Abstract
The purpose of this study is to compare intervertebral bone fusion and clinical outcomes in L4–5 posterior lumbar interbody fusion (PLIF) using the same posterior instrumentation with four combinations of one of three types of interbody cage with one of two bone grafts, iliac and local or only local. In 67 patients who underwent L4–5 PLIF, 19 patients had the Brantigan cage and iliac and local bone graft, 18 with the TELAMON C cage and iliac and local bone graft, 16 with the TELAMON C cage and local bone graft (TL), and 14 with the OIC PEEK cage and local bone graft. Clinical assessments were based on Japanese Orthopaedic Association (JOA) scores and on the visual analogue scale (VAS). The bone fusion assessments were based on radiography and CT scans according to the Brantigan, Steffee, and Fraser criteria. More than 2 years after surgery, these assessments were made. In the results, the fusion outcome for the group receiving TL was significantly less than those for the other three groups. In TL, multivariate logistic regression analysis showed that the inside volume of the cage of ≥2.0 mL was the only significant factor for incomplete fusion. Moreover, the VAS (low back pain) score was significantly higher for TL than for the other three groups. In conclusions, we believe that the large volume inside the cage (≥2.0 mL) with local bone graft may lead incomplete interbody bone fusion and residual postsurgical low back pain after PLIF.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In a 1985 review, Cloward [4], who had been the first to develop the use of autologous iliac bone grafts (IBGs) for posterior lumbar interbody fusion (PLIF), asserted that PLIF is the answer to the treatment of diseases of the lumbar spine and may be the operation of the future. During the past 10 years, less invasive surgery using local bone grafts (LBGs) obtained from the laminectomy has been used instead of IBG for the interbody fusion [10, 13, 19, 24], achieving fusion outcomes similar to those with IBG. However, various materials, such as allografts [1], platelet gels [3, 25], ceramic synthetic bone-graft substitutes [5, 25], demineralized bone matrix [18], and recombinant human bone morphogenetic proteins 2 [2, 6, 9] and 7 [23], have been used to obtain more complete interbody fusion. Therefore, fusion outcome may be related not only to the type of bone graft, but also to the types of cage and posterior instrumentation, or to surgical skill. This retrospective cohort study compared fusion and clinical outcomes achieved at more than two postsurgical years by the same surgeon who did L4–L5 single-level PLIFs using the same posterior instrumentation with four combinations of one of three types of interbody cage with one of two sources of bone graft (iliac and local, or only local).
Materials and methods
Case selection
Records of patients who had undergone L4–L5 single-level PLIF performed by the same surgeon (M.K.) at the university hospital from April 2004 through December 2009 were reviewed. The indication for PLIF was based on a diagnosis of lumbar spinal stenosis with spondylolisthesis from clinical and radiographic assessments. The Japanese Orthopaedic Association (JOA) score [14, 20] was used for clinical assessment. The visual analogue scale (VAS) [12] graded on a 100-mm scale to evaluate low back pain and leg pain was also used for clinical assessment.
Radiologic assessment was based on reviewing plain radiographs. A finding of greater than 10 mm sagittal translation in the lateral radiographic view of the patient in the lumbar neutral position, or of greater than 5° local kyphosis in the lateral radiographic view of the patient in the lumbar flexion position, constituted diagnosis of lumbar instability. Cases of trauma, infection, previous lumbar surgery, and surgery at other levels were excluded.
PLIF procedure and postsurgical treatment
Two thirds of the upper lamina toward bony lesion was removed while maintaining integrity of the interspinous and supraspinous ligaments. A pair of TSRH-RP pedicle screws (Texas Scottish Rite Hospital spinal system, Medtronic Sofamor Danek, Memphis, TN, USA) was inserted from the entry point at the lateral border of the superior facet where it intersected the midportion of the transverse process at the bilateral L4 and L5, and two screws, another pair of TSRH-RP pedicle screws, were inserted on the other side. The pairs of screws were connected by a titanium rod [17]. Disc material and cartilaginous endplates were excised to make the graft bed. Bone grafts were harvested from the posterior iliac crest or were removed from the lamina and crushed by bone milling. Two cages of the same type were filled with crushed bone graft (Fig. 1a). The extent of the volume of the cages filled with bone graft was determined according to the cage manufacturer’s instructions. Then, the cages were implanted into the lateral and anterior portions of the interbody space (Fig. 1b), then the remaining bone chips were inserted outside of the cage (Fig. 1c). Additional materials, such as allografts, platelet gels, ceramics, demineralized bone matrix, or bone morphogenetic proteins were not used.
Postsurgically, patients were required to wear a fitted, rigid brace for at least 3 months.
Postsurgical radiologic and clinical assessments
For radiologic assessment, anteroposterior (AP) and lateral radiographic views with the patient in flexion and extension positions were examined by two independent observers who had not participated in PLIF surgery and who were blinded at the more than 2-year postsurgical assessment. Any movement detected between the vertebral bodies or lucency with a diameter greater than 1 mm observed within the cage or at the cage-bone interface on lateral radiographs was considered a sign of non-fusion.
CT scanning for sagittal and coronal image reconstruction of the involved lumbar segments and the bony structure was done to evaluate the success of fusion at the more than 2-year postsurgical assessment. Classification of interbody fusion success was evaluated according to criteria based on Brantigan, Steffee, and Fraser (BSF) criteria; BSF-1: Radiographic pseudarthrosis (non-fusion) is indicated by collapse of the construct, loss of disc height, vertebral slip, broken screws, displacement of the carbon cage, or significant resorption of the bone graft or lucency visible around the periphery of the graft or cage. BSF-2: Radiographic locked pseudarthrosis (questionable fusion) is indicated by lucency visible in the middle of the cages with solid bone growing into the cage from each vertebral endplate. BSF-3: Radiographic fusion is indicated by bone bridges at least half of the fusion area with at least the density originally achieved at surgery. Radiographic fusion through one cage (half of the fusion area) is considered to be mechanically solid fusion even if there is lucency on the opposite side [8]. At the same time, the VAS and the JOA score were for repeated clinical assessment.
Statistical analysis
Statistical tests used to compare differences among treatment groups are identified on the tables and the figure presenting results. For incomplete fusion group, to assess the factors affecting fusion, multivariate logistic regression models were used to estimate odds ratios (ORs), P values, and associated 95 % confidence intervals, and these following factors were examined: smoking (positive), sex (woman), surgical time and blood loss, and the inner volumes of various cage sizes. The kappa statistic was used to determine interrater reliability for the postsurgical radiographic assessments [16].
Data were analyzed with SPSS (version 16; SPSS, Chicago, Illinois, USA). Alpha was set at 0.05.
Study approval, informed consent, and funding
The study was approved by the institutional review board of the university hospital, and study subjects provided informed consent. The study did not receive any external funding, and authors do not have any disclosures to declare.
Results
Demographics and surgical details
Review of records showed that 67 patients (24 men and 43 women) underwent L4–L5 single-level PLIF by the same surgeon (M.K.) during April 2004 through December 2009. Their median (range) age was 67 (36 to 80) years, and the median (range) duration of follow-up was 34 (24 to 62) months. The records were sorted into four groups according to cage type and bone graft (Table 1). Age, sex, follow-up times, smoker or nonsmoker, and surgical time and blood loss were not statistically significantly different among the four groups (Table 2).
Fusion outcomes
Outcomes were compared by groups taken in two different ways: by the four groups according to the four different combinations of cages and bone graft and by the two groups according to different sources of bone graft (iliac and local or only local) regardless of the type of cage.
As evaluated at final follow-up by observing AP and lateral flexion-extension radiographic images, a patient in the BI (Brantigan I/F cage; iliac and local bone graft) group had no fusion. Based on BSF classification, fusion for the TL (TELAMON C cage; local bone graft) group was statistically significantly less than those for the BI (P = 0.02, χ 2 test) and TI (TELAMON C cage; iliac and local bone graft) groups (P = 0.03, χ 2 test) and OL (OIC PEEK Interbody; local bone graft) group (P = 0.03, χ 2 test) (Tables 3). Fusions in the iliac and local bone graft (IBG) and local bone graft (LBG) groups on the BSF criteria after surgery were not significantly different (P = 0.09, χ 2 test, Table 4). The concordance of interrater reliability for these evaluations was 81 %, which was almost perfect [16].
Clinical assessment at final follow-up
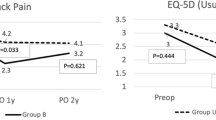
Presurgically, the JOA score, the VAS (low back pain), and the VAS (leg pain) scores were not significantly different when compared by the four combinations of source of bone graft. At final follow-up, the JOA score and the VAS (leg pain) scores were also not significantly different. However, the VAS (low back pain) score was significantly higher for the TL group than for the other three groups (P = 0.01, ANOVA, Table 5). In the TL group, the change of the JOA score and the VAS score for low back pain and the VAS for leg pain score between presurgical and final follow-up time were not significantly among BSF fusion classification (JOA score; P = 0.51, VAS for low back pain; P = 0.59, VAS for leg pain; P = 0.50, two-way ANOVA).
Multivariate logistic regression models for the TL group
In the factors affecting poor fusion and poor clinical outcome, multivariate logistic regression analysis in the TL group showed that the inside volume of cage of ≥2.0 mL was the only significant factor for incomplete fusion 34 months after PLIF (Table 6).
Discussion
We found that at a 34-month follow-up, fusion outcomes were the same for iliac bone grafts and local bone grafts used for L4–L5 single-level PLIF. However, the fusion outcomes at a 34-month follow-up for the TL group were statistically significantly less than those of the BI and TI groups and the OL group. As for clinical assessment, we found that the VAS (low back pain) score was significantly higher for the TL group compared with the other three groups. Therefore, it is very likely that the TELAMON C cage with local bone graft lead to incomplete fusion or inadequate clinical outcome.
We considered two reasons why the TELAMON C cage with local bone graft led incomplete interbody fusion. First, there might be a relationship between bone fusion and the amount of bone grafts, because the fusion rate in the TELAMON C cage with local bone graft was significantly less than that for the TELAMON C cage with iliac and local bone graft. Second, inside volume of cages might also affect the bone fusion. Because by using different inside volumes of cages with same local bone graft, there was a significant worse bone fusion in TL group compared with OL group. The volume inside the cage we used from catalogues was not significant between BI, TI, and TL; however, the cages in the OL group had significantly less volume than the cages of the other three groups (Fig. 2). Moreover, in our result, multivariate logistic regression models of incomplete fusion in the TL group showed that larger inside volume of the cage was statistically significantly related to incomplete fusion. Quantities of laminar bone chips for local bone grafts (LBG) were limited, and we conjectured that the larger volume of the TELAMON C cage compared with that of the OIC cage meant that less LBG was available to transplant around the TELAMON C cage. Shar et al. indicated that bony fusion is most likely be observed at the lateral zone in the intervertebral disc space where PLIF is performed [22]. They argued that for successful outcomes of PLIF, the amount of bone transplanted needs to be sufficient for the insertion into the intervertebral disc space posterior and lateral to the cages. For all of these reasons, we speculated that large volume inside the cage with only local bone graft led incomplete interbody fusion because of a lack of the bone transplantation around the cage.
As to clinical outcomes at the 34-month follow-up, among the four combinations of cage and source of bone graft, the improvement of the VAS (low back pain) was significantly less in the TL group than in the other three groups. Thus, the TL group, which had more incomplete fusion than the other three groups, also had more back pain at final follow-up. Some previous reports presented that non-union did not affect the clinical result in the short term [7, 11]. By contrast, other previous reports noted that some patients with incomplete fusion have persistent or recurrent symptoms after surgery [15, 21]. The relationship between incomplete bone fusion and clinical outcomes has been controversial. In our results, it is probable that the large volume cages (TELAMON C cage) with local bone graft leading the incomplete fusion will be related to residual postsurgical low back pain. Thus, we believe that the large volume cages with local bone graft may be the cause of the residual postsurgical low back pain, and the type of the graft with amount is important for fusion rates for PLIF.
This study had limitations. It was a retrospective comparison, not a prospective, randomized controlled trial. Only three types of cages were used. If more types had been used, stronger results might have been obtained.
Conclusions
The type of cage or the amount of grafts used in PLIF could lead to incomplete fusion or inadequate clinical outcome. We believe that iliac bone grafts should be added to local bone grafts if the volume inside the cage is ≥2.0 mL. We recommend that a cage with a small inside volume be used to transplant the bone chips around the cage if local bone grafting is used. Incomplete interbody bone fusion after PLIF may be related to residual postsurgical low back pain.
References
An HS, Lynch K, Toth J (1995) Prospective comparison of autograft vs. allograft for adult posterolateral lumbar spine fusion: differences among freeze-dried, frozen, and mixed grafts. J Spinal Disord 8:1341–135
Boden SD, Kang J, Sandhu H, Heller JG (2002) Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine (Phila Pa 1976) 27:2662–2673
Carreon LY, Glassman SD, Anekstein Y, Puno RM (2005) Platelet gel (AGF) fails to increase fusion rates in instrumented posterolateral fusions. Spine (Phila Pa 1976) 30:E243–6, discussion E247
Cloward RB (1985) Posterior lumbar interbody fusion updated. Clin Orthop Relat Res 193:16–19
Dai LY, Jiang LS (2008) Single-level instrumented posterolateral fusion of lumbar spine with beta-tricalcium phosphate versus autograft: a prospective, randomized study with 3-year follow-up. Spine (Phila Pa 1976) 33:1299–1304
Dimar JR 2nd, Glassman SD, Burkus JK, Pryor PW, Hardacker JW, Carreon LY (2009) Clinical and radiographic analysis of an optimized rhBMP-2 formulation as an autograft replacement in posterolateral lumbar spine arthrodesis. J Bone Joint Surg Am 91:1377–1386
Fischgrund JS, Mackay M, Herkowitz HN, Brower R, Montgomery DM, Kurz LT (1997) 1997 Volvo Award winner in clinical studies. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine (Phila Pa 1976) 22:2807–2812
Fogel GR, Toohey JS, Neidre A, Brantigan JW (2008) Fusion assessment of posterior lumbar interbody fusion using radiolucent cages: X-ray films and helical computed tomography scans compared with surgical exploration of fusion. Spine J 8:570–577
Haid RW Jr, Branch CL Jr, Alexander JT, Burkus JK (2004) Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J 4:527–538, discussion 538–539
Hashimoto T, Oha F, Shigenobu K, Kanayama M, Harada M, Ohkoshi Y, Tada H, Yamamoto K, Yamane S (2001) Mid-term clinical results of Graf stabilization for lumbar degenerative pathologies. a minimum 2-year follow-up. Spine J 1:283–289
Herkowitz HN, Kurz LT (1991) Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg Am 73:802–808
Huskisson EC (1974) Measurement of pain. Lancet 2:1127–1131
Ito Z, Matsuyama Y, Sakai Y, Imagama S, Wakao N, Ando K, Hirano K, Tauchi R, Muramoto A, Matsui H, Matsumoto T, Kanemura T, Yoshida G, Ishikawa Y, Ishiguro N (2010) Bone union rate with autologous iliac bone versus local bone graft in posterior lumbar interbody fusion. Spine (Phila Pa 1976) 35:E1101–1105
Izumida S, Inoue S (1986) Assessment of treatment for low back pain. J Jpn Orthop Assoc (in Japanese) 60:391–394
Korovessis P, Repantis T, Papazisis Z, Iliopoulos P (2010) Effect of sagittal spinal balance, levels of posterior instrumentation, and length of follow-up on low back pain in patients undergoing posterior decompression and instrumented fusion for degenerative lumbar spine disease: a multifactorial analysis. Spine (Phila Pa 1976) 35:898–905
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 35:159–174
Magerl FP (1984) Stabilization of the lower thoracic and lumbar spine with external skeletal fixation. Clin Orthop Relat Res 189:125–141
Martin GJ Jr, Boden SD, Titus L, Scarborough NL (1999) New formulations of demineralized bone matrix as a more effective graft alternative in experimental posterolateral lumbar spine arthrodesis. Spine (Phila Pa 1976) 24:637–645
Miura Y, Imagama S, Yoda M, Mitsuguchi H, Kachi H (2003) Is local bone viable as a source of bone graft in posterior lumbar interbody fusion? Spine (Phila Pa 1976) 28:2386–2389
Nakashima H, Yukawa Y, Ito K, Horie Y, Machino M, Kanbara S, Morita D, Imagama S, Ishiguro N, Kato F (2011) Extension CT scan: its suitability for assessing fusion after posterior lumbar interbody fusion. Eur Spine J 20:1496–1502
Rothman SL, Glenn WV Jr (1985) CT evaluation of interbody fusion. Clin Orthop Relat Res 193:47–56
Shah RR, Mohammed S, Saifuddin A, Taylor BA (2003) Comparison of plain radiographs with CT scan to evaluate interbody fusion following the use of titanium interbody cages and transpedicular instrumentation. Eur Spine J 12:378–385
Vaccaro AR, Whang PG, Patel T, Phillips FM, Anderson DG, Albert TJ, Hilibrand AS, Brower RS, Kurd MF, Appannagari A, Patel M, Fischgrund JS (2008) The safety and efficacy of OP-1 (rhBMP-7) as a replacement for iliac crest autograft for posterolateral lumbar arthrodesis: minimum 4-year follow-up of a pilot study. Spine J 8:457–465
Violas P, Chapuis M, Bracq H (2004) Local autograft bone in the surgical management of adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 29:189–192
Walsh WR, Loefler A, Nicklin S, Arm D, Stanford RE, Yu Y, Harris R, Gillies RM (2004) Spinal fusion using an autologous growth factor gel and a porous resorbable ceramic. Eur Spine J 13:359–366
Acknowledgments
The authors contracted with Michael S. Altus, PhD, ELS, of Intensive Care Communications, Inc., Baltimore, MD, USA, to edit their initial manuscript. They thank Dr Altus for his excellent services. The authors maintained complete control over the direction and content of the manuscript.
Conflict of interest
The authors report no conflicts of interest related to the materials or methods used or the findings of this study. No benefits in any form have been or will be received from a commercial entity related directly or indirectly to the subject of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments
H. Selim Karabekir, Izmir, Turkey
First, I will begin to thank Prof. Bertalanffy for giving me a chance to comment. Takeuchi et al. were postulated in their study “the large volume inside the cage (≥2.0 mL) with local bone graft may lead incomplete bone fusion and residual postsurgical low back pain after PLIF.” They used Brantigan, TELAMON C, and OIC PEEK cages with iliac and local bone grafts and evaluated the patients by JOA and VAS. The bone fusion assessments were based on radiography and CT scans according to Brantigan, Steffee, and Frasier criteria. The follow-up period was more than 2 years. At the end, their result related to fusion outcome was significantly less at TL group (TELAMON C cage with local bone graft.
In the literature, we know that the graft surface is important for fusion. If it increases, the fusion chance increases too. Also, the type of the graft is important for fusion. So, the volume of the cage (≥2.0 mL) may not be the one cause of the lower fusion rate. The factors affecting poor fusion rates may be the type of surgery, the preparation of the graft bed, the material of the cages, the type of grafts, patient co-morbidities, patient smoking, etc. The authors determined that there were no differences between smoking or non-smoking patients, but we know that from the English literature, smoking is a factor of poor fusion rate. The large volume may be a factor of residual postsurgical low back pain after posterior lumbar interbody fusion, but the possibility of poor fusion rate may not be a scientific reality. Because the same type of cage (TELAMON C) was used in the study and the only significant difference between two study groups was using different types of graft (TI; iliac and local bone graft, TL; local bone graft), this study may be designed as two more groups using local bone graft with Brantigan and iliac + local bone graft with OIC PEEK cages. So, the authors’ hypothesis must be supported by new studies.
Rights and permissions
About this article
Cite this article
Takeuchi, M., Kamiya, M., Wakao, N. et al. Large volume inside the cage leading incomplete interbody bone fusion and residual back pain after posterior lumbar interbody fusion. Neurosurg Rev 38, 573–578 (2015). https://doi.org/10.1007/s10143-015-0610-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-015-0610-x