Abstract
In medical refractory temporal lobe epilepsy (TLE), the epileptogenic zone can be difficult to identify and therefore difficult to treat, especially in the absence of clear MRI pathologies and specific results from presurgical evaluation. Invasive monitoring with stereo-electroencephalography (sEEG) is a tool for a better determination of the epileptogenic zone. Here, we investigate the impact of sEEG on decision-making in temporal lobe epilepsy surgery. We reviewed patients with TLE who underwent further investigation with sEEG in our epilepsy unit. We examined specifically how sEEG findings influenced our decision regarding indication for a surgical procedure and resection volume. From 2013 to 2017, we performed 152 temporal resections in epilepsy patients. Twenty-one of these patients were designated for further preoperative investigation with sEEG due to incongruent findings in presurgical evaluation. Six patients were implanted bitemporally. In five cases, the hypothesis for the epileptogenic zone and localization had to be changed due to sEEG findings and resulted in a different tailored resection than intended. In three cases, sEEG findings led to the cancelation of the originally intended temporal resection as the epileptogenic zone was not definable or bilateral. In another three cases, the prognosis for reduction of seizures postoperatively had to be reduced due to the sEEG findings. However, the resection was performed after interdisciplinary discussion and informed consent of the patient. The examination by sEEG led to a change of plan for further treatment in 13 patients (61.9%) suffering TLE in total. Invasive monitoring with sEEG electrodes had a strong impact on decision-making for further treatment in patients suffering from temporal lobe epilepsy with incongruent findings in presurgical examination designated for epilepsy surgery. This applies to resection volumes as well as to prediction of seizure outcome.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Refractory temporal lobe epilepsy (TLE) is generally a domain of epilepsy surgery due to good seizure outcome. Invasive diagnostic is seldom necessary [1]. However, when the combination of preoperative clinical and diagnostic data is incongruent or relevant MRI features are missing, invasive monitoring like stereo-electroencephalography (sEEG) becomes necessary. This is also the case when lateralization is questionable or bilateral seizure onset is assumed. Furthermore, sEEG is a useful tool to discriminate between the different mesial subtypes described by Bartolomei et al. [2, 3]. The goal of sEEG is to delineate the epileptogenic zone in a clinically useful way [4]. Surgical planning must take into account not only the epileptogenic zone but also the eloquent cortex areas all of which can be determined by sEEG. Recently, guidelines for the indication and use of sEEG were published [5]. A number of studies already describe the seizure outcome after sEEG recording [6, 7]. However, there is hardly any data on how the immense amount of data obtained from sEEG exploration is influencing daily practice in an appropriate way and how this affects our surgical planning in TLE. In this paper, we present an approach to interpreting sEEG data in TLE and the consequences that can be drawn from it.
Methods
All patients suffering from pharmaco-resistant TLE who were designated for further invasive monitoring by stereo- electroencephalography between June 2013 and December 2017 were included for evaluation. Patients had already undergone evaluation, performed at our Epilepsy Center, comprised of history of seizure, semiology, video electroencephalography (EEG) monitoring, 3 Tesla magnetic resonance imaging (MRI), neuropsychological testing, and partially single photon emission computed tomography (SPECT) and/or positron emission tomography (PET); language lateralization was determined by functional transcranial Doppler sonography [8]. MRI involved a T2-weighted space dark fluid, a T1-weighted magnetization-prepared rapid gradient-echo (MP-RAGE), and a FLAIR volume dataset. Likewise, a T2-weighted turbo spin echo (TSE) coronal, a T1-weighted turbo inversion recovery (TIR) coronal, and a proton density (PD)/T2-weighted axial dataset were acquired. Planning of sEEG trajectories and electrode implantations were performed by WH and JAK in cooperation with ML and TM at the University Medical Center Hamburg-Eppendorf. All patients received a postoperative MRI during their hospital stay. The patients were followed up through clinical investigations and EEG at our Epilepsy Center. Seizure outcome was evaluated according to the ILAE classification system whereby class 1–4 were defined as improvement [9]. Analysis of correlative clinical data was conducted retrospectively by chart review. Informed consent for clinical data evaluation was provided by all patients. The indication for surgical treatment was set in an interdisciplinary presurgical conference with epileptologists, neurosurgeons, and neuropsychologists. Qualitative variables are outlined as numbers and percentages. Statistical analysis of the data was performed by a univariate analysis (Fisher’s exact test) to examine correlations between the parameters using IBM® SPSS® Statistics 22 (IBM Corporation, Armonk, NY, USA). Our epilepsy data register is approved by the local ethic committee at the medical council of the state of Hamburg (Ethik-Kommission der Ärztekammer Hamburg – PV-4585).
Results
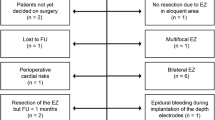
Out of 152 surgical candidates suffering temporal lobe epilepsy, 21 (13.8%) patients were designated for investigation by sEEG. In our center, patients selected for sEEG have undergone non-invasive investigations consisting of seizure history, semiology, video-EEG, MRI, and neuropsychological exam. In a second step, functional imaging like PET and SPECT are conducted. If the collected results are still incongruent, sEEG is advised (Fig. 1). Bilateral implantation was mostly indicated for patients with temporal seizures with no clear lateralization in semiology and a bilateral pathological EEG, and in patients with a temporal lesion and an incongruent EEG finding. sEEG planning required a hypothesis for the individual epileptogenic zone which was established during presurgical conference. Localization and number of electrodes are summarized in Table 1. The results of presurgical evaluation are summarized in Table 2. Our analysis showed that the hypothesis regarding the epileptogenic zone was confirmed more frequently in patients with bilaterally implanted electrodes than in patients with only unilateral implantation (85.7% vs. 28.6%, p = 0.024). Using a non-parametric Kursal-Wallis test, we found no significant association between surgical outcome (significant reduction of seizure frequency, ILAE class I) and the number of implanted electrodes (p = 0.260).
Flow-chart illustrating the consecutive steps in the work-up for epilepsy surgery (a). Decision-tree. Group I (“surgery as planned”): n = 8, ILAE 1: 75.0%; group II (“diff. tailored resection”): n = 6, ILAE 1: 33.3%; group III (“declined prognosis”): n = 4, ILAE 1: 25.0%; group IV (“no surgery”): n = 3 (b)
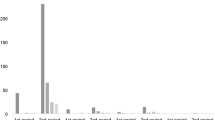
MRI abnormalities were found in 11 patients. This includes hippocampal sclerosis (n = 4), cavernoma (n = 1), heterotopia (n = 1), residual lesions (n = 4), and hemosiderin (n = 1). The detection of a lesion in 3-T MRI showed no correlation with the probability of a confirmed hypothesis (Fig. 2; p = 0.055, regression analysis).
Seventeen patients (81%) were found eligible for surgery after sEEG. Based on the sEEG results, we were able to define four different groups based on the original hypothesis (Fig. 1). Group I is defined by the confirmation of the preliminary hypothesis through sEEG (n = 8, 38.1%). No further investigation is needed, and the planned surgery is carried out. Groups II–IV are characterized by their divergence from the original hypothesis. Group II (n = 6, 28.6%) is characterized by a different epileptogenic zone than expected which leads to a different tailored surgical resection. Group III (n = 4, 19.0%) has a main epileptogenic zone but also at least one recorded epileptogenic focus distant from it. Surgery will be suggested as planned but with a declined prognosis. Group IV (n = 3, 14.3%) shows a bitemporal or indefinable seizure onset zone. No epilepsy surgery will be performed. Examples are presented in Figs. 3 and 4. Hence, 13 patients (61.9%) received a different treatment than initially planned. Nine patients showed a significant reduction in seizure frequency after epilepsy surgery after 1 year. In three of them, sEEG did not confirm the original hypothesis. Histological work-up revealed hippocampal sclerosis (HS; n = 3), focal cortical dysplasia (FCD; n = 5), tumor (n = 2), and gliosis (n = 1). No histological diagnosis correlated with an increased use of sEEG. These results are summarized in Table 3.
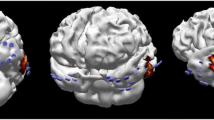
Group II example: MRI revealed a paraventricular heterotopia left (yellow ellipse). sEEG on the other hand diagnosed a clear seizure zone in the left hippocampus (red star). Both pathologies were then resected. The functional coupling between these lesions has already been described [10]
Seizure outcome was evaluated according to the ILAE classification and mean follow-up was 18 months. ILAE class 1 was found in 75.0% of patients in group I, 33.3% of patients in group II, and 25.0% of patients in group III. Group IV patients were all designated to deep-brain stimulation in the course (Table 4). One patient showed an intracerebral hemorrhage after sEEG explantation. In our own cohort of TLE, patients treated by surgery without prior sEEG evaluation 71.1% achieved ILAE class 1.
Discussion
There are several series evaluating the role of sEEG in different types of epilepsy in the literature [11, 12]. We focused on temporal lobe electrodes only in order to achieve as homogenous a cohort as possible. A rate of resection of 81.0% after sEEG recordings is comparable to others [6]. Of those, 12 patients (57.1%) were treated differently or were confronted with a declined seizure prognosis in the course of sEEG recordings which led to a logic step-by-step treatment-decision pattern for results in stereo-electroencephalography in temporal lobe epilepsy. Four groups based on the sEEG findings and the initial seizure-hypothesis could be established. The first group, group I, is characterized by the confirmation of the hypothesis by sEEG. Groups II–IV are distinguished by sEEG findings incongruent to the initial hypothesis and thereby leading to different treatment strategies. Furthermore, patients can be advised individually according to sEEG results. This could already be shown for frontal lobe epilepsies [13]. The classification in four different groups is not only feasible for patient communication but also for communication between the different specialties in epilepsy surgery as proposed by the ILAE routinely [14]. Sindou et al. already briefly addressed the influence of sEEG on TLE surgery but did not clearly define the resulting subgroups [15].
The use of bilateral sEEG electrodes in TLE correlated positively with the confirmation of the hypothesis. This seems odd at first glance, since bilateral sEEG might suggest a very vague hypothesis. On the other hand, bilateral electrodes can contribute to a much more precise picture of the course of seizure development by ruling out a contralateral propagation and thereby lead to a better prediction regarding successful epilepsy surgery [16, 17]. Bilateral recording might also lead to a better seizure outcome in otherwise poorly to localize epilepsy [18]. Temporal lesions visible on MRI did not contribute to a higher percentage of confirmed hypotheses. This can be explained by a mismatch of the lesions’ localization and the additional findings during diagnostic evaluation, pointing to a different organized epileptogenic zone comparable to the temporal plus epilepsies [19]. On the other hand, MR seemed to have an impact on outcome while narrowly missing statistical significance. Structural lesions in drug-resistant epilepsy are generally associated with a favorable seizure outcome after surgery [20]. An impact on confirmation of the hypothesis or seizure outcome could not be established due to the heterogeneous findings.
Seizure freedom in 75.0% of group I patients is comparable to seizure outcome after epilepsy surgery without preceding invasive sEEG and also to our own cohort of TLE patients treated by surgery where 71.1% achieved ILAE class 1. This supports that a strong pre-implantation hypothesis is crucial for the patients.
TLE is predisposed to sEEG due to the densely folded nature of the temporal lobe. Subdural grid electrodes would not be able to record adequately the temporomesial structures and carry a greater risk for perioperative morbidity [21, 22].
This data presents a comprehensible tool for decision-making in TLE after sEEG. This paradigm is important and useful for giving advice to the patient and/or guardian during the process of finding the right treatment strategy. Furthermore, it helps to assess the seizure outcome for the patient which is perhaps one of the most important facts to discuss with the individual when decisions regarding epilepsy surgery have to be made.
Conclusion
We could show that investigation by sEEG has a strong impact on daily decision-making regarding further treatment in TLE for patients with incongruent findings in presurgical evaluation. The sEEG results helped to tailor personalized therapy regimens and to identify patients where epilepsy surgery would be without benefit.
References
de Tisi J, Bell GS, Peacock JL, McEvoy AW, Harkness WF, Sander JW, Duncan JS (2011) The long-term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: a cohort study. Lancet 378:1388–1395. https://doi.org/10.1016/S0140-6736(11)60890-8
Bartolomei F, Wendling F, Vignal JP, Kochen S, Bellanger JJ, Badier JM, Le Bouquin-Jeannes R, Chauvel P (1999) Seizures of temporal lobe epilepsy: identification of subtypes by coherence analysis using stereo-electro-encephalography. Clin Neurophysiol 110:1741–1754
Kahane P, Bartolomei F (2010) Temporal lobe epilepsy and hippocampal sclerosis: lessons from depth EEG recordings. Epilepsia 51(Suppl 1):59–62. https://doi.org/10.1111/j.1528-1167.2009.02448.x
Luders HO, Najm I, Nair D, Widdess-Walsh P, Bingman W (2006) The epileptogenic zone: general principles. Epileptic Disord 8(Suppl 2):S1–S9
Jayakar P, Gotman J, Harvey AS, Palmini A, Tassi L, Schomer D, Dubeau F, Bartolomei F, Yu A, Krsek P, Velis D, Kahane P (2016) Diagnostic utility of invasive EEG for epilepsy surgery: indications, modalities, and techniques. Epilepsia 57:1735–1747. https://doi.org/10.1111/epi.13515
Cossu M, Cardinale F, Castana L, Citterio A, Francione S, Tassi L, Benabid AL, Lo Russo G (2005) Stereoelectroencephalography in the presurgical evaluation of focal epilepsy: a retrospective analysis of 215 procedures. Neurosurgery 57:706–718
Gonzalez-Martinez J, Bulacio J, Alexopoulos A, Jehi L, Bingaman W, Najm I (2013) Stereoelectroencephalography in the “difficult to localize” refractory focal epilepsy: early experience from a north American epilepsy center. Epilepsia 54:323–330. https://doi.org/10.1111/j.1528-1167.2012.03672.x
House PM, Bruckner KE, Lohmann HH (2011) Presurgical functional transcranial Doppler sonography (fTCD) with intravenous echo enhancing agent SonoVue enables determination of language lateralization in epilepsy patients with poor temporal bone windows. Epilepsia 52:636–639. https://doi.org/10.1111/j.1528-1167.2010.02951.x
Wieser HG, Blume WT, Fish D, Goldensohn E, Hufnagel A, King D, Sperling MR, Luders H, Pedley TA (2001) Commission on Neurosurgery of the International League Against E ILAE commission report. Proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery Epilepsia 42:282–286
Kitaura H, Oishi M, Takei N, Fu YJ, Hiraishi T, Fukuda M, Takahashi H, Shibuki K, Fujii Y, Kakita A (2012) Periventricular nodular heterotopia functionally couples with the overlying hippocampus. Epilepsia 53:e127–e131. https://doi.org/10.1111/j.1528-1167.2012.03509.x
Bonini F, McGonigal A, Scavarda D, Carron R, Regis J, Dufour H, Peragut JC, Laguitton V, Villeneuve N, Chauvel P, Giusiano B, Trebuchon A, Bartolomei F (2018) Predictive factors of surgical outcome in frontal lobe epilepsy explored with stereoelectroencephalography. Neurosurgery 83:217–225. https://doi.org/10.1093/neuros/nyx342
Maillard LG, Tassi L, Bartolomei F, Catenoix H, Dubeau F, Szurhaj W, Kahane P, Nica A, Marusic P, Mindruta I, Chassoux F, Ramantani G (2017) Stereoelectroencephalography and surgical outcome in polymicrogyria-related epilepsy: a multicentric study. Ann Neurol 82:781–794. https://doi.org/10.1002/ana.25081
Holtkamp M, Sharan A, Sperling MR (2012) Intracranial EEG in predicting surgical outcome in frontal lobe epilepsy. Epilepsia 53:1739–1745. https://doi.org/10.1111/j.1528-1167.2012.03600.x
Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, Hirsch E, Jain S, Mathern GW, Moshe SL, Nordli DR, Perucca E, Tomson T, Wiebe S, Zhang YH, Zuberi SM (2017) ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia 58:512–521. https://doi.org/10.1111/epi.13709
Sindou M, Guenot M, Isnard J, Ryvlin P, Fischer C, Mauguiere F (2006) Temporo-mesial epilepsy surgery: outcome and complications in 100 consecutive adult patients. Acta Neurochir 148:39–45. https://doi.org/10.1007/s00701-005-0644-x
Diehl B, Luders HO (2000) Temporal lobe epilepsy: when are invasive recordings needed? Epilepsia 41(Suppl 3):S61–S74
Steriade C, Martins W, Bulacio J, Morita-Sherman ME, Nair D, Gupta A, Bingaman W, Gonzalez-Martinez J, Najm I, Jehi L (2019) Localization yield and seizure outcome in patients undergoing bilateral SEEG exploration. Epilepsia 60:107–120. https://doi.org/10.1111/epi.14624
Hill TC, Rubin BA, Tyagi V, Theobald J, Silverberg A, Miceli M, Dugan P, Carlson C, Doyle WK (2017) The value of diagnostic bilateral intracranial electroencephalography in treatment-resistant focal epilepsy. World Neurosurg 103:1–10. https://doi.org/10.1016/j.wneu.2017.01.093
Barba C, Rheims S, Minotti L, Guenot M, Hoffmann D, Chabardes S, Isnard J, Kahane P, Ryvlin P (2016) Temporal plus epilepsy is a major determinant of temporal lobe surgery failures. Brain 139:444–451. https://doi.org/10.1093/brain/awv372
Jones AL, Cascino GD (2016) Evidence on use of neuroimaging for surgical treatment of temporal lobe epilepsy: a systematic review. JAMA Neurol 73:464–470. https://doi.org/10.1001/jamaneurol.2015.4996
Arya R, Mangano FT, Horn PS, Holland KD, Rose DF, Glauser TA (2013) Adverse events related to extraoperative invasive EEG monitoring with subdural grid electrodes: a systematic review and meta-analysis. Epilepsia 54:828–839. https://doi.org/10.1111/epi.12073
Mullin JP, Shriver M, Alomar S, Najm I, Bulacio J, Chauvel P, Gonzalez-Martinez J (2016) Is SEEG safe? A systematic review and meta-analysis of stereo-electroencephalography-related complications. Epilepsia 57:386–401. https://doi.org/10.1111/epi.13298
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dührsen, L., Sauvigny, T., Ricklefs, F.L. et al. Decision-making in temporal lobe epilepsy surgery based on invasive stereo-electroencephalography (sEEG). Neurosurg Rev 43, 1403–1408 (2020). https://doi.org/10.1007/s10143-019-01175-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-019-01175-4