Abstract
The aim of this study is to discuss the state of the art with regard to established or promising bioelectric therapies meant to alter or control neurologic function. We present recent reports on bioelectric technologies that interface with the nervous system at three potential sites—(1) the end organ, (2) the peripheral nervous system, and (3) the central nervous system—while exploring practical and clinical considerations. A literature search was executed on PubMed, IEEE, and Web of Science databases. A review of the current literature was conducted to examine functional and histomorphological effects of neuroprosthetic interfaces with a focus on end-organ, peripheral, and central nervous system interfaces. Innovations in bioelectric technologies are providing increasing selectivity in stimulating distinct nerve fiber populations in order to activate discrete muscles. Significant advances in electrode array design focus on increasing selectivity, stability, and functionality of implantable neuroprosthetics. The application of neuroprosthetics to paretic nerves or even directly stimulating or recording from the central nervous system holds great potential in advancing the field of nerve and tissue bioelectric engineering and contributing to clinical care. Although current physiotherapeutic and surgical treatments seek to restore function, structure, or comfort, they bear significant limitations in enabling cosmetic or functional recovery. Instead, the introduction of bioelectric technology may play a role in the restoration of function in patients with neurologic deficits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The application of bioelectric stimulation to the nervous system has proven to be an effective option for restoring or augmenting some degree of function in patients with neurologic dysfunction [98]. In particular, functional stimulation of paretic nerves is a clinically vital and promising area of research that warrants significant investigation. At present, a variety of implantable nerve stimulators have been clinically employed and demonstrated to be efficacious in alleviating numerous pathologies. Several implantable devices are already in routine use, including hypoglossal nerve stimulation in patients with severe obstructive sleep apnea [76, 137]; chronic spinal cord stimulation in patients with severe neuropathic pain [153, 160]; direct electrical stimulation of peripheral nerves in patients with bladder, bowel, and sexual dysfunction [30, 40]; and even transcutaneous stimulation of the trigeminal nerve in migraine patients [35, 36, 75, 107, 125, 136, 142]. Deep brain stimulation (DBS) is arguably the most successful example of a bioelectric technology that has transitioned into the clinical realm to treat a variety of neurological disorders with an incredible degree of efficacy [59, 108]. DBS and other neuroprosthetic technologies represent only one example of the rapidly developing field of bioelectronic medicine, which broadly seeks to diagnose and treat disease by integrating the fields of neuroscience, bioengineering, and computer science with medicine [102]. Bioelectronic medicine, as a field, developed from work on modulation of neural networks to treat pathologies (e.g., vagus nerve stimulation has been used to successfully activate the inflammatory reflex and improve rheumatoid arthritis symptoms) [62]. Other examples of bioelectronic medicine include implantation of electrodes on the cortex itself to decode neural signals and drive movement of prosthetic limbs [16, 17].
This manuscript reviews the state of the art with regard to established or promising bioelectric therapies meant to alter or control neurologic function. First, we address the three potential sites for bioelectric technologies to interface with the nervous system—both peripheral and central—while exploring practical and clinical considerations. The three sites include, from distal to proximal, (1) the end organ, (2) the peripheral nervous system, and (3) the central nervous system. At each of these sites, electrodes can either record activity, deliver electrical current, or both, allowing for a broad degree of modulation of physiological activity to ideally mitigate dysfunction from a variety of pathological processes. We also discuss the emerging technologies that have potential in advancing the field of nerve and tissue bioelectric engineering to address a variety of clinical conditions. Lastly, we discuss the legal, engineering, and biological hurdles limiting the translation of bioelectric technologies to the clinical realm.
Materials and methods
A systematic search was executed on PubMed, IEEE, and Web of Science databases from database creation to August 2017. PubMed, Ovid, and Cochrane databases were queried using the following keywords: (“bioelectric” or “stimulation” or “technologies” or “electrode” or “recording” or “neuroprosthetic” or “implant” or “interface”) and (“paresis” or “paralysis” or “neural” or “muscle” or “end organ” or “peripheral” or “nervous” or “system” or “central” or “brain” or “computer interface” or “nerve”). References of each manuscript were checked for additional manuscripts that were of potential relevance to our review. Three investigators independently screened each article according to title and abstract and included any article relating to the application of bioelectric technologies or neuroprosthetics in the peripheral or central nervous system. The full text of each selected article was obtained and analyzed. Because no patient information or animals were involved, Institutional Review Board approval and Institutional Animal Care and Use Committee approval were not required.
Results and discussion
Interfacing with the end organ
End-organ stimulation has gained recent prominence within the field of bioelectric technology and aims to stimulate or augment organ function (e.g., muscles, visceral organs) through electrodes implanted in the organ itself. The end organ can be a receptor organ that subsequently stimulates afferent nerve fibers (e.g., cochlea of the auditory system) or an effector organ (e.g., muscle). In both cases, an electrode can be placed either directly on the end organ or placed on the nerve associated with that organ (Fig. 1). In this section, the former approach will be discussed, while the latter approach will be explored in the section on peripheral nervous system stimulation. One benefit of interfacing with the end organ is that recorded signals are up to 100 times greater when compared to signals from peripheral nerve axons, facilitating signal input acquisition [63]. Further, the end organ is often better able to structurally interface with the mechanical composition of an electrode when compared to soft and delicate neural tissue [63] and oftentimes is more readily accessible than the associated nerve.
A cartoon example of end-organ stimulation within the endplate, exaggerated in size, of a neuromuscular junction (NMJ) is shown. Here, the electrode is not in direct contact with the axonal nerve ending, which is depolarized through current spread. Muscle-electrode interfaces have proven more effective with sensory signal clarity due to an increased biocompatibility of the hard metal electrode and the tough mesodermal tissue [63]
Muscle stimulation
Electrical stimulation of end organs, such as muscles, can be non-invasive, as in the case of transcutaneous electrical stimulation, or invasive, as in the case of implanted electrodes. For example, McDonnall et al. used transcutaneous electrodes to activate the orbicularis oculi muscle and restore blink function in patients with facial paralysis, while simultaneously minimizing painful sensations associated with the stimulation [81]. Similarly, others have implemented transcutaneous muscle stimulation to treat post-surgical pain [24], disuse atrophy [44], as well as chronic back pain [57]. However, transcutaneous muscle stimulation to manage pain has yielded conflicting results, and the efficacy of this approach remains uncertain [34, 112].
For decades, implantable cardiac pacemakers and defibrillators have been in widespread clinical use and have proven efficacious in the long-term treatment of many cardiac disorders [1, 50, 71]. Recently, this technology has been adapted by Mueller et al. to serve as a laryngeal pacemaker system implanted directly into laryngeal muscles in patients with bilateral vocal fold paralysis to improve breathing and swallowing, without compromising vocalization [94]. In a rodent model, electrodes implanted within the gastrocnemius muscle reduced muscle atrophy without affecting motor reinnervation following tibial nerve transection and repair [159]. Electrodes inserted within muscles may also be able to record movements associated with limb tremors and stimulate the muscles to mitigate the tremors. End-organ stimulation of effector organs offers tremendous potential, but many of the advancements in the field are still within the realm of clinical research and are not yet routinely used in clinical practice. In contrast, end-organ bioelectric interfaces of receptor organs are already in routine clinical use, particularly within the auditory system. With the advent of miniature pacemakers, such as Medtronic’s® leadless intracardiac transcatheter pacing system [119] or Nan et al.’s wirelessly chargeable ferromagnetic-piezoelectric antenna [96], we foresee increased versatility and wider application of such technologies in the near future.
Cochlear, retinal, and vestibular implants
In recent decades, electrodes implanted directly in the cochlea have restored hearing in patients with substantial sensorineural hearing loss, which is typically the result of irreversible damage to the cochlear sensory epithelium and auditory nerve. Cochlear implants (CIs) consist of a multi-channel electrode array that is surgically inserted deep into the scala tympani within the cochlea and connected to a receiver stimulator implanted beneath post-auricular soft tissue (Fig. 2). Electric current is delivered from select platinum electrode contacts to the spiral ganglion neurons within Rosenthal’s canal, depolarizing these cells and generating a neural signal at the desired frequency to be propagated along the auditory pathway to the auditory cortex [109]. Approximately a half million deaf or hard-of-hearing children and adults have undergone cochlear implantation worldwide, leading to incredible personal, financial, and societal benefits [26, 56, 82, 139].
A typical cochlear implant system consists of (1) an external sound processor, which accurately converts pressure changes in the air (soundwaves) into electromagnetic signals, (2) an internal implant, which converts the electromagnetic field into electrical current, and (3) an intracochlear multi-electrode array, with delivers the current and depolarizes the first-order auditory neurons. The current from the electrode bypasses damaged cochlear hair cells and stimulates (4) the cochlear nerve, leading to sound perception and hearing. Images provided by Advanced Bionics, Inc. and modified
Retinal implants have also made their way into the clinical realm, with three retinal implant approaches in clinical trials: epiretinal, subretinal, and suprachoroidal implants, all of which relay visual input to the retina to stimulate surviving retinal neurons [99, 146]. Retinal implants are meant to treat degenerative disorders such as retinitis pigmentosa and age-related macular degeneration, which destroy photoreceptors in the retina. Although the functional restoration of vision is limited in resolution, these implants currently allow patients to perceive light and provide some degree of object recognition. However, further refinements and innovations will likely enhance the functional utility of retinal implants [23].
Bilateral vestibulopathy, or Dandy’s syndrome, is a debilitating condition characterized by oscillopsia and unsteadiness during locomotion. Unsteadiness, due to a deficient vestibulo-spinal reflex, and oscillopsia, caused by bilaterally impaired vestibulo-ocular reflexes (VORs), both lead to severe impairment of postural control and image stabilization during head and body movement [60]. In recent years, cochlear implants have been modified to be used as vestibular prosthetics to restore inner ear balance and function in animal models [33, 83, 124]. Many of these experimental devices utilize inertial sensors to detect accelerations of the head, data from which is then used to provide specific electrical signals and patterns to the vestibular system in a compensatory manner.
Pelizzone et al. have recently translated this work to human trials in an effort to restore the VOR [106]. Using a modified cochlear implant with a standard intracochlear array and three additional electrode arrays, each of which to be implanted within the ampullae of the three semicircular canals (lateral, superior, and posterior), the authors electrically stimulated the vestibular end organs based on acceleration forces of the head detected by a gyroscope within the device. Electrical stimulation with the prosthesis, at 1 Hz, provided a significant VOR gain in three implanted patients with vestibular damage, reaching up to 98% of the average VOR gain in healthy patients. A similar clinical study is underway at Johns Hopkins University [19].
Interfacing with the peripheral nervous system
Both transcutaneous and invasive electrical interfaces with peripheral nerves have been implemented into routine clinical practice. From percutaneous cranial nerve stimulation to treat chronic [35, 36, 75, 136, 142] and episodic [107, 125] migraines and neuralgia, to epidural placement of leads in the dorsal root ganglion (DRG) for treatment of neuropathic pain [68], and to direct sacral anterior root stimulation to enhance bowel function post-spinal injury [117], electrical stimulation of nerves or nerve roots has been demonstrated to be a safe and efficacious clinical intervention. Although long-term repercussions of implantable neurostimulators have yet to be fully elucidated, the major shortcomings of implantable devices arise from the fibrotic foreign body response that develops following implantation, particularly following implantation within the peripheral or central nervous system [10]. Restricting the neuroprosthetic device to the epineurium can mitigate any ensuing foreign body response; however, long-term use with this approach can induce histological changes (e.g., fibrosis, perineural thickening, decrease in axon myelination) at the site of electrode lead placement [6]. The safest approach would be the utilization of minimally invasive transcutaneous devices, which at most cause minor irritation or an allergic reaction on the skin [130]. We will now explore several of these transcutaneous, epineural, and intraneural interfaces.
Transcutaneous nerve stimulators
The most neurologically relevant transcutaneous devices are Cefaly® Technology’s cranial transcutaneous nerve stimulators (TNS) that treat two forms of neuralgia: episodic and chronic migraine. All three of their devices, the external trigeminal nerve stimulator [107], supraorbital transcutaneous nerve stimulator [35, 75], and the external occipital nerve stimulator [142], have been demonstrated to be efficacious in decreasing migraine intensity, frequency, and associated pain medication consumption [36]. The electrical stimulation from these devices is thought to block ascending impulses of trigeminal nerve nociceptors and may decrease metabolic activity of the orbitofrontal and anterior cingulate cortices, hence reducing pain signal generation and subsequent pain sensation [74, 125]. Deep neuromodulation has also been used to successfully treat primary headache. Although deep neuromodulation utilizes invasive electrode implantation (e.g., vagus nerve stimulation and sphenopalatine ganglion stimulation), it nonetheless is another approach being used to modulate autonomic pathways underlying the pathophysiological headache mechanisms [88, 114].
Other forms of TNS have been employed by Frigerio and colleagues in stimulating the distal facial nerve branches to elicit blinking of the eye [23]. Similarly, Antonio et al. used TNS to modulate activity within the auricular branch of the vagus nerve and treat spontaneous cardiac baroreflex sensitivity [7]. Additionally, transcutaneous vagus nerve stimulation has been demonstrated to reduce atrial fibrillation in humans [144].
Cuff electrodes
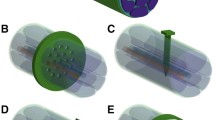
Cuff electrodes are the most basic type of epineural recording or stimulation device. The typical cuff electrode is comprised of a self-coiling, double-layer silicone cuff embedded with two to three platinum foil strips and is wrapped around the outer surface of the nerve, thus providing a direct interface for electrical stimulation (Fig. 3) [46, 73, 97, 128, 148, 149]. The electrode can operate at low stimulus thresholds, thus reducing the likelihood of detrimental nerve damage. Unfortunately, the cuff electrode generally elicits all-or-none neural activity and has a limited ability to target individual fascicles within the nerve fiber [63]. Cuff electrodes have been used safely for years, and although one study in rabbits found that long-term use of cuff electrodes had damaged myelinated axons, these axons were able to regenerate [64]. Furthermore, impedance and stimulation thresholds of cuff electrodes are stable over time, maintaining functionality for over 12 years in peroneal nerve stimulation experiments in hemiplegic patients [4, 149, 155, 158]. Peroneal nerve stimulation can also be accomplished via functional electrical stimulation (i.e., transcutaneous stimulation) of the peroneal nerve, which has been demonstrated to improve gait quality following stroke to the same degree as ankle foot orthotics [11, 135, 141].
a Cuff electrodes and b flat interface nerve electrodes (FINEs) are the most common epineural nerve-electrode interfaces: FINE compresses the nerve and thus has a higher resolution to excite specific fascicles. c Longitudinal intrafascicular electrodes (LIFEs) and d transverse intrafascicular electrodes (TIMEs) are the most common intraneural nerve-electrode interfaces. e Multi-channel electrodes have been used by our group to selectively activate fascicles within cranial nerves. For example, if implanted within the facial nerve, specific channels on the electrode array can activate specific fascicles and corresponding facial muscles
The cuff electrode is routinely used in the clinical setting for vagus nerve stimulation (VNS), which has been shown to be effective in the management of epilepsy [31, 104], resistant depression [127], blood pressure control [110], as well as prevention of heart failure in patients with reduced ejection fractions [32, 43, 113]. Additionally, the Inspire® hypoglossal nerve stimulator, a Food and Drug Administration (FDA)-approved device used for obstructive sleep apnea, utilizes a cuff electrode wrapped around the hypoglossal nerve (Fig. 4). This implant is under clinical trials to augment the oropharyngeal airway by stimulating the motor nerve and lowering the tongue in coordination with the breathing cycle [54, 95]. Multiple mechanisms of action have been proposed to explain the various therapeutic effects of VNS, from emission of diffuse energy centrally towards the brain to disrupt the aberrant signals that contribute to uncontrolled epileptic seizures [13] to sympathetic and parasympathetic modulation of heart rate to prevent arrhythmia [32].
Diagram of the Inspire Upper Airway Stimulation (UAS) system. This is an implantable system that stimulates the hypoglossal nerve to treat obstructive sleep apnea (OSA). The components of the system include an implantable pulse generator (IPG), which is normally placed in the chest and is connected to two leads. The first lead is the sensing lead and is placed into the fourth intercostal space. This senses intercostal muscle contraction and activates the stimulating lead, which is interfaced with the hypoglossal nerve. Additionally, external components that include the physician and patient programmer (sleep remote) make up the system. Image provided by Inspire Medical Systems, Inc.
For purposes of sensory nerve stimulation, a tripolar or a modified monopolar or bipolar electrode would likely be required. Such designs can facilitate unidirectional action potential propagation while mitigating undesired signals in the opposing direction, though this has been difficult to reliably induce in practice. This contrasts with conventional monopolar or bipolar electrodes, which are impractical for administration of current to a single site (Fig. 5) [93]. Nonetheless, with regard to motor stimulation, bidirectional action potential propagation in stimulated efferent neurons can reliably elicit muscle activation and is less of an issue than with sensory nerve stimulation.
a Monopolar electrode configuration yields bidirectional action potential propagation due to uncontrolled depolarization of the contact point. b Bipolar electrode configuration aims to block one direction of action potential propagation by hyperpolarization of the nerve at the anode site. However, such magnitudes of anodic current induce a virtual cathode and negate the cancellation effect, thus yielding a bipolar action potential. c An important aspect of tripolar electrodes is the platinum placements (d2 > d1). Due to a time-shifted hyperpolarization at the further anode (d2), a virtual cathode beyond the closer anode (d1) will not form. However, a virtual cathode will be formed beyond the further anode, which results in a unidirectional action potential
Flat interface nerve electrodes
The flat interface nerve electrode (FINE) is a modified version of the cuff electrode. FINE can be designed in a multi-channel configuration and, moreover, compresses and reshapes the nerve into a flatter conformation, thereby allowing the central fascicles to be closer to the surface and providing more selective axonal population activation (Fig. 3) [63, 66, 105, 151, 161]. Additionally, intraoperative studies in the human femoral nerve showed that muscles innervated by the femoral nerve could be independently and selectively stimulated with a FINE device [133]. Surgical placement of FINE around the femoral trunk leads to selective activation of leg muscles, thereby aiding patients who suffer from lower trunk paralysis to stand from a sitting position [134]. Furthermore, the US Department of Defense has investigated the use of FINE in controlling neural prostheses in amputees [90]. Although FINEs have been used in several human studies without any deleterious consequences, FINEs have the potential to compress the nerve, reduce blood flow, or even cause neural damage [116].
Longitudinal and transverse intrafascicular electrode
In contrast to FINE, intrafascicular electrodes pierce the protective epineurium of the nerve and can stimulate or record from peripheral nerves with higher sensitivity [89, 115]. Notably, a polymer-based, thin-film longitudinal intrafascicular electrode (tfLIFE/polyLIFE) demonstrated no deleterious effects on nerve fiber count, diameter, or myelin thickness while providing higher recording selectivity than standard metal LIFE following 6 months of implantation in rabbit sciatic nerves [65]. LIFE has also been used to detect neural impulses from the median and ulnar nerves in an amputee to manipulate a robotic hand by the human patient [85, 122]. Transverse intrafascicular multi-channel electrodes (TIMEs) provide superior spatial selectivity than LIFE by enabling contact beyond one fascicle (Fig. 3) [14]. Specifically, TIME was developed to manage phantom limb pain in patients who required simultaneous excitation of multiple parallel fascicles within a nerve. This feat previously required the implantation of multiple LIFEs [14].
Penetrating multi-channel arrays
Penetrating intraneural multi-channel arrays offer researchers vast access to diverse neural pathways (Fig. 3). Of note, investigators at the University of Utah have developed two types of multi-array electrodes: the Utah electrode array (UEA) and the Utah slanted electrode array (USEA) [25, 123]. Both are designed with the capacity to contain up to 100 microneedles which are implanted into neural tissue. Previous experiments with the UEA have reported successful recording of volitional motor commands from the central nervous system, among other accomplishments [101]. However, because this technique is highly invasive, it carries the risk of permanent neural damage [38]. Generally, the more invasive the neuroprosthetic interface, the greater the degree of selectivity of neural fiber activation at the sacrifice of potential injury to the nerve. As previously discussed, cuff electrodes, which gently wrap the nerve, generally activate the nerve in an “all-or-none” fashion with limited selectivity, while intraneural multi-channel microelectrode arrays violate the perineurium and may induce glial scarring, but offer exquisite selectivity of neural fiber activation.
Using a penetrating multi-electrode array developed at the University of Michigan, Middlebrooks et al. investigated intraneural cochlear nerve stimulation while recording the downstream auditory pathway output at the brainstem’s inferior colliculus [86, 87]. The NeuroNexus (Ann Arbor, MI) stimulating array consists of 16 iridium-plated electrode contacts placed along a silicon shank, and in every critical auditory electrophysiological measurement in anesthetized cats, the penetrating intraneural array consistently outperformed a conventional intracochlear array. Furthermore, our group recently described the role of intraneural stimulation for facial nerve reanimation in the feline model. In short-term experiments, we achieved selective stimulation of facial muscles with the NeuroNexus multi-electrode array implanted into the main trunk of the facial nerve (Fig. 6) [129]. Electromyography (EMG) responses of four facial muscles were recorded following stimulation through the 16-channel penetrating array. Electrical pulses from each channel of the array selectively activated discrete neural populations within the facial nerve, resulting in independent activation of specific facial muscles. Increases in stimulation current levels resulted in corresponding increases in EMG voltage response. Moreover, long-term presence of the microelectrode array in the facial nerve confirmed the efficacy and stability of this intraneural stimulation approach [131]. Intraneural stimulation of restricted neural populations with one or more multi-channel arrays may play a role in reanimating the face for patients with permanent facial paralysis and may also help rehabilitate other motor and sensory functions throughout the central and peripheral nervous systems [129].
Diagram depicting intraneural stimulation of the facial nerve with a multi-channel electrode array that can selectively activate discrete nerve fiber populations within the facial nerve and elicit selective stimulation of facial muscle groups. Image credit: Modified from Patrick J. Lynch, medical illustrator; C. Carl Jaffe, MD
Interfacing with the central nervous system
Spinal cord stimulation
Spinal cord stimulation (SCS) is an established therapy that has been readily investigated in the treatment of chronic severe pain. The complexity of the neural circuitry at the level of the spinal cord, however, has made it challenging to successfully achieve 50% or greater self-reported pain relief [163]. Beyond pain relief, SCS has been employed in the treatment of movement disorders. Epidural SCS has been successfully demonstrated to disrupt pathological circuit behavior and ultimately mitigates the motor symptoms of Parkinson’s disease in marmosets [132]. This approach is less invasive than deep brain stimulation and may augment pharmacological dopamine replacement therapy in the treatment of Parkinson’s patients. Furthermore, in human trials, restoration of voluntary movement following epidural SCS was achieved in patients with complete motor and sensory lesions [5]. Despite these successes, electrode migration is a common complication of SCS, and if stimulator reprogramming is insufficient in restoring function, surgical repositioning may be required [9]. Although SCS is a safe and effective approach to bioelectric restoration of function, electrode failure/fracture, cerebrospinal fluid leakage, spinal epidural hematoma, and other complications may hinder the functional performance and ultimate utility of SCS [9].
Brain computer interfaces
Brain computer interfaces (BCIs) have been investigated for decades, leading to advancements in recording from various parts of the brain to restore a variety of functions [100, 152]. In essence, a variety of recording devices such as electroencephalogram (EEG), magnetoencephalogram (MEG), electrocorticography (ECoG), functional magnetic resonance imaging (fMRI), and functional near infrared spectroscopy (fNIRS) detect brain activity (e.g., electrical activity, magnetic activity, blood flow, metabolism) that serves as an input signal to a computer algorithm whose output affects the external environment. The spatiotemporal resolution and therefore the clinical applicability of such devices are dependent on invasive implantation within neural tissue [103]. Currently, non-invasive BCIs have been demonstrated to have therapeutic potential in enabling software-aided communication [20] and robot-assisted object manipulation (e.g., picking up a glass of water) [143] in advanced amyotrophic lateral sclerosis (ALS) patients with locked-in syndrome. Additionally, BCIs have exhibited great potential in aiding paraplegics with recovery [120, 162]. Nenadic et al. successfully used an EEG exoskeleton coupled with an augmented reality training platform to provide superior physical rehabilitation to a paraplegic spinal cord injury patient that enabled him to regain some gait function [61, 157]. Furthermore, Nenadic and colleagues have miniaturized their BCI system as well as decreased associated costs of production [78, 79]. Their goal is to advance their BCI into an implantable system for the complete recovery of gait post-spinal injury or stroke [79, 80].
Non-clinically, BCIs have been used by able-bodied users to control drones [58], smart homes [126], and augmented reality gaming [53]. In theory, non-invasive BCIs hold tremendous potential for human augmentation and alleviation of disability, specifically as battery and other hardware/software technologies evolve; however, as of now, such devices are heavily hindered by signal processing algorithms, input signal differentiation and noise cancelation, and input-output signal mapping that conforms to unique individuals [103].
Intracranial brain computer interfaces (iBCIs) are invasive BCIs that directly interface with neurons to decode motor intent and elicit a response (e.g., artificial limb movement) [51]. iBCIs utilize microelectrodes implanted into the cortical surface, or ECoG grids to either bypass disrupted neural pathways and directly stimulate nerves and muscles or directly control artificial limbs. For example, tetraplegic patients have been able to volitionally control robotic arms [29, 49], while other patients have been able to control their own limbs via iBCI-based approaches [37, 91]. In addition to restoration of motor movement, enhancing communication, as in the case of ALS patients, for example, can also be achieved by iBCI [15, 42, 55].
Regardless of the higher spatiotemporal resolution of invasive iBCIs, no current electrode system can read and interact with individual neurons within the brain. Such a task, however, is anticipated with the introduction of a neural lace by Elon Musk’s Neuralink (San Francisco, CA) [39], which is an ultra-thin cortical mesh ultimately meant to enhance cognition by fusing the human mind with the internet [147]. This feat is not achievable with current technologies, and there is no clear understanding of how this system would process enormous bandwidths of neural activity, how it would be powered, and how it would chronically interface with neurons without damaging them; however, a proof of concept has been presented by Lieber et al., who achieved over 8 months of neural recording in a murine model via a syringe-injected nanoscopic self-assembling intracranial mesh, without causing neurological side effects [72]. Future research efforts directed at incorporating sensory feedback in iBCI setups and improving both recording and stimulation parameters is expected, as it can improve the efficacy of iBCI approaches in the clinical and human augmentation realms.
Cortical stimulation
Additionally, intraparenchymal or subdural cortical electrodes have proven to be stable and effective implants for the treatment of chronic pain and long-term monitoring for seizure identification [67]. Non-invasive cortical stimulation has been repeatedly demonstrated to improve hand function following chronic stroke and has been utilized to varying degrees of success in neurorehabilitation regimens [52, 118]. More invasive subdural cortical stimulation, with a responsive neurostimulator connected to predetermined seizure foci, has been demonstrated to reduce the frequency of debilitating partial seizures in patients with medically intractable epilepsy [92]. Furthermore, peri-infarct stimulation following cortical ischemic stroke, combined with rehabilitation, has been demonstrated to alleviate chronic motor deficits and enhance recovery in a primate model of ischemic stroke [111]. Recently, cortical stimulation combined with brain-state detection and haptic feedback has been demonstrated to augment iBCIs in neurorehabilitation of stroke patients with minimal hand function [41]. Although numerous histological changes (e.g., non-reactive, reactive, and toxic) at the brain implant boundary occur following chronic implantation of cortical electrodes, efforts to mitigate the fibrogliotic encapsulation of electrode leads may expedite the implementation of cortical stimulation technologies in the clinic [145].
Auditory nucleus and ganglion stimulation
For patients with trauma or deformations of the cochlea or auditory nerve, the CI, which is designed to be placed in the cochlea and electrically activate the auditory nerve, is not an option for auditory rehabilitation. Most notably, patients with neurofibromatosis type-2 (NF2) suffer from bilateral vestibular schwannomas and as a result require more central activation of the auditory pathway at the brainstem’s cochlear nucleus [84]. The auditory brainstem implant (ABI), again a modified cochlear implant with a flat array consisting of 12 (ABI by Med-El) to 21 (ABI by Cochlear Ltd.) platinum electrodes on a Dacron mesh backing, was created for NF2 patients [84]. To date, however, ABIs have proven most beneficial for adults without NF2 and in children with cochlear malformations [22, 28, 140]. In one study involving 48 non-NF2 (non-tumor) patients, a mean score of 59% was achieved on open-set speech perception tests [27]. For children suffering from bilateral, ossified cochlea secondary to meningitis, five out of the nine patients experienced a meaningful benefit from ABI, with access to conversational speech and sound field thresholds varying from 25 to 50 dB [8]. Although historically NF2 patients have had exceedingly limited benefit from ABIs due to the detrimental nature of the tumors, more recent studies from Europe have reported considerable success in ABI performance in NF2 patients, including a mean open-set speech perception of 41% at 24 months post-ABI activation in 18 NF2 patients [77]. Notably, a penetrating ABI and an auditory midbrain implant inserted into the inferior colliculus did not lead to an increased benefit over the ABI [69, 70, 138].
Deep brain stimulation
DBS involves the implantation of microelectrodes into specific regions of the brain. An electrical pulse generator is connected to the multiple microelectrodes via microwires and, in the treatment of Parkinson’s disease, will deliver biphasic current pulses (up to 30 microC/cm2) to the subthalamic nucleus (STN) or globus pallidus interna [47]. Surgical treatments for Parkinson’s disease were first described in the 1940s, but it was not until the 1990s that DBS was utilized to treat Parkinson’s by targeting the STN [18]. Since the initial approval of DBS in 2002 by the Food and Drug Administration, over 70,000 patients have been treated with DBS to augment pharmacological dopamine replacement therapy [18]. Technical and surgical issues with DBS hardware are not uncommon, though a lack of standardized adverse event reporting protocols makes it difficult to fully understand the extent of DBS failure infection, lead migration, lead fracture, or skin erosion [18]. Refinements in lead design, placement, and stimulator programming may improve DBS efficacy, battery life, stimulation patterns, and electromagnetic field interference.
DBS has also been successfully used in the management of tremors, depression, obsessive-compulsive disorder, tinnitus, and other conditions, depending on the location of electrode implantation [21, 108, 156]. Developments in DBS technology, such as closed-loop stimulation strategies, have resulted in significantly enhanced management of Parkinson-related motor dysfunction [121]. Unlike traditional DBS approaches which deliver electrical impulses independent of neural feedback, closed-loop approaches constantly monitor neuronal activity to modulate the delivery of electrical stimulation [12]. Furthermore, coordinated reset of neural subpopulations (i.e., administering high-frequency pulse trains in a coordinated way to abolish pathological synchronization) has also been investigated to battle Parkinson’s disease and essential tremor and may even be applicable in epilepsy [48, 150].
Hurdles and conclusions
Despite the promise of the previously discussed bioelectric interfaces, a variety of biological and engineering hurdles must be addressed to implement and improve their clinical utility. Following implantation of any bioelectronic device, the host foreign body response will result in rapid protein adsorption onto the material surface, resulting in a cascade of inflammatory events mediated by cytokines, chemoattractants, and growth factors released by neutrophils, monocytes, and lymphocytes [2, 45]. The acute polymorphonuclear cell-mediated inflammatory phase transcends into a chronic inflammatory phase mediated by monocytes and lymphocytes. Fibrous encapsulation of the implanted electrode and the formation of a biofilm containing reactive oxygen species and degradative enzymes result in a variety of functional changes to electrical stimulation and recording, as well as possible structural failures [3]. For a more in-depth overview of the biological effects of chronic implantation of bioelectronic devices, we suggest a recent review by Sahyouni et al. [130].
In addition to mitigating the chronic foreign body response to implanted biomaterials, the development and optimization of hardware/software with a user-friendly and programmable interface remains an engineering challenge [154]. Developing closed-loop devices that can record real-time neural or physiological data and instantaneously modulate stimulation, or recording parameters, will help optimize the functional utility of bioelectronic devices. Ultimately, a multi-disciplinary approach between engineers, industry, and medical professionals will help expedite the translation of bioelectronic devices to the clinic. Applying the lessons learned from successful technologies, such as DBS, that have been implemented into routine clinical practice, will help expedite future technologies that target new pathologies. Although interfacing with the central and peripheral nervous systems offers incredible resolution and has tremendous potential in treating a wide variety of neurological disorders, the complexity and sensitivity of the brain, spinal cord, and nerves makes targeting the end organ an attractive alternative. With successes in the field of cochlear implantation, the extension of end-organ interfaces to effector organs (e.g., muscles) may be fully realized in the near future.
In conclusion, the application of neuroprosthetics to paretic nerves holds great potential in advancing the field of nerve and tissue bioelectric engineering and contributing to clinical care. Although current physiotherapeutic and surgical treatments seek to restore function, structure, or comfort, they bear significant limitations in enabling full recovery. Instead, the introduction of bioelectric technology may play a role in the restoration of volitional function in patients with nerve deficits.
References
Achilli A, Sassara M, Ficili S, Pontillo D, Achilli P, Alessi C, De Spirito S, Guerra R, Patruno N, Serra F (2003) Long-term effectiveness of cardiac resynchronization therapy in patients with refractory heart failure and “narrow” QRS. J Am Coll Cardiol 42:2117–2124
Anderson JM (2001) Biological responses to materials. Annu Rev Mater Res 31:81–110
Anderson JM, Rodriguez A, Chang DT (2008) Foreign body reaction to biomaterials. In: Seminars in immunology. vol 2. Elsevier, pp 86–100
Andreasen LN, Struijk JJ, Lawrence S (2000) Measurement of the performance of nerve cuff electrodes for recording. Med Biol Eng Comput 38:447–453
Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ (2014) Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 137:1394–1409. https://doi.org/10.1093/brain/awu038
Anholt TA, Ayal S, Goldberg JA (2011) Recruitment and blocking properties of the CardioFit stimulation lead. J Neural Eng 8:034004. https://doi.org/10.1088/1741-2560/8/3/034004
Antonino D, Teixeira AL, Maia-Lopes PM, Souza MC, Sabino-Carvalho JL, Murray AR, Deuchars J, Vianna LC (2017) Non-invasive vagus nerve stimulation acutely improves spontaneous cardiac baroreflex sensitivity in healthy young men: a randomized placebo-controlled trial. Brain Stimul 10:875–881. https://doi.org/10.1016/j.brs.2017.05.006
Bayazit Y, Kosaner J, Celenk F, Somdas M, Yilmaz I, Altin G, Cevizci R, Yavuz H, Ozluoglu L (2016) Auditory brainstem implant in postlingual postmeningitic patients. Laryngoscope 126:1889–1892. https://doi.org/10.1002/lary.25731
Bendersky D, Yampolsky C (2014) Is spinal cord stimulation safe? A review of its complications. World Neurosurg 82:1359–1368. https://doi.org/10.1016/j.wneu.2013.06.012
Benfield J, Maknojia A, Epstein F (2016) Progressive paraplegia from spinal cord stimulator lead fibrotic encapsulation: a case report. Am J Phys Med Rehabil 95:e30–e33. https://doi.org/10.1097/PHM.0000000000000411
Bethoux F, Rogers HL, Nolan KJ, Abrams GM, Annaswamy TM, Brandstater M, Browne B, Burnfield JM, Feng W, Freed MJ, Geis C, Greenberg J, Gudesblatt M, Ikramuddin F, Jayaraman A, Kautz SA, Lutsep HL, Madhavan S, Meilahn J, Pease WS, Rao N, Seetharama S, Sethi P, Turk MA, Wallis RA, Kufta C (2014) The effects of peroneal nerve functional electrical stimulation versus ankle-foot orthosis in patients with chronic stroke: a randomized controlled trial. Neurorehabil Neural Repair 28:688–697. https://doi.org/10.1177/1545968314521007
Beuter A, Lefaucheur JP, Modolo J (2014) Closed-loop cortical neuromodulation in Parkinson’s disease: an alternative to deep brain stimulation? Clin Neurophysiol 125:874–885. https://doi.org/10.1016/j.clinph.2014.01.006
Bonaz B, Picq C, Sinniger V, Mayol JF, Clarencon D (2013) Vagus nerve stimulation: from epilepsy to the cholinergic anti-inflammatory pathway. Neurogastroenterol Motil 25:208–221. https://doi.org/10.1111/nmo.12076
Boretius T, Badia J, Pascual-Font A, Schuettler M, Navarro X, Yoshida K, Stieglitz T (2010) A transverse intrafascicular multichannel electrode (TIME) to interface with the peripheral nerve. Biosens Bioelectron 26:62–69. https://doi.org/10.1016/j.bios.2010.05.010
Bouchard KE, Mesgarani N, Johnson K, Chang EF (2013) Functional organization of human sensorimotor cortex for speech articulation. Nature 495:327–332. https://doi.org/10.1038/nature11911
Bouton C (2017) Cracking the neural code, treating paralysis and the future of bioelectronic medicine. J Intern Med 282:37–45. https://doi.org/10.1111/joim.12610
Bouton CE, Shaikhouni A, Annetta NV, Bockbrader MA, Friedenberg DA, Nielson DM, Sharma G, Sederberg PB, Glenn BC, Mysiw WJ, Morgan AG, Deogaonkar M, Rezai AR (2016) Restoring cortical control of functional movement in a human with quadriplegia. Nature 533:247–250. https://doi.org/10.1038/nature17435
Bronstein JM, Tagliati M, Alterman RL, Lozano AM, Volkmann J, Stefani A, Horak FB, Okun MS, Foote KD, Krack P, Pahwa R, Henderson JM, Hariz MI, Bakay RA, Rezai A, Marks WJ Jr, Moro E, Vitek JL, Weaver FM, Gross RE, DeLong MR (2011) Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues. Arch Neurol 68:165. https://doi.org/10.1001/archneurol.2010.260
Carey J (2016) Multichannel vestibular implant early feasibility study. Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT02725463?cond=Multichannel+Vestibular+Implant+Early+Feasibility+Study&rank=1. Accessed 11 Jul 2017
Chaudhary U, Xia B, Silvoni S, Cohen LG, Birbaumer N (2017) Brain-computer interface-based communication in the completely locked-in state. PLoS Biol 15:e1002593. https://doi.org/10.1371/journal.pbio.1002593
Chen XL, Xiong YY, GL X, Liu XF (2013) Deep brain stimulation. Interv Neurol 1:200–212. https://doi.org/10.1159/000353121
Choi JY, Song MH, Jeon JH, Lee WS, Chang JW (2011) Early surgical results of auditory brainstem implantation in nontumor patients. Laryngoscope 121:2610–2618. https://doi.org/10.1002/lary.22137
Chuang AT, Margo CE, Greenberg PB (2014) Retinal implants: a systematic review. Br J Ophthalmol 98:852–856. https://doi.org/10.1136/bjophthalmol-2013-303708
Cipriano G Jr, de Camargo Carvalho AC, Bernardelli GF, Tayar Peres PA (2008) Short-term transcutaneous electrical nerve stimulation after cardiac surgery: effect on pain, pulmonary function and electrical muscle activity. Interact Cardiovasc Thorac Surg 7:539–543. https://doi.org/10.1510/icvts.2007.168542
Clark GA, Ledbetter NM, Warren DJ, Harrison RR (2011) Recording sensory and motor information from peripheral nerves with Utah slanted electrode arrays. Conf Proc: Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Conf 2011:4641–4644. https://doi.org/10.1109/IEMBS.2011.6091149
Clinkard D, Barbic S, Amoodi H, Shipp D, Lin V (2015) The economic and societal benefits of adult cochlear implant implantation: a pilot exploratory study. Cochlear Implants Int 16:181–185. https://doi.org/10.1179/1754762814Y.0000000096
Colletti V, Shannon R, Carner M, Veronese S, Colletti L (2009) Outcomes in nontumor adults fitted with the auditory brainstem implant: 10 years’ experience. Otol Neurotol 30:614–618. https://doi.org/10.1097/MAO.0b013e3181a864f2
Colletti V, Shannon RV (2005) Open set speech perception with auditory brainstem implant? Laryngoscope 115:1974–1978. https://doi.org/10.1097/01.mlg.0000178327.42926.ec
Collinger JL, Wodlinger B, Downey JE, Wang W, Tyler-Kabara EC, Weber DJ, McMorland AJ, Velliste M, Boninger ML, Schwartz AB (2013) High-performance neuroprosthetic control by an individual with tetraplegia. Lancet 381:557–564. https://doi.org/10.1016/S0140-6736(12)61816-9
Creasey GH, Craggs MD (2012) Functional electrical stimulation for bladder, bowel, and sexual function. Handb Clin Neurol 109:247–257. https://doi.org/10.1016/B978-0-444-52137-8.00015-2
Cukiert A (2015) Vagus nerve stimulation for epilepsy: an evidence-based approach. Prog Neurol Surg 29:39–52. https://doi.org/10.1159/000434654
De Ferrari GM (2014) Vagal stimulation in heart failure. J Cardiovasc Transl Res 7:310–320. https://doi.org/10.1007/s12265-014-9540-1
Della Santina CC, Migliaccio AA, Patel AH (2007) A multichannel semicircular canal neural prosthesis using electrical stimulation to restore 3-d vestibular sensation. IEEE Trans Biomed Eng 54:1016–1030. https://doi.org/10.1109/TBME.2007.894629
Deyo RA, Walsh NE, Martin DC, Schoenfeld LS, Ramamurthy S (1990) A controlled trial of transcutaneous electrical nerve stimulation (TENS) and exercise for chronic low back pain. New Engl J Med 322:1627–1634
Di Fiore P, Bussone G, Galli A, Didier H, Peccarisi C, D’Amico D, Frediani F (2017) Transcutaneous supraorbital neurostimulation for the prevention of chronic migraine: a prospective, open-label preliminary trial. Neurol Sci 38:201–206. https://doi.org/10.1007/s10072-017-2916-7
Diener HC, Charles A, Goadsby PJ, Holle D (2015) New therapeutic approaches for the prevention and treatment of migraine. Lancet Neurol 14:1010–1022. https://doi.org/10.1016/S1474-4422(15)00198-2
Ethier C, Oby ER, Bauman MJ, Miller LE (2012) Restoration of grasp following paralysis through brain-controlled stimulation of muscles. Nature 485:368–371. https://doi.org/10.1038/nature10987
Fernandez E, Greger B, House PA, Aranda I, Botella C, Albisua J, Soto-Sanchez C, Alfaro A, Normann RA (2014) Acute human brain responses to intracortical microelectrode arrays: challenges and future prospects. Front Neuroeng 7:24. https://doi.org/10.3389/fneng.2014.00024
Gaggioli A (2017) Cyborg-Psychology. Cyberpsychol Behav Soc Netw 20:458–458
George AT, Dudding TC, Gurmany S, Kamm MA, Nicholls RJ, Vaizey CJ (2014) Pudendal nerve stimulation for bowel dysfunction in complete cauda equina syndrome. Ann Surg 259:502–507. https://doi.org/10.1097/SLA.0b013e31828e7602
Gharabaghi A, Kraus D, Leao MT, Spuler M, Walter A, Bogdan M, Rosenstiel W, Naros G, Ziemann U (2014) Coupling brain-machine interfaces with cortical stimulation for brain-state dependent stimulation: enhancing motor cortex excitability for neurorehabilitation. Front Hum Neurosci 8:122. https://doi.org/10.3389/fnhum.2014.00122
Gilja V, Pandarinath C, Blabe CH, Nuyujukian P, Simeral JD, Sarma AA, Sorice BL, Perge JA, Jarosiewicz B, Hochberg LR, Shenoy KV, Henderson JM (2015) Clinical translation of a high-performance neural prosthesis. Nat Med 21:1142–1145. https://doi.org/10.1038/nm.3953
Gold MR, Van Veldhuisen DJ, Hauptman PJ, Borggrefe M, Kubo SH, Lieberman RA, Milasinovic G, Berman BJ, Djordjevic S, Neelagaru S, Schwartz PJ, Starling RC, Mann DL (2016) Vagus nerve stimulation for the treatment of heart failure. The INOVATE-HF Trial J Am Coll Cardiol 68:149–158. https://doi.org/10.1016/j.jacc.2016.03.525
Gould N, Donnermeyer D, Gammon GG, Pope M, Ashikaga T (1983) Transcutaneous muscle stimulation to retard disuse atrophy after open meniscectomy. Clin Orthop Relat Res 178:190–197
Gretzer C, Emanuelsson L, Liljensten E, Thomsen P (2006) The inflammatory cell influx and cytokines changes during transition from acute inflammation to fibrous repair around implanted materials. J Biomater Sci Polym Ed 17:669–687
Grill WM, Mortimer JT (1998) Stability of the input-output properties of chronically implanted multiple contact nerve cuff stimulating electrodes. IEEE Trans Rehabil Eng 6:364–373
Grill WM, Wei XF (2009) High efficiency electrodes for deep brain stimulation. Conf Proc: Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Conf 2009:3298–3301. https://doi.org/10.1109/IEMBS.2009.5333774
Hauptmann C, Popovych O, Tass PA (2005) Effectively desynchronizing deep brain stimulation based on a coordinated delayed feedback stimulation via several sites: a computational study. Biol Cybern 93:463–470. https://doi.org/10.1007/s00422-005-0020-1
Hochberg LR, Bacher D, Jarosiewicz B, Masse NY, Simeral JD, Vogel J, Haddadin S, Liu J, Cash SS, van der Smagt P, Donoghue JP (2012) Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature 485:372–375. https://doi.org/10.1038/nature11076
Hochleitner M, Hortnagl H, Hortnagl H, Fridrich L, Gschnitzer F (1992) Long-term efficacy of physiologic dual-chamber pacing in the treatment of end-stage idiopathic dilated cardiomyopathy. Am J Cardiol 70:1320–1325
Hu K, Bounni F, Williams Z (2017) Editorial. Advancement in brain-machine interfaces for patients with tetraplegia: neurosurgical perspective. Neurosurg Focus 43:E5. https://doi.org/10.3171/2017.5.FOCUS17244
Hummel F, Celnik P, Giraux P, Floel A, WH W, Gerloff C, Cohen LG (2005) Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain 128:490–499. https://doi.org/10.1093/brain/awh369
Iidal Y, Tsutsumi D, Saeki S, Ootsuka Y, Hashimoto T, Horie R (2017) The effect of immersive head mounted display on a brain computer interface game. In: Advances in Affective and Pleasurable Design. Springer, pp 211–219
Inspire Medical Systems Inc (2016) Stimulation therapy for apnea reduction. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02725463. Accessed 11 Jul 2017
Jarosiewicz B, Sarma AA, Bacher D, Masse NY, Simeral JD, Sorice B, Oakley EM, Blabe C, Pandarinath C, Gilja V, Cash SS, Eskandar EN, Friehs G, Henderson JM, Shenoy KV, Donoghue JP, Hochberg LR (2015) Virtual typing by people with tetraplegia using a self-calibrating intracortical brain-computer interface. Sci Transl Med 7:313ra179. https://doi.org/10.1126/scitranslmed.aac7328
Jung D, Bhattacharyya N (2012) Association of hearing loss with decreased employment and income among adults in the United States. Ann Otol Rhinol Laryngol 121:771–775. https://doi.org/10.1177/000348941212101201
Khadilkar A, Milne S, Brosseau L, Wells G, Tugwell P, Robinson V, Shea B, Saginur M (2005) Transcutaneous electrical nerve stimulation for the treatment of chronic low back pain: A systematic review. Spine 30:2657–2666. https://doi.org/10.1097/01.brs.0000188189.21202.0f
Khan MJ, Hong KS (2017) Hybrid EEG-fNIRS-based eight-command decoding for BCI: application to quadcopter control. Front Neurorobot 11:6. https://doi.org/10.3389/fnbot.2017.00006
Khanna VK Implantable medical electronics : prosthetics, drug delivery, and health monitoring. Springer. https://doi.org/10.1007/978-3-319-25448-7
Kim S, Oh YM, Koo JW, Kim JS (2011) Bilateral vestibulopathy: clinical characteristics and diagnostic criteria. Otol Neurotol 32:812–817. https://doi.org/10.1097/MAO.0b013e31821a3b7d
King CE, Wang PT, McCrimmon CM, Chou CC, Do AH, Nenadic Z (2015) The feasibility of a brain-computer interface functional electrical stimulation system for the restoration of overground walking after paraplegia. J Neuroeng Rehabil 12:80. https://doi.org/10.1186/s12984-015-0068-7
Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, Mehta AD, Levine YA, Faltys M, Zitnik R, Tracey KJ, Tak PP (2016) Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci U S A 113:8284–8289. https://doi.org/10.1073/pnas.1605635113
Langhals NB, Urbanchek MG, Ray A, Brenner MJ (2014) Update in facial nerve paralysis: tissue engineering and new technologies. Curr Opin Otolaryngol Head Neck Surg 22:291–299. https://doi.org/10.1097/MOO.0000000000000062
Larsen JO, Thomsen M, Haugland M, Sinkjaer T (1998) Degeneration and regeneration in rabbit peripheral nerve with long-term nerve cuff electrode implant: a stereological study of myelinated and unmyelinated axons. Acta Neuropathol 96:365–378
Lawrence SM, Larsen JO, Horch KW, Riso R, Sinkjaer T (2002) Long-term biocompatibility of implanted polymer-based intrafascicular electrodes. J Biomed Mater Res 63:501–506. https://doi.org/10.1002/jbm.10303
Lertmanorat Z, Montague FW, Durand DM (2009) A flat interface nerve electrode with integrated multiplexer. IEEE Trans Neural Syst Rehabil Eng 17:176–182. https://doi.org/10.1109/TNSRE.2008.2009307
Leuthardt EC, Schalk G, Moran D, Ojemann JG (2006) The emerging world of motor neuroprosthetics: a neurosurgical perspective. Neurosurgery 59:1–14; discussion 11-14. https://doi.org/10.1227/01.NEU.0000221506.06947.AC
Liem L, Russo M, Huygen FJ, Van Buyten JP, Smet I, Verrills P, Cousins M, Brooker C, Levy R, Deer T, Kramer J (2015) One-year outcomes of spinal cord stimulation of the dorsal root ganglion in the treatment of chronic neuropathic pain. Neuromodulation 18:41–48; discussion 48-49. https://doi.org/10.1111/ner.12228
Lim HH, Lenarz M, Lenarz T (2009) Auditory midbrain implant: a review. Trends Amplif 13:149–180. https://doi.org/10.1177/1084713809348372
Lim HH, Lenarz T (2015) Auditory midbrain implant: research and development towards a second clinical trial. Hear Res 322:212–223. https://doi.org/10.1016/j.heares.2015.01.006
Linde C, Leclercq C, Rex S, Garrigue S, Lavergne T, Cazeau S, McKenna W, Fitzgerald M, Deharo JC, Alonso C, Walker S, Braunschweig F, Bailleul C, Daubert JC (2002) Long-term benefits of biventricular pacing in congestive heart failure: results from the MUltisite STimulation in cardiomyopathy (MUSTIC) study. J Am Coll Cardiol 40:111–118
Liu J, TM F, Cheng Z, Hong G, Zhou T, Jin L, Duvvuri M, Jiang Z, Kruskal P, Xie C, Suo Z, Fang Y, Lieber CM (2015) Syringe-injectable electronics. Nat Nanotechnol 10:629–636. https://doi.org/10.1038/nnano.2015.115
Loeb GE, Peck RA (1996) Cuff electrodes for chronic stimulation and recording of peripheral nerve activity. J Neurosci Methods 64:95–103
Magis D, D’Ostilio K, Thibaut A, De Pasqua V, Gerard P, Hustinx R, Laureys S, Schoenen J (2017) Cerebral metabolism before and after external trigeminal nerve stimulation in episodic migraine. Cephalalgia 37:881–891. https://doi.org/10.1177/0333102416656118
Magis D, Sava S, d’Elia TS, Baschi R, Schoenen J (2013) Safety and patients’ satisfaction of transcutaneous supraorbital neurostimulation (tSNS) with the Cefaly(R) device in headache treatment: a survey of 2,313 headache sufferers in the general population. J Headache Pain 14:95. https://doi.org/10.1186/1129-2377-14-95
Malhotra A (2014) Hypoglossal-nerve stimulation for obstructive sleep apnea. N Engl J Med 370:170–171. https://doi.org/10.1056/NEJMe1314084
Matthies C, Brill S, Varallyay C, Solymosi L, Gelbrich G, Roosen K, Ernestus RI, Helms J, Hagen R, Mlynski R, Shehata-Dieler W, Muller J (2014) Auditory brainstem implants in neurofibromatosis type 2: is open speech perception feasible? J Neurosurg 120:546–558. https://doi.org/10.3171/2013.9.JNS12686
McCrimmon C, Fu J, Wang M, Lopes LS, Wang P, Karimi-Bidhendi A, Liu C, Heydari P, Nenadic Z, Do A (2017) Performance assessment of a custom, portable and low-cost brain-computer interface platform. IEEE Trans Biomed Eng 64(10):2313–2320
McCrimmon CM, Ming W, Silva Lopes L, Wang PT, Karimi-Bidhendi A, Liu CY, Heydari P, Nenadic Z, Do AH (2016) A small, portable, battery-powered brain-computer interface system for motor rehabilitation. Conf Proc: Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Conf 2016:2776–2779. https://doi.org/10.1109/EMBC.2016.7591306
McCrimmon CM, Wang PT, Heydari P, Nguyen A, Shaw SJ, Gong H, Chui LA, Liu CY, Nenadic Z, Do AH (2017) Electrocorticographic encoding of human gait in the leg primary motor cortex. Cereb Cortex 11:1–11
McDonnall D, Guillory KS, Gossman MD (2009) Restoration of blink in facial paralysis patients using FES. In: Neural Engineering, NER'09. 4th International IEEE/EMBS Conference on, 2009. IEEE, pp 76–79
McKinnon BJ (2014) Cost effectiveness of cochlear implants. Curr Opin Otolaryngol Head Neck Surg 22:344–348. https://doi.org/10.1097/MOO.0000000000000091
Merfeld DM, Haburcakova C, Gong W, Lewis RF (2007) Chronic vestibulo-ocular reflexes evoked by a vestibular prosthesis. IEEE Trans Biomed Eng 54:1005–1015. https://doi.org/10.1109/TBME.2007.891943
Merkus P, Di Lella F, Di Trapani G, Pasanisi E, Beltrame MA, Zanetti D, Negri M, Sanna M (2014) Indications and contraindications of auditory brainstem implants: systematic review and illustrative cases. Eur Arch Otorhinolaryngol 271:3–13. https://doi.org/10.1007/s00405-013-2378-3
Micera S, Citi L, Rigosa J, Carpaneto J, Raspopovic S, Di Pino G, Rossini L, Yoshida K, Denaro L, Dario P (2010) Decoding information from neural signals recorded using intraneural electrodes: toward the development of a neurocontrolled hand prosthesis. Proc IEEE 98:407–417
Middlebrooks JC, Snyder RL (2008) Intraneural stimulation for auditory prosthesis: modiolar trunk and intracranial stimulation sites. Hear Res 242:52–63. https://doi.org/10.1016/j.heares.2008.04.001
Middlebrooks JC, Snyder RL (2010) Selective electrical stimulation of the auditory nerve activates a pathway specialized for high temporal acuity. J Neurosci 30:1937–1946. https://doi.org/10.1523/JNEUROSCI.4949-09.2010
Miller S, Matharu MS (2017) The use of electroceuticals and neuromodulation in the treatment of migraine and other headaches. In: Electroceuticals. Springer, pp 1–33
Minev IR, Chew DJ, Delivopoulos E, Fawcett JW, Lacour SP (2012) High sensitivity recording of afferent nerve activity using ultra-compliant microchannel electrodes: an acute in vivo validation. J Neural Eng 9:026005. https://doi.org/10.1088/1741-2560/9/2/026005
Miranda RA, Casebeer WD, Hein AM, Judy JW, Krotkov EP, Laabs TL, Manzo JE, Pankratz KG, Pratt GA, Sanchez JC, Weber DJ, Wheeler TL, Ling GS (2015) DARPA-funded efforts in the development of novel brain-computer interface technologies. J Neurosci Methods 244:52–67. https://doi.org/10.1016/j.jneumeth.2014.07.019
Moritz CT, Perlmutter SI, Fetz EE (2008) Direct control of paralysed muscles by cortical neurons. Nature 456:639–642. https://doi.org/10.1038/nature07418
Morrell MJ, Group RNSSiES (2011) Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 77:1295–1304. https://doi.org/10.1212/WNL.0b013e3182302056
Mortimer JT, Bhadra N (2004) Peripheral nerve and muscle stimulation. In: Neuroprosthetics: theory and practice, World Scientific Publishing Company River Edge, NJ, pp 638–682
Mueller AH, Hagen R, Foerster G, Grossmann W, Baumbusch K, Pototschnig C (2016) Laryngeal pacing via an implantable stimulator for the rehabilitation of subjects suffering from bilateral vocal fold paralysis: a prospective first-in-human study. Laryngoscope 126:1810–1816. https://doi.org/10.1002/lary.25792
Mwenge GB, Rombaux P, Lengele B, Rodenstein D (2015) Hypoglossal nerve stimulation for obstructive sleep apnea. Prog Neurol Surg 29:94–105. https://doi.org/10.1159/000434660
Nan T, Lin H, Gao Y, Matyushov A, Yu G, Chen H, Sun N, Wei S, Wang Z, Li M, Wang X, Belkessam A, Guo R, Chen B, Zhou J, Qian Z, Hui Y, Rinaldi M, McConney ME, Howe BM, Hu Z, Jones JG, Brown GJ, Sun NX (2017) Acoustically actuated ultra-compact NEMS magnetoelectric antennas. Nat Commun 8:296. https://doi.org/10.1038/s41467-017-00343-8
Naples GG, Mortimer JT, Scheiner A, Sweeney JD (1988) A spiral nerve cuff electrode for peripheral nerve stimulation. IEEE Trans Biomed Eng 35:905–916. https://doi.org/10.1109/10.8670
Navarro X, Krueger TB, Lago N, Micera S, Stieglitz T, Dario P (2005) A critical review of interfaces with the peripheral nervous system for the control of neuroprostheses and hybrid bionic systems. J Peripher Nerv Syst 10:229–258. https://doi.org/10.1111/j.1085-9489.2005.10303.x
Nazari H, Falabella P, Yue L, Weiland J, Humayun MS (2017) Retinal prostheses: a clinical perspective. J VitreoRetinal Dis 1:204–213
Nicolas-Alonso LF, Gomez-Gil J (2012) Brain computer interfaces, a review. Sensors 12:1211–1279
Normann RA, Fernandez E (2016) Clinical applications of penetrating neural interfaces and Utah electrode array technologies. J Neural Eng 13:061003. https://doi.org/10.1088/1741-2560/13/6/061003
Olofsson PS, Tracey KJ (2017) Bioelectronic medicine: technology targeting molecular mechanisms for therapy. J Intern Med 282:3–4. https://doi.org/10.1111/joim.12624
Ordikhani-Seyedlar M, Lebedev MA, Sorensen HB, Puthusserypady S (2016) Neurofeedback therapy for enhancing visual attention: state-of-the-art and challenges. Front Neurosci 10:352. https://doi.org/10.3389/fnins.2016.00352
Orosz I, McCormick D, Zamponi N, Varadkar S, Feucht M, Parain D, Griens R, Vallee L, Boon P, Rittey C, Jayewardene AK, Bunker M, Arzimanoglou A, Lagae L (2014) Vagus nerve stimulation for drug-resistant epilepsy: a European long-term study up to 24 months in 347 children. Epilepsia 55:1576–1584. https://doi.org/10.1111/epi.12762
Park HJ, Durand DM (2015) Motion control of the rabbit ankle joint with a flat interface nerve electrode. Muscle Nerve 52:1088–1095. https://doi.org/10.1002/mus.24654
Pelizzone M, Fornos AP, Guinand N, van de Berg R, Kos I, Stokroos R, Kingma H, Guyot JP (2014) First functional rehabilitation via vestibular implants. Cochlear Implants Int 15(Suppl 1):S62–S64. https://doi.org/10.1179/1467010014Z.000000000165
Penning S, Schoenen J (2017) A survey on migraine attack treatment with the CEFALY(R) device in regular users. Acta Neurol Belg 117:547–549. https://doi.org/10.1007/s13760-017-0757-z
Perlmutter JS, Mink JW (2006) Deep brain stimulation. Annu Rev Neurosci 29:229–257. https://doi.org/10.1146/annurev.neuro.29.051605.112824
Peterson NR, Pisoni DB, Miyamoto RT (2010) Cochlear implants and spoken language processing abilities: review and assessment of the literature. Restor Neurol Neurosci 28:237–250. https://doi.org/10.3233/RNN-2010-0535
Plachta DT, Gierthmuehlen M, Cota O, Espinosa N, Boeser F, Herrera TC, Stieglitz T, Zentner J (2014) Blood pressure control with selective vagal nerve stimulation and minimal side effects. J Neural Eng 11:036011. https://doi.org/10.1088/1741-2560/11/3/036011
Plautz EJ, Barbay S, Frost SB, Friel KM, Dancause N, Zoubina EV, Stowe AM, Quaney BM, Nudo RJ (2003) Post-infarct cortical plasticity and behavioral recovery using concurrent cortical stimulation and rehabilitative training: a feasibility study in primates. Neurol Res 25:801–810. https://doi.org/10.1179/016164103771953880
Pope MH, Phillips RB, Haugh LD, Hsieh CY, MacDonald L, Haldeman S (1994) A prospective randomized three-week trial of spinal manipulation, transcutaneous muscle stimulation, massage and corset in the treatment of subacute low back pain. Spine (Phila Pa 1976) 19:2571–2577
Premchand RK, Sharma K, Mittal S, Monteiro R, Dixit S, Libbus I, DiCarlo LA, Ardell JL, Rector TS, Amurthur B, KenKnight BH, Anand IS (2014) Autonomic regulation therapy via left or right cervical vagus nerve stimulation in patients with chronic heart failure: results of the ANTHEM-HF trial. J Card Fail 20:808–816. https://doi.org/10.1016/j.cardfail.2014.08.009
Puledda F, Goadsby PJ (2016) Current approaches to neuromodulation in primary headaches: focus on vagal nerve and sphenopalatine ganglion stimulation. Curr Pain Headache Rep 20:47. https://doi.org/10.1007/s11916-016-0577-5
Qiao S, Yoshida K (2013) Influence of unit distance and conduction velocity on the spectra of extracellular action potentials recorded with intrafascicular electrodes. Med Eng Phys 35:116–124. https://doi.org/10.1016/j.medengphy.2012.04.008
Raffe MR (1985) Principles of peripheral nerve repair. In: Newton CD, Nunamaker DM (eds) Textbook of Small Animal Orthopaedics. Lippincott Williams & Wilkins, Philadelphia
Rasmussen MM, Kutzenberger J, Krogh K, Zepke F, Bodin C, Domurath B, Christensen P (2015) Sacral anterior root stimulation improves bowel function in subjects with spinal cord injury. Spinal Cord 53:297–301. https://doi.org/10.1038/sc.2015.2
Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, Celnik PA, Krakauer JW (2009) Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci U S A 106:1590–1595. https://doi.org/10.1073/pnas.0805413106
Reynolds D, Duray GZ, Omar R, Soejima K, Neuzil P, Zhang S, Narasimhan C, Steinwender C, Brugada J, Lloyd M, Roberts PR, Sagi V, Hummel J, Bongiorni MG, Knops RE, Ellis CR, Gornick CC, Bernabei MA, Laager V, Stromberg K, Williams ER, Hudnall JH, Ritter P, Micra Transcatheter Pacing Study G (2016) A leadless intracardiac transcatheter pacing system. N Engl J Med 374:533–541. https://doi.org/10.1056/NEJMoa1511643
Rosenfeld JV, Wong YT (2017) Neurobionics and the brain-computer interface: current applications and future horizons. Med J Aust 206:363–368
Rosin B, Slovik M, Mitelman R, Rivlin-Etzion M, Haber SN, Israel Z, Vaadia E, Bergman H (2011) Closed-loop deep brain stimulation is superior in ameliorating parkinsonism. Neuron 72:370–384. https://doi.org/10.1016/j.neuron.2011.08.023
Rossini PM, Micera S, Benvenuto A, Carpaneto J, Cavallo G, Citi L, Cipriani C, Denaro L, Denaro V, Di Pino G, Ferreri F, Guglielmelli E, Hoffmann KP, Raspopovic S, Rigosa J, Rossini L, Tombini M, Dario P (2010) Double nerve intraneural interface implant on a human amputee for robotic hand control. Clin Neurophysiol 121:777–783. https://doi.org/10.1016/j.clinph.2010.01.001
Rousche PJ, Normann RA (1998) Chronic recording capability of the Utah intracortical electrode array in cat sensory cortex. J Neurosci Methods 82:1–15
Rubinstein JT, Bierer S, Kaneko C, Ling L, Nie K, Oxford T, Newlands S, Santos F, Risi F, Abbas PJ, Phillips JO (2012) Implantation of the semicircular canals with preservation of hearing and rotational sensitivity: a vestibular neurostimulator suitable for clinical research. Otol Neurotol 33:789–796. https://doi.org/10.1097/MAO.0b013e318254ec24
Russo A, Tessitore A, Esposito F, Di Nardo F, Silvestro M, Trojsi F, De Micco R, Marcuccio L, Schoenen J, Tedeschi G (2017) Functional changes of the perigenual part of the anterior cingulate cortex after external trigeminal neurostimulation in migraine patients. Front Neurol 8:282. https://doi.org/10.3389/fneur.2017.00282
Saboor A, Rezeika A, Stawicki P, Gembler F, Benda M, Grunenberg T (2017) Volosyak I SSVEP-based BCI in a smart home scenario. In: International Work-Conference on Artificial Neural Networks, Springer, pp 474–485
Sackeim HA, Rush AJ, George MS, Marangell LB, Husain MM, Nahas Z, Johnson CR, Seidman S, Giller C, Haines S, Simpson RK Jr, Goodman RR (2001) Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology 25:713–728. https://doi.org/10.1016/S0893-133X(01)00271-8
Sahin M, Haxhiu MA, Durand DM, Dreshaj IA (1997) Spiral nerve cuff electrode for recordings of respiratory output. J Appl Physiol (1985) 83:317–322
Sahyouni R, Bhatt J, Djalilian HR, Tang WC, Middlebrooks JC, Lin HW (2017) Selective stimulation of facial muscles with a penetrating electrode array in the feline model. Laryngoscope 127:460–465. https://doi.org/10.1002/lary.26078
Sahyouni R, Chang DT, Moshtaghi O, Mahmoodi A, Djalilian HR, Lin HW (2017) Functional and histological effects of chronic neural electrode implantation. Laryngoscope investigative. Otolaryngology 2:80–93
Sahyouni R, Haidar YM, Moshtaghi O, Wang BY, Djalilian HR, Middlebrooks JC, Lin HW (2017) Selective stimulation of facial muscles following chronic intraneural electrode array implantation and facial nerve injury in the feline model. Otol Neurotol. https://doi.org/10.1097/MAO.0000000000001545
Santana MB, Halje P, Simplicio H, Richter U, Freire MAM, Petersson P, Fuentes R, Nicolelis MAL (2014) Spinal cord stimulation alleviates motor deficits in a primate model of Parkinson disease. Neuron 84:716–722. https://doi.org/10.1016/j.neuron.2014.08.061
Schiefer MA, Polasek KH, Triolo RJ, Pinault GC, Tyler DJ (2010) Selective stimulation of the human femoral nerve with a flat interface nerve electrode. J Neural Eng 7:26006. https://doi.org/10.1088/1741-2560/7/2/026006
Schiefer MA, Triolo RJ, Tyler DJ (2008) A model of selective activation of the femoral nerve with a flat interface nerve electrode for a lower extremity neuroprosthesis. IEEE Trans Neural Syst Rehabil Eng 16:195–204. https://doi.org/10.1109/TNSRE.2008.918425
Schiemanck S, Berenpas F, van Swigchem R, van den Munckhof P, de Vries J, Beelen A, Nollet F, Geurts AC (2015) Effects of implantable peroneal nerve stimulation on gait quality, energy expenditure, participation and user satisfaction in patients with post-stroke drop foot using an ankle-foot orthosis. Restor Neurol Neurosci 33:795–807. https://doi.org/10.3233/RNN-150501
Schoenen JE (2016) Migraine prevention with a supraorbital transcutaneous stimulator: a randomized controlled trial. Neurology 86:201–202. https://doi.org/10.1212/01.wnl.0000479686.32453.cc
Schwartz AR, Bennett ML, Smith PL, De Backer W, Hedner J, Boudewyns A, Van de Heyning P, Ejnell H, Hochban W, Knaack L, Podszus T, Penzel T, Peter JH, Goding GS, Erickson DJ, Testerman R, Ottenhoff F, Eisele DW (2001) Therapeutic electrical stimulation of the hypoglossal nerve in obstructive sleep apnea. Arch Otolaryngol Head Neck Surg 127:1216–1223
Schwartz MS, Otto SR, Shannon RV, Hitselberger WE, Brackmann DE (2008) Auditory brainstem implants. Neurotherapeutics 5:128–136. https://doi.org/10.1016/j.nurt.2007.10.068
Semenov YR, Yeh ST, Seshamani M, Wang NY, Tobey EA, Eisenberg LS, Quittner AL, Frick KD, Niparko JK, Team CDI (2013) Age-dependent cost-utility of pediatric cochlear implantation. Ear Hear 34:402–412. https://doi.org/10.1097/AUD.0b013e3182772c66
Sennaroglu L, Ziyal I, Atas A, Sennaroglu G, Yucel E, Sevinc S, Ekin MC, Sarac S, Atay G, Ozgen B, Ozcan OE, Belgin E, Colletti V, Turan E (2009) Preliminary results of auditory brainstem implantation in prelingually deaf children with inner ear malformations including severe stenosis of the cochlear aperture and aplasia of the cochlear nerve. Otol Neurotol 30:708–715. https://doi.org/10.1097/MAO.0b013e3181b07d41
Sheffler LR, Taylor PN, Gunzler DD, Buurke JH, Ijzerman MJ, Chae J (2013) Randomized controlled trial of surface peroneal nerve stimulation for motor relearning in lower limb hemiparesis. Arch Phys Med Rehabil 94:1007–1014. https://doi.org/10.1016/j.apmr.2013.01.024
Silberstein SD, Dodick DW, Saper J, Huh B, Slavin KV, Sharan A, Reed K, Narouze S, Mogilner A, Goldstein J, Trentman T, Vaisman J, Ordia J, Weber P, Deer T, Levy R, Diaz RL, Washburn SN, Mekhail N (2012) Safety and efficacy of peripheral nerve stimulation of the occipital nerves for the management of chronic migraine: results from a randomized, multicenter, double-blinded, controlled study. Cephalalgia 32:1165–1179. https://doi.org/10.1177/0333102412462642
Spataro R, Chella A, Allison B, Giardina M, Sorbello R, Tramonte S, Guger C, La Bella V (2017) Reaching and grasping a glass of water by locked-in ALS patients through a BCI-controlled humanoid robot. Front Hum Neurosci 11:68. https://doi.org/10.3389/fnhum.2017.00068
Stavrakis S, Humphrey MB, Scherlag BJ, Hu Y, Jackman WM, Nakagawa H, Lockwood D, Lazzara R, Po SS (2015) Low-level transcutaneous electrical vagus nerve stimulation suppresses atrial fibrillation. J Am Coll Cardiol 65:867–875. https://doi.org/10.1016/j.jacc.2014.12.026
Stensaas SS, Stensaas LJ (1978) Histopathological evaluation of materials implanted in the cerebral cortex. Acta Neuropathol 41:145–155
Stingl K, Schippert R, Bartz-Schmidt KU, Besch D, Cottriall CL, Edwards TL, Gekeler F, Greppmaier U, Kiel K, Koitschev A, Kuhlewein L, MacLaren RE, Ramsden JD, Roider J, Rothermel A, Sachs H, Schroder GS, Tode J, Troelenberg N, Zrenner E (2017) Interim results of a multicenter trial with the new electronic subretinal implant alpha AMS in 15 patients blind from inherited retinal degenerations. Front Neurosci 11:445. https://doi.org/10.3389/fnins.2017.00445
Strickland E (2017) Silicon valley’s latest craze: brain tech [news]. IEEE Spectr 54:8–9
Struijk JJ, Thomsen M (1995) Tripolar nerve cuff recording: stimulus artifact, EMG and the recorded nerve signal. In: Engineering in Medicine and Biology Society, 1995., IEEE 17th annual conference, IEEE, pp 1105–1106
Struijk JJ, Thomsen M, Larsen JO, Sinkjaer T (1999) Cuff electrodes for long-term recording of natural sensory information. IEEE Eng Med Biol Mag 18:91–98
Tass PA (2003) A model of desynchronizing deep brain stimulation with a demand-controlled coordinated reset of neural subpopulations. Biol Cybern 89:81–88. https://doi.org/10.1007/s00422-003-0425-7
Tyler DJ, Durand DM (2002) Functionally selective peripheral nerve stimulation with a flat interface nerve electrode. IEEE Trans Neural Syst Rehabil Eng 10:294–303. https://doi.org/10.1109/TNSRE.2002.806840
Vallabhaneni A, Wang T, He B (2005) Brain—computer interface. In: He. B (ed) Neural Engineering. Bioelectric Engineering, Springer, Boston, p 85–121. https://doi.org/10.1007/0-306-48610-_3
Van Buyten JP, Al-Kaisy A, Smet I, Palmisani S, Smith T (2013) High-frequency spinal cord stimulation for the treatment of chronic back pain patients: results of a prospective multicenter European clinical study. Neuromodulation 16:59–65; discussion 65-56. https://doi.org/10.1111/ner.12006
van den Brand R, Mignardot JB, von Zitzewitz J, Le Goff C, Fumeaux N, Wagner F, Capogrosso M, Martin Moraud E, Micera S, Schurch B, Curt A, Carda S, Bloch J, Courtine G (2015) Neuroprosthetic technologies to augment the impact of neurorehabilitation after spinal cord injury. Ann Phys Rehabil Med 58:232–237. https://doi.org/10.1016/j.rehab.2015.04.003
Veraart C, Grill WM, Mortimer JT (1993) Selective control of muscle activation with a multipolar nerve cuff electrode. IEEE Trans Biomed Eng 40:640–653. https://doi.org/10.1109/10.237694
Volkmann J, Herzog J, Kopper F, Deuschl G (2002) Introduction to the programming of deep brain stimulators. Mov Disord 17(Suppl 3):S181–S187
Wang PT, King C, Chui LA, Nenadic Z, Do A (2010) BCI controlled walking simulator for a BCI driven FES device. In: Proc of RESNA Ann Conf, Arlington: RESNA
Waters RL, McNeal DR, Faloon W, Clifford B (1985) Functional electrical stimulation of the peroneal nerve for hemiplegia. Long-term clinical follow-up. J Bone Joint Surg Am 67:792–793
Willand MP, Holmes M, Bain JR, Fahnestock M, De Bruin H (2013) Electrical muscle stimulation after immediate nerve repair reduces muscle atrophy without affecting reinnervation. Muscle Nerve 48:219–225. https://doi.org/10.1002/mus.23726
Wolter T (2014) Spinal cord stimulation for neuropathic pain: current perspectives. J Pain Res 7:651–663. https://doi.org/10.2147/JPR.S37589
Yoo PB, Durand DM (2005) Selective recording of the canine hypoglossal nerve using a multicontact flat interface nerve electrode. IEEE Trans Biomed Eng 52:1461–1469. https://doi.org/10.1109/TBME.2005.851482
Zhang R, Wang Q, Li K, He S, Qin S, Feng Z, Chen Y, Song P, Yang T, Zhang Y, Yu Z, Hu Y, Shao M, Li Y (2017) A BCI-based environmental control system for patients with severe spinal cord injuries. IEEE Trans Biomed Eng. https://doi.org/10.1109/TBME.2016.2628861
Zhang TC, Janik JJ, Grill WM (2014) Mechanisms and models of spinal cord stimulation for the treatment of neuropathic pain. Brain Res 1569:19–31. https://doi.org/10.1016/j.brainres.2014.04.039
Funding
This project was supported by grants from the American Neurotology Society, and the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1 TR001414.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This research did not involve any animals; therefore, Institutional Animal Care and Use Committee approval was not required.
Informed consent
This research did not involve human participants or any patient information; thus, Institutional Review Board approval was not required.
Rights and permissions
About this article
Cite this article
Sahyouni, R., Mahmoodi, A., Chen, J.W. et al. Interfacing with the nervous system: a review of current bioelectric technologies. Neurosurg Rev 42, 227–241 (2019). https://doi.org/10.1007/s10143-017-0920-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-017-0920-2