOOSE and STROBE checklists, when evaluated with respect to the original study design and our PICOS question (Supplementary Table 2).
Demographics and presentation
Collectively, the included studies reported outcomes in 1010 TBI adult patients managed by decompressive craniectomy (Table 1). There were 211 (21%) females; overall median age was 37.5 years (range 33–53). On TBI presentation, median GCS was 7 (range, 5–8), while subarachnoid or intraventricular hemorrhage was reported in 61–94% or 5–29% of patients, respectively. Across three studies [9, 18, 23] that reported mechanism of trauma, incidence of motor vehicle accidents ranged from 44 to 80%, and mechanical fall ranged from 17 to 40%. The number of patients in each study undergoing unilateral (versus bilateral) decompressive craniectomy for management ranged from 49 to 100%. Radiographic CT imaging was obtained for all cases within the first 24 h after craniectomy as standard postoperative protocol and throughout hospitalization. Overall, the median minimum time for PTH surveillance post-craniectomy was 6 months (range 3–6).
Clinical features
All studies defined PTH using the radiographic ventriculomegaly criteria described by Evans et al. [13] or Huh et al. [14] (Table 2). By sum, PTH was reported in 228/840 (27%) cases across all studies. Subdural hygroma was consistently defined as a subdural CSF collection, but no standardized minimal depth threshold was identified, with one of the earlier studies by Honeybul et al. [8] utilizing a 1 cm threshold, whereas three later studies [18, 22, 23] utilized a 0.5 cm threshold (Table 2). Five studies [9, 18, 22, 23, 33] incorporated a mandatory minimum survival threshold for inclusion of > 7 days post-craniectomy. Overall, subdural hygroma was noted in 449/1010 (44%) cases across all studies. As lateral and interhemispheric subdural hygroma could co-present, the incidences by location in descending order were ipsilateral (214/770, 28%), contralateral (132/770, 17%), interhemispheric (108/672, 16%), and bilateral (23/369, 6%) when reported.
Time from craniectomy to subdural hygroma detection was reported by two studies: Yuan et al. [18] reported an average time of 7 days for ipsilateral hygromas, and Vedantam et al. [35] reported average times of 7 and 12 days for ipsilateral and interhemispheric hygromas. Finally, after craniectomy, the proportion of PTH patients that proceeded to cranioplasty with concurrent shunting was reported by three studies: 34/37 (92%) by Nasi et al. [33], 21/24 (88%) by Ki et al. [22], and 10/15 (67%) by Vedantam et al. [35], with only Ki et al. [22] reported a mean time of 4 months from craniectomy to cranioplasty.
Predicting post-traumatic hydrocephalus
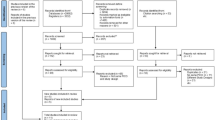
The presence of post-craniectomy subdural hygroma in any location was independently and significantly predictive of PTH in TBI patients treated with decompressive craniectomy, based on pooled HRs from all studies which ranged from 0.66 to 62.6 (Table 3). All but one [7] of the 11 included studies individually indicated that subdural hygroma was an independent and significant predictor of PTH based on multivariable Cox regression analyses, with no log-rank values reported. Overall, the pooled HR was 7.1 (95% CI, 3.3–15.1; I2 = 73%; P-heterogeneity < 0.01) (Fig. 2).
A forest plot of the pooled hazard ratios (HRs) using a random-effects model and their corresponding 95% confidence intervals (95% CIs) of all cohorts investigating prognostication of subdural hygroma for post-traumatic hydrocephalus in traumatic brain injury patients managed by decompressive craniectomy. All hygroma positions were considered. The weighted HR, the 95% CI, and the relative weightings are represented by the middle of the square, the horizontal line, and the relative size of the square, respectively
Certainty assessment
The certainty of how predictive subdural hygroma was for PTH deemed low as per the GRADE criteria primarily due to anatomical and clinical heterogeneity concerns (Table 4).
Bias assessment
No significant asymmetry was noted on funnel plot analysis (Supplementary Fig. 1). Begg (P = 0.77) and Egger (P = 0.19) regression analyses were similarly non-significant. Nevertheless, we performed a trim-and-fill analysis of overall subdural hygroma to address bias concerns related to the wide 95% CI reported by several included studies. This resulted in five studies being trimmed-and-filled, and an associated pooled HR of 2.7 (95% CI, 1.2–6.0; P-heterogeneity < 0.01) (Supplementary Fig. 2).
Discussion
TBI and its sequelae represent a complex network of interrelated pathophysiologic processes, many of which remain poorly understood from a mechanistic perspective. In this context, there has been an increasing awareness that decompressive craniectomy in the setting of TBI may be associated with significantly increased incidences of subdural hygroma and PTH. [8, 18, 22, 23] Indeed, based on our pooled statistic deriving from multivariate regression, the potential of subdural hygroma to predict PTH occurrence appears to have a strong effect size even after accounting for major clinical and demographic parameters, although the certainty of this association was deemed very low based on current literature quality.
The meaningful clinical impact of monitoring subdural hygroma is highly dependent on context, particularly given that a considerable fraction of post-craniectomy, TBI-associated, subdural hygroma will not progress to PTH—a companion population to those patients who develop PTH without preceding hygroma or craniectomy. [1, 10] Although correlations between subdural hygroma and PTH have been reported in this meta-analysis and its component studies, little in the way of causative evidence has been reported. Some authors have suggested that the simplest interpretation is that subdural hygroma and PTH are essentially expressions of the same underlying dysfunction in CSF hydrodynamics, with PTH representing the more severe form of the disease. [7, 22] A related theory holds that these hygromas may represent a focal disruption of CSF homeostasis, whereas PTH indicates a more global dysfunction, which is more likely to occur in patients with established subdural hygroma, but that may also arise independently. [7]
One proposed mechanism linking decompressive craniectomy to PTH in the setting of TBI centers on the intracranial dura-arachnoid interface, where shearing forces from the primary injury may critically interrupt the CSF resorption systems. [22] When such a disruption is then followed by the characteristically large craniectomy required for a trauma management, the abnormal resulting trans-cerebral and intracranial pressure gradients allow for a marked expansion of all the subdural spaces, given the pressure-dependent nature of the proposed mechanism. [36] How exactly subdural hygroma location modulates these pressures with respect to the PTH development remain an area for future investigation, as our study identified multiple different locations at a single-study level confer risk. Nevertheless, with the empiric observation that opening the cranial vault alters intracranial pressures, CSF dynamics, glymphatic drainage, cerebral compliance, and a number of other physiologic parameters, the most likely reality is that PTH results from a polyfactorial concert of variables, including those pertinent to the patient, the injury, and the management strategies—including decompressive craniectomy. [7,8,9,10]
In spite of the lack-of-clarity regarding a truly causal relationship between subdural hygroma and PTH, the independent and statistically significant association between the variables in multiple single institutional studies as well as the current meta-analysis emphasizes the potential importance of subdural hygroma as a clinically relevant risk factor. [8, 9, 18, 22, 23, 33,34,35] Indeed, the limited data would imply that hygroma evolution is a phenomenon that on average occurs within the first 2 weeks following craniectomy. Correspondingly, increased postoperative surveillance for PTH is likely warranted in patients with established subdural hygroma irrespective of location, particularly given the apparent benefit in terms of neurologic outcome following early detection and treatment of PTH via CSF diversion. [37] Notwithstanding, this recommendation is cautiously proposed, given the marked degree of between-study heterogeneity observed with respect to definitions of essentially all the critical variables, including subdural hygroma, PTH, and neurologic status both at baseline and in follow-up. [15]
There is a paucity in current literature about the possible clinical impact of proceeding with cranioplasty after decompressive craniectomy upon PTH risk in TBI patients, with many possible avenues that may confound or augment our subdural hygroma finding. Timing is one concern, where delayed cranioplasty (> 3 months) has been associated with increased hazard for PTH. [33] Another concern is that staged VP shunting after cranioplasty may increase long-term risk of subdural hygroma when compared to simultaneous procedures. [38] Unfortunately, these post-cranioplasty elements were not reported in detail among our included studies as their focus was evaluating acute post-craniectomy subdural hygromas. Nevertheless, charting the longer-term clinical course poses a target for upcoming studies to better understand if the prognostic nature of subdural hygromas for PTH persists after cranioplasty in this particular setting.
Future studies are required to establish consensus definitions, and to provide more robust evidence regarding the true benefits of increased surveillance after decompressive craniectomy and aggressive CSF diversion protocols, with greater accountability given to co-presenting intracranial complications such as hemorrhages and fractures. [39] Additionally, whether or not the preceding presence of subdural hygroma affects optimal PTH management strategies has not been robustly explored. Nasi et al. [33] was the only study to specify VP shunt insertion in 90% of their PTH cases preceded by subdural hygroma, which all resulted in satisfactory outcomes. Vedantam et al. [35] did allude to not all their PTH cases requiring VP shunting, however, did not elaborate if the precession by subdural hygroma affected their decision-making. Greater clinical granularity and post-PTH follow-up will better disclose if PTH preceded by subdural hygroma (versus not) does indeed follow a different clinical course requiring different management sequence.
Strengths and limitations of the literature
This study strictly adhered to the PRIMSA guidelines and focused solely on the pre-specified association between subdural hygroma as an independent predictor of PTH. By only considering outcomes reported by multivariate analyses, we partially adjusted for selected potential confounders of incident PTH that were assessed in the included studies. However, the retrospective nature of these studies precluded controlling for this intrinsic heterogeneity beyond our trim-and-fill approach.
There are limitations to the quantitative nature of this study. First, neurocritical care is a heterogeneous subspecialty, characterized by considerable local variation in patient population parameters, medical and surgical practice environments, institutional biases, and follow-up protocols. Tendencies to monitor for hygroma evolution using CT imaging may vary between studies, resulting in it being unclear if all possible subdural hygromas were truly detected across all studies as the typical evolution course appears to be in the order of weeks, but that itself requires greater clarity too. The impact of this collective clinical heterogeneity risk is apparent in the wide confidence intervals reported by the meta-analyses, which emphasize the degree of caution one must incorporate when interpreting the results despite our statistical adjustments and risk-of-bias assessments we have incorporated to optimize the present data. Second, the included studies also adversely impact interpretation of the current study results due to the lack of clarity or consistency in a number of critical definitions, more importantly PTH, and to a lesser degree subdural hygroma, neurologic outcome, and TBI severity assessments beyond GCS. For example, the PTH definition by Huh et al. [14] involves a quantitative component which is likely more objective and reliable compared to the descriptive definition by Evans et al. [13]—it is difficult to be sure that presentations defined as PTH by one definition would also be defined as PTH by the other across the included studies.
Finally, many of the included studies did not provide adequately granular, standardized data, essentially obviating the possibility of meaningfully analyzing anatomic location of the hygroma and mechanism-of-injury, two potentially key factors contributing to the development of PTH. Longer follow-up would have also been ideal to chart better the longer-term relevance of our subdural hygroma prognostication both with respect to VP shunt management and cranioplasty. These concerns emphasize again the need for standardization in future reporting, ideally via a prospective multicenter registry, or formal consensus guidelines for related research endeavors.
Conclusions
Subdural hygroma appears to indicate a markedly increased risk of PTH after decompressive craniectomy in the treatment of TBI, a finding that we anticipate will inform improved surveillance protocols and potentially neurologic outcomes in this critical patient population. Greater standardization in subdural hygroma and PTH definitions and treatment protocols, as well as focused translational investigation into the intracranial hydrodynamics following decompressive craniectomy in the setting of TBI, are required to improve understanding of this challenging, complex, and impactful neurosurgical disease.
References
Aarabi B, Hesdorffer DC, Ahn ES, Aresco C, Scalea TM, Eisenberg HM (2006) Outcome following decompressive craniectomy for malignant swelling due to severe head injury. J Neurosurg 104:469–479. https://doi.org/10.3171/jns.2006.104.4.469
De Bonis P, Pompucci A, Mangiola A, D'Alessandris QG, Rigante L, Anile C (2010) Decompressive craniectomy for the treatment of traumatic brain injury: does an age limit exist? J Neurosurg 112:1150–1153. https://doi.org/10.3171/2009.7.Jns09505
Guerra WK, Gaab MR, Dietz H, Mueller JU, Piek J, Fritsch MJ (1999) Surgical decompression for traumatic brain swelling: indications and results. J Neurosurg 90:187–196. https://doi.org/10.3171/jns.1999.90.2.0187
Honeybul S (2017) Decompressive craniectomy for severe traumatic brain injury reduces mortality but increases survival with severe disability. Evidence-based medicine 22:61. https://doi.org/10.1136/ebmed-2016-110616
Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J, Anderson I, Bulters DO, Belli A, Eynon CA, Wadley J, Mendelow AD, Mitchell PM, Wilson MH, Critchley G, Sahuquillo J, Unterberg A, Servadei F, Teasdale GM, Pickard JD, Menon DK, Murray GD, Kirkpatrick PJ (2016) Trial of Decompressive Craniectomy for traumatic intracranial hypertension. N Engl J Med 375:1119–1130. https://doi.org/10.1056/NEJMoa1605215
Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D'Urso P, Kossmann T, Ponsford J, Seppelt I, Reilly P, Wolfe R (2011) Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med 364:1493–1502. https://doi.org/10.1056/NEJMoa1102077
De Bonis P, Sturiale CL, Anile C, Gaudino S, Mangiola A, Martucci M, Colosimo C, Rigante L, Pompucci A (2013) Decompressive craniectomy, interhemispheric hygroma and hydrocephalus: a timeline of events? Clin Neurol Neurosurg 115:1308–1312. https://doi.org/10.1016/j.clineuro.2012.12.011
Honeybul S, Ho KM (2012) Incidence and risk factors for post-traumatic hydrocephalus following decompressive craniectomy for intractable intracranial hypertension and evacuation of mass lesions. J Neurotrauma 29:1872–1878. https://doi.org/10.1089/neu.2012.2356
Kaen A, Jimenez-Roldan L, Alday R, Gomez PA, Lagares A, Alen JF, Lobato RD (2010) Interhemispheric hygroma after decompressive craniectomy: does it predict posttraumatic hydrocephalus? J Neurosurg 113:1287–1293. https://doi.org/10.3171/2010.4.Jns10132
Stiver SI (2009) Complications of decompressive craniectomy for traumatic brain injury. Neurosurg Focus 26:E7. https://doi.org/10.3171/2009.4.Focus0965
Beaumont A, Marmarou A (1999) Treatment of raised intracranial pressure following traumatic brain injury. Critical reviews in neurosurgery : CR 9:207–216
Di G, Zhang Y, Liu H, Jiang X, Liu Y, Yang K, Chen J, Liu H (2019) Postoperative complications influencing the long-term outcome of head-injured patients after decompressive craniectomy. Brain Behav:9. https://doi.org/10.1002/brb3.1179
Evans WA Jr (1942) An encephalographic ratio for estimating ventricular enlargement and cerebral atrophy. Arch Neurol Psychiatr 47:931–937. https://doi.org/10.1001/archneurpsyc.1942.02290060069004
Huh PW, Yoo DS, Cho KS, Park CK, Kang SG, Park YS, Kim DS, Kim MC (2006) Diagnostic method for differentiating external hydrocephalus from simple subdural hygroma. J Neurosurg 105:65–70. https://doi.org/10.3171/jns.2006.105.1.65
Licata C, Cristofori L, Gambin R, Vivenza C (2001) Post-traumatic hydrocephalus/comment. J Neurosurg Sci 45:141
Sun S, Zhou H, Ding ZZ, Shi H (2018) Risk factors associated with the outcome of post-traumatic hydrocephalus. Scandinavian journal of surgery : SJS : official organ for the Finnish surgical society and the Scandinavian surgical society:1457496918812210. doi:https://doi.org/10.1177/1457496918812210
Eguchi S, Aihara Y, Hori T, Okada Y (2011) Postoperative extra-axial cerebrospinal fluid collection--its pathophysiology and clinical management. Pediatr Neurosurg 47:125–132. https://doi.org/10.1159/000330543
Yuan Q, Wu X, Yu J, Sun Y, Li Z, Du Z, Wu X, Zhou L, Hu J (2015) Subdural hygroma following decompressive craniectomy or non-decompressive craniectomy in patients with traumatic brain injury: clinical features and risk factors. Brain Inj 29:971–980. https://doi.org/10.3109/02699052.2015.1004760
Aarabi B, Hesdorffer DC, Simard JM, Ahn ES, Aresco C, Eisenberg HM, McCunn M, Scalea T (2009) Comparative study of decompressive craniectomy after mass lesion evacuation in severe head injury. Neurosurgery 64:927–939. https://doi.org/10.1227/01.NEU.0000341907.30831.D2
Choi I, Park HK, Chang JC, Cho SJ, Choi SK, Byun BJ (2008) Clinical factors for the development of posttraumatic hydrocephalus after decompressive craniectomy. Journal of Korean Neurosurgical Society 43:227–231. https://doi.org/10.3340/jkns.2008.43.5.227
Yang XF, Wen L, Gong JB, Zhan RY (2010) Subdural effusion secondary to decompressive craniectomy in patients with severe traumatic brain injury. Acta Neurochir 152:555–556. https://doi.org/10.1007/s00701-009-0475-2
Ki HJ, Lee H-J, Lee H-J, Yi J-S, Yang J-H, Lee I-W (2015) The Risk Factors for Hydrocephalus and Subdural Hygroma after Decompressive Craniectomy in Head Injured Patients. Journal of Korean Neurosurgical Society 58:254–261. https://doi.org/10.3340/jkns.2015.58.3.254
Su T-M, Lan C-M, Lee T-H, Hsu S-W, Tsai N-W, Lu C-H (2019) Risk factors for the development of posttraumatic hydrocephalus after unilateral decompressive craniectomy in patients with traumatic brain injury. J Clin Neurosci 63:62–67. https://doi.org/10.1016/j.jocn.2019.02.006
Moher D, Liberati A, Tetzlaff J, Althman D (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097. https://doi.org/10.1159/000320313
Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ : British Medical Journal 327:557–560
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O'Connell D, Oxman AD, Phillips B, Schunemann HJ, Edejer T, Varonen H, Vist GE, Williams JW Jr, Zaza S (2004) Grading quality of evidence and strength of recommendations. BMJ (Clinical research ed) 328:1490. https://doi.org/10.1136/bmj.328.7454.1490
Phan K, Tian DH, Cao C, Black D, Yan TD (2015) Systematic review and meta-analysis: techniques and a guide for the academic surgeon. Ann Cardiothorac Surg 4:112–122. https://doi.org/10.3978/j.issn.2225-319X.2015.02.04
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. Jama 283:2008–2012
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, Initiative S (2007) Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 335:806–808. https://doi.org/10.1136/bmj.39335.541782.AD
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed) 315:629–634
Duval S, Tweedie R (2000) Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56:455–463
Nasi D, Gladi M, Di Rienzo A, di Somma L, Moriconi E, Iacoangeli M, Dobran M (2018) Risk factors for post-traumatic hydrocephalus following decompressive craniectomy. Acta Neurochir 160:1691–1698. https://doi.org/10.1007/s00701-018-3639-0
Silva Neto AR, Valenca MM (2019) Transcalvarial brain herniation volume as a predictor of posttraumatic hydrocephalus after decompressive craniectomy. Clin Neurol Neurosurg 182:73–78. https://doi.org/10.1016/j.clineuro.2019.05.003
Vedantam A, Yamal J-M, Hwang H, Robertson CS, Gopinath SP (2018) Factors associated with shunt-dependent hydrocephalus after decompressive craniectomy for traumatic brain injury. J Neurosurg 128:1547–1552. https://doi.org/10.3171/2017.1.JNS162721
Kilincer C, Hamamcioglu MK (2010) Contralateral subdural effusion secondary to decompressive craniectomy: differences in patients with large hemispheric infarctions and traumatic brain injury. Medical principles and practice : international journal of the Kuwait University, Health Science Centre 19:499; author reply 500. doi:https://doi.org/10.1159/000320313
Kowalski RG, Weintraub AH, Rubin BA, Gerber DJ, Olsen AJ (2018) Impact of timing of ventriculoperitoneal shunt placement on outcome in posttraumatic hydrocephalus. J Neurosurg:1–12. https://doi.org/10.3171/2017.7.Jns17555
Schuss P, Borger V, Güresir Á, Vatter H, Güresir E (2015) Cranioplasty and Ventriculoperitoneal shunt placement after Decompressive Craniectomy: staged surgery is associated with fewer postoperative complications. World neurosurgery 84:1051–1054. https://doi.org/10.1016/j.wneu.2015.05.066
Honeybul S, Ho KM (2014) Decompressive craniectomy for severe traumatic brain injury: the relationship between surgical complications and the prediction of an unfavourable outcome. Injury 45:1332–1339. https://doi.org/10.1016/j.injury.2014.03.007
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not required.
Informed consent
Not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 96 kb)
Rights and permissions
About this article
Cite this article
Lu, V.M., Carlstrom, L.P., Perry, A. et al. Prognostic significance of subdural hygroma for post-traumatic hydrocephalus after decompressive craniectomy in the traumatic brain injury setting: a systematic review and meta-analysis. Neurosurg Rev 44, 129–138 (2021). https://doi.org/10.1007/s10143-019-01223-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-019-01223-z