Abstract
Augmenting healing through a single application of an exogenous growth factor or bone morphogenetic protein is not a new concept. The use of autologous growth factors through platelet isolation and concentration provides multiple endogenous growth factors to the healing site. A posterolateral fusion model in aged sheep (5- to 6-year-old ewes) was used to examine the effects of the addition of growth factors through autologous platelet isolation on the biomechanic and histologic properties of the fusion using a resorbable coral bone graft substitute. At 6 months the combination of autologous growth factors to the Pro Osteon 500R plus aspirated bone marrow resulted in the greatest bending stiffness but not ultimate load. Autologous growth factors can be isolated from platelets and concentrated to provide multiple growth factors to the fusion site to aid in spinal fusion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biomaterial bone graft substitutes, including a variety of hydroxyapatite [3, 24, 25, 27, 36] and composite-based materials, [36, 43] have been developed to replace autogenous bone, with varied success. Whilst bone graft substitutes can provide a matrix for cellular ingrowth/ongrowth or a scaffold for new bone formation, they lack the biological molecules and cells present in the normal healing environment [20]. Bone morphogenetic proteins (BMPs) and a variety of growth factors have been identified to play a role in the cascade of fusion and fracture healing [9, 18]. Bone grafting using autologous corticocancellous or cancellous bone graft due to its unique composition remains the most effective material because it provides the three essential elements for bone regeneration; an osteoconductive matrix, osteoinductive factors as well as osteogenic cells [20].
Many studies have examined the effect of exogenous application of a single osteoinductive protein or growth factor to enhance bone repair and regeneration [6, 7, 14, 23]. A potential alternative to the exogenous application of a single factor is a system designed to deliver the cells that have the factors themselves to the desired site. Platelet degranulation provides growth factors to the healing site (PDGF, TGF-β, bFGF, and VEGF) as part of the normal cascade. Platelets can be sequestered and concentrated using gradient density centrifugation [37, 40]. Recent reports suggest that platelet-rich plasma may enhance the formation of new bone when used in combination with autogenous graft materials [1, 32, 34].
A tissue engineering approach combining an osteoconductive biomaterial scaffold and bone marrow mesenchymal stem cells has also been examined to heal or augment bone regeneration [10, 15, 16, 17, 19, 38, 39]. This study examined the effects of autologous biological therapy through isolation of multiple growth factors using a two-stage buffy coat (BC) sequestration protocol followed by concentration using an ultrafiltration device. This concentrate, termed AGF, was mixed with autogenous bone or a resorbable porous ceramic bone graft substitute with bone marrow aspirate in a sheep posterolateral spinal fusion model [43].
Materials and methods
Approval of the University of New South Wales Animal Care and Ethics Committee was obtained prior to the start of the study. Twenty-four aged cross-bred wethers (5–6 years old) were used in a posterior lateral L3–L4 spinal fusion model stabilised with 5.5 mm diameter pedicle screws [43]. A resorbable hydroxyapatite-calcium carbonate bone graft substitute derived from coral (Pro Osteon 500R, Interpore-Cross) or autograft were used alone and in combination with the BC isolation and the AGF concentrate with and without aspirated bone marrow. Four groups (n=6 per group) were examined in this study and euthanased at 6 months; (1) autograft alone (AUTO), (2) autograft + AGF (AUTO AGF), (3) Pro Osteon 500R with AGF (500R AGF), (4) Pro Osteon 500R + AGF and marrow aspirate (500R AGF MA). The autograft was harvested from the iliac crest.
Animals were anaesthetized and given approximately 400–500 ml of fluids (Hartmann’s solution) prior to withdrawing 350–400 ml of blood into a citrate-anticoagulated donor bag from the carotid artery. An optimized two-stage sequestration protocol was performed using a cell separator (Medtronic Sequestra 1000) to isolate 50 ml of buffy coat (BC). The BC contained the platelets, and white blood cells were processed into 20 ml of autologous growth factor concentrate (AGF) using an Interpore UltraConcentrator (Fig. 1a). 10 ml of AGF was introduced into Pro Osteon 500R, autogenous bone graft chips or Pro Osteon 500R mixed with 3–4 ml of bone marrow aspirate from the iliac crest in a 10 ml syringe with simultaneous injection with 1 ml of thrombin (100 units/ml) to allow gel to occur (Fig. 1b). This was performed in duplicate and both sides of the fusion site received the contents of the 10-ml syringe.
The contents of the gelled bone graft syringes were removed for placement into the L3–L4 inter-transverse surgical site adjacent to the vertebral facets. Animals received their red cells and plasma from the cell separator prior to completion of the surgery, so blood volume loss was minimal. Samples of blood and AGF extracts were obtained for platelet count.
Spines were harvested from L2 to L5. Attached muscle tissue was removed to leave the posterolateral fusion masses intact. All specimens were radiographed in a dorso-ventral plane. Two trained observers who were blinded to the surgical treatment examined the radiographs. The regions between successive transverse processes on each side were inspected. Qualitative observations were made regarding the radiographic appearance of the graft material and bony fusion. Signs of bone fusion were continuous cortex formation and/or a confluent trabecular pattern between successive transverse processes. The presence or absence of an incomplete neocortex alongside the fusion masses was noted. Two of six animals from each group underwent computed tomography (CT) (Toshiba, Tokyo, Japan) to examine the fusion.
Dual energy X-ray absorptiometry (DEXA) was performed using a pencil beam Lunar DPXL (Lunar Systems, USA) on all specimens prior to mechanical testing. Regions of interest between the transverse processes were analyzed for DEXA bone mineral density (g/cm2). The spines were scanned following the removal of the pedicle screws to assess the fusion density.
The L3–L4 segments were mechanically tested in posteroanterior three-point bending [43]. The L3–L4 vertebrae were potted in a metal alloy and tested using an MTS 858 Mini Bionix (Material Testing Systems, Eden Prairie, MN). The interspinous ligament and disc at the L3–L4 level were sectioned prior to testing. Samples were tested to failure in the posteroanterior plane at 50 mm/min [43]. The peak load (N) and stiffness (N/mm) were obtained from the load-displacement data. The mechanical and DEXA data were analyzed using a one-way analysis of variance (ANOVA) followed by the Tukey HSD post-hoc test using Statistica (Statsoft, Tulsa, OK).
Histology was performed on all animals following mechanical testing. Mechanically tested samples were fixed in phosphate-buffered formalin for 48 h immediately following testing. The L3–L4 segments were sectioned sagitally into two halves. The left half was dehydrated in ethanol, and embedded in PMMA for hard tissue histology and electron microscopy. The right half was decalcified in 10% formic acid/formalin, embedded in paraffin and sectioned for routine histology.
Results
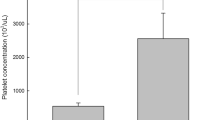
No surgical complications relating to the platelet isolation or spinal fusion surgery were encountered. Up to a 10-fold increase in platelet concentration compared to whole blood was found in the AGF concentrate after ultra-concentration using the Medtronic Sequestra 1000.
Faxitron radiographs provided excellent visualization of the fusion masses demonstrating resorption of the calcium carbonate phase of Pro Osteon 500R at 6 months, with new bone formation at the transverse process interfaces creeping into the centre of the fusion mass along the vertebral body. Radiographic results are presented in Table 1. Radiographic bilateral fusion occurred in one of six cases in sheep treated solely with Pro Osteon 500R + AGF. The addition of aspirated bone marrow to Pro Osteon 500R + AGF resulted in fusion in four of six cases with significant bone. This was equivalent to the four out of six bilateral fusion rate observed in the autograft group.
Fusion was obtained in 100% of cases (6/6) in the autograft + AGF group. Examples of faxitron images are presented in Fig. 2. CT confirmed the radiographic findings and revealed reformed cortices in the middle of the fusion mass in all groups (Fig. 3). Some resorption of the autograft during the study may also account for the smaller fusion masses seen in the autograft alone and autograft + AGF.
DEXA analysis did not reveal any significant differences between the groups although the radiographic fusion rates differed. The presence of residual bone graft or Pro Osteon 500R may have accounted for the lack of any differences. Failure during mechanical testing occurred through the fusion masses between the transverse processes in all specimens at 6 months. The combination of AGF and autograft obtained the greatest peak load compared to autograft alone and Pro Osteon + AGF but was not significant (p>0.05) (Table 2). The addition of bone marrow aspirate to Pro Osteon 500R + AGF significantly increased stiffness (p<0.05) (Table 2).
The histological appearance at 6 months in the aged animals demonstrated maturation of the bone and evidence of remodelling. A neocortex had formed between the transverse processes in all four groups. Hematoxylin and eosin staining at 4× and 20× for each group are presented in Fig. 4. The newly formed bone was thicker throughout the fusion mass in the groups treated with AGF compared to autograft alone. Resorption of the calcium carbonate on the porous ceramic was noted at 6 months with new bone formation and remodelling in the porous domains of the resorbable ceramic. New bone formation was also noted on the remaining autograft, with evidence of cement lines in the autograft alone (group 1) and the autograft + AGF (group 2).
Discussion
Bone graft substitutes have been traditionally considered as osteoconductive scaffolds, which support the growth of new bone due to their surface properties, chemistry and ultrastructure. Coralline-derived bone graft substitutes provide a scaffold for bone growth similar to that of native trabecular bone [4, 11, 27, 41, 42, 45]. Unlike autograft, osteoconductive bone graft substitutes are limited since they neither contain the native osteoinductive molecules (BMPs and growth factors) present in autologous bone nor the undifferentiated mesenchymal cells and blood-related products that facilitate healing [20]. The present study examined the effects of adding AGFs as well as bone marrow aspirate on posterolateral spinal fusion using a sheep model [43].
Augmentation of spinal fusion has been examined using BMPs [4, 5, 6, 7, 8, 13, 23, 35, 46], demineralised bone [26] as well as non-invasive techniques of ultrasound [21] or pulsed electromagnetic fields [28, 30]. Collectively, these studies and many others support the concept of stimulating or accelerating healing through the application of a single molecule, non-invasive stimulation and more recently gene therapy. The biological and mechanical effects of such treatments remain to be completely characterised.
The isolation of platelet-rich plasma is by no means a new idea [32, 37, 40]. This concept has been recently reintroduced in alveolar bone regeneration [1, 32] and bone grafts [34]. Platelet-rich plasma has also been used as an autologous source of a fibrin glue [44]. The use of concentrated platelets placed directly into the fusion site has merit considering one of the most immediate responses to bony trauma is the migration and degranulation of platelets at the injury site.
The alpha granules of platelets contain a number of growth factors that have been shown to affect bone formation, including PDGF, TGF-β, bFGF, IGF, and VEGF. These factors act to enhance vascular tissue ingrowth, encourage migration of osteoblasts and osteoprogenitor cells from surrounding areas, cause an increase in the mitogenic proliferation of these bone-forming cells, influence differentiation down the osteoblastic lineage, and enhance the synthesis of the extracellular matrix. Studies support that these factors do not act individually, but rather in combination with other factors by both synergistic and modulatory interactions [31].
Canalis [12] showed that treatment of fetal osteoblasts with platelets directly stimulated osteogenic activity. More recently, Marx et al. [34] has proposed a specific mechanism by which these growth factors released from platelets may stimulate new bone formation. Though the exact mechanism of the synergistic contribution of these factors to bony healing is not completely determined, one effect is the initiation of proliferation and differentiation of mesenchymal progenitor cells [29].
PDGF has been shown to be chemotactic and induce mitogenesis in osteoblasts and bone progenitor cells, as well as stimulate synthesis of some connective tissue matrix components such as collagen [33]. TGF-β also increases synthesis of the extracellular matrix, and affects differentiation of mesenchymal cells into osteoblasts and chondrocytes [22]. Vascular endothelial growth factor (VEGF) released from the alpha granules of platelets have also been implicated in angiogenesis and wound healing [2].
In this study, plasma rich in platelets and white cells was concentrated to obtain AGFs. AGF differs from platelet-rich plasma by the amount of platelets (6–10x baseline blood levels compared to 2–3x baseline blood levels) as well as in approximately 3 times the fibrinogen content. The high fibrinogen concentration enables formation of a firm gel by the addition of a small amount of thrombin.
The older animals and 6-month time point were thought to provide a more challenging model than younger animals that heal well and could demonstrate the benefit of AGF treatment. The grafts were placed adjacent to the vertebral body and in contact with the transverse processes. This may have enhanced the ability of creeping substitution from the decorticated bone beds [42].
The iliac crest corticocancellous autograft was reduced from strip form to particulates similar in size to Pro Osteon 500R prior to surgical placement. The radiographs and CTs at 6 months revealed a well-formed fusion mass and neocortex (Fig. 3). Peak loads and stiffness in this study with particulate autograft were significantly greater than the strip form of autograft in the younger populations at 6 months following fusion in our previous study [43]. This may reflect the benefit of reducing the autograft to particulate form, which increases the surface area for cellular attachment and placement onto a decorticated bone bed.
The addition of aspirated bone marrow in combination with AGF had a significant effect on the mechanical and histology results in the present study. The addition of bone marrow alone to a collagen-mineral composite bone graft substitute in our previous study [43] did not provide any additional benefit in the younger animal model. The combination of Pro Osteon 500R, AGF and bone marrow cell aspirate may provide a composite material that contains the appropriate cells, signalling molecules, and local environment favourable for healing.
These results are in agreement with work of Erbe and colleagues [19] who reported positive results with a tricalcium phosphate bone graft substitute and bone marrow aspirate cells. Indeed, the stiffness of the Pro Osteon 500R + AGF + marrow in aged animals was the greatest in the current study and nearly double compared to our previous work [43]. This data suggests that the combination of Pro Osteon 500R + AGF + marrow may be a viable alternative to autografts where donor site morbidity would remain a problem.
In terms of structural properties, the stiffness (load per unit deflection) rather than ultimate load differentiated the treatment groups at 6 months. Both strength and stiffness would increase with time and ultimately reach an equilibrium following remodelling of the tissue. The resorbable nature of Pro Osteon 500R, a combination of a calcium carbonate core and hydroxyapatite coating, may provide an increased local concentration of calcium and phosphate ions at the interface during new bone formation. New bone formation was observed in the regions where the resorbable ceramic was present but to a lesser degree in the autograft alone group.
Using the patient’s own growth factors through platelet sequestration and ultra-concentration offers a promising alternative to recombinant technology in healing augmentation. Clinically, this represents an exciting technique to provide an autologous biological boost to the bone graft substitute [1].
References
Anitua E (1999) Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants 14(4):529–535
Banks RE, Forbes MA, Kinsey SE, Stanley A, Ingham E, Walters C, Selby PJ (1998) Release of the angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: significance for VEGF measurements and cancer biology. Br J Cancer 77(6):956–964
Baramki HG, Steffen T, Lander P, Chang M, Marchesi D (2000) The efficacy of interconnected porous hydroxyapatite in achieving posterolateral lumbar fusion in sheep. Spine 25(9):1053–1060
Boden SD, Martin GJ Jr, Morone MA, Ugbo JL, Moskovitz PA (1999) Posterolateral lumbar intertransverse process spine arthrodesis with recombinant human bone morphogenetic protein 2/hydroxyapatite-tricalcium phosphate after laminectomy in the nonhuman primate. Spine 24(12):1179–1185
Boden SD, Martin GJ Jr, Morone MA, Ugbo JL, Titus L, Hutton WC (1999) The use of coralline hydroxyapatite with bone marrow, autogenous bone graft, or osteoinductive bone protein extract for posterolateral lumbar spine fusion. Spine 24(4):320–327
Boden SD, Schimandle JH (1995) Biologic enhancement of spinal fusion. Spine 20 [Suppl 24]:113S–123S
Boden SD, Schimandle JH, Hutton WC (1995) 1995 Volvo Award in basic sciences. The use of an osteoinductive growth factor for lumbar spinal fusion. II. Study of dose, carrier, and species. Spine 20(24):2633–2644
Boden SD, Titus L, Hair G, Liu Y, Viggeswarapu M, Nanes MS, Baranowski C (1998) Lumbar spine fusion by local gene therapy with a cDNA encoding a novel osteoinductive protein (LMP-1). Spine 23(23):2486–2492
Bolander ME (1992) Regulation of fracture repair by growth factors. Proc Soc Exp Biol Med 200(2):165–170
Boo JS, Yamada Y, Okazaki Y, Hibino Y, Okada K, Hata K, Yoshikawa T, Sugiura Y, Ueda M (2002) Tissue-engineered bone using mesenchymal stem cells and a biodegradable scaffold. J Craniofac Surg 13(2):231–239; discussion 240–243
Bozic KJ, Glazer PA, Zurakowski D, Simon BJ, Lipson SJ, Hayes WC (1999) In vivo evaluation of coralline hydroxyapatite and direct current electrical stimulation in lumbar spinal fusion. Spine 24(20):2127–2133
Canalis E (1985) Effect of growth factors on bone cell replication and differentiation. Clin Orthop 193:246–263
Cook SD, Dalton JE, Tan EH, Whitecloud TS 3rd, Rueger DC (1994) In vivo evaluation of recombinant human osteogenic protein (rhOP-1) implants as a bone graft substitute for spinal fusions. Spine 19(15):1655–1663
Cook SD, Rueger DC (1996) Osteogenic protein-1: biology and applications. Clin Orthop 324:29–38
Dong J, Kojima H, Uemura T, Kikuchi M, Tateishi T, Tanaka J (2001) In vivo evaluation of a novel porous hydroxyapatite to sustain osteogenesis of transplanted bone marrow-derived osteoblastic cells. J Biomed Mater Res 57(2):208–216
Dong J, Uemura T, Kikuchi M, Tanaka J, Tateishi T (2002) Long-term durability of porous hydroxyapatite with low-pressure system to support osteogenesis of mesenchymal stem cells. Biomed Mater Eng 12(2):203–209
Dong J, Uemura T, Shirasaki Y, Tateishi T (2002) Promotion of bone formation using highly pure porous beta-TCP combined with bone marrow-derived osteoprogenitor cells. Biomaterials 23(23):4493–4502
Einhorn TA (1998) The cell and molecular biology of fracture healing. Clin Orthop 355 [Suppl]:S7–21
Erbe EM, Marx JG, Clineff TD, Bellincampi LD (2001) Potential of an ultraporous beta-tricalcium phosphate synthetic cancellous bone void filler and bone marrow aspirate composite graft. Eur Spine J 10 [Suppl 2]:S141–146
Gazdag AR, Lane JM, Glaser D, Forster RA (1995) Alternatives to autogenous bone graft: efficacy and indications. J Am Acad Orthop Surg 3(1):1–8
Glazer PA, Heilmann MR, Lotz JC, Bradford DS (1998) Use of ultrasound in spinal arthrodesis. A rabbit model. Spine 23(10):1142–1148
Gombotz WR, Pankey SC, Bouchard LS, Phan DH, Puolakkainen PA (1994) Stimulation of bone healing by transforming growth factor-beta 1 released from polymeric or ceramic implants. J Appl Biomater 5(2):141–150
Grauer JN, Patel TC, Erulkar JS, Troiano NW, Panjabi MM, Friedlaender GE (2001) 2000 Young Investigator Research Award winner. Evaluation of OP-1 as a graft substitute for intertransverse process lumbar fusion. Spine 26(2):127–133
Guigui P, Plais PY, Flautre B, Viguier E, Blary MC, Chopin D, Lavaste F, Hardouin P (1994) Experimental model of posterolateral spinal arthrodesis in sheep. Part 2. Application of the model: evaluation of vertebral fusion obtained with coral (Porites) or with a biphasic ceramic (Triosite). Spine 19(24):2798–2803
Guigui P, Plais PY, Flautre B, Viguier E, Blary MC, Sales De Gauzy J, Chopin D, Lavaste F, Hardouin P (1994) Experimental model of posterolateral spinal arthrodesis in sheep. Part 1. Experimental procedures and results with autologous bone graft. Spine 19(24):2791–2797
Helm GA, Sheehan JM, Sheehan JP, Jane JA Jr, diPierro CG, Simmons NE, Gillies GT, Kallmes DF, Sweeney TM (1997) Utilization of type I collagen gel, demineralized bone matrix, and bone morphogenetic protein-2 to enhance autologous bone lumbar spinal fusion. J Neurosurg 86(1):93–100
Holmes RE (1979) Bone regeneration within a coralline hydroxyapatite implant. Plast Reconstr Surg 63(5):626–633
Ito M, Fay LA, Ito Y, Yuan MR, Edwards WT, Yuan HA (1997) The effect of pulsed electromagnetic fields on instrumented posterolateral spinal fusion and device-related stress shielding. Spine 22(4):382–388
Joyce ME, Terek RM, Jingushi S, Bolander ME (1990) Role of transforming growth factor-beta in fracture repair. Ann N Y Acad Sci 593:107–123
Kahanovitz N, Arnoczky SP, Nemzek J, Shores A (1994) The effect of electromagnetic pulsing on posterior lumbar spinal fusions in dogs. Spine 19(6):705–709
Kasperk CH, Wergedal JE, Mohan S, Long DL, Lau KH, Baylink DJ (1990) Interactions of growth factors present in bone matrix with bone cells: effects on DNA synthesis and alkaline phosphatase. Growth Factors 3(2):147–158
Kassolis JD, Rosen PS, Reynolds MA (2000) Alveolar ridge and sinus augmentation utilizing platelet-rich plasma in combination with freeze-dried bone allograft: case series. J Periodontol 71(10):1654–1661
Kiritsy CP, Lynch AB, Lynch SE (1993) Role of growth factors in cutaneous wound healing: a review. Crit Rev Oral Biol Med 4(5):729–760
Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR (1998) Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 85(6):638–646
Muschler GF, Hyodo A, Manning T, Kambic H, Easley K (1994) Evaluation of human bone morphogenetic protein 2 in a canine spinal fusion model. Clin Orthop 308:229–240
Muschler GF, Negami S, Hyodo A, Gaisser D, Easley K, Kambic H (1996) Evaluation of collagen ceramic composite graft materials in a spinal fusion model. Clin Orthop 328: 250–260
Nanu A, Taneja N, Sood SK (1980) Preparation and standardisation of platelet rich plasma and platelet concentrates in a developing blood bank. Indian J Med Res 71:661–7
Noshi T, Yoshikawa T, Dohi Y, Ikeuchi M, Horiuchi K, Ichijima K, Sugimura M, Yonemasu K, Ohgushi H (2001) Recombinant human bone morphogenetic protein-2 potentiates the in vivo osteogenic ability of marrow/hydroxyapatite composites. Artif Organs 25(3):201–208
Ohgushi H, Okumura M, Tamai S, Shors EC, Caplan A (1990) Marrow cell induced osteogenesis in porous hydroxyapatite and tricalcium phosphate: a comparative histomorphometric study of ectopic bone formation. J Biomed Mater Res 24(12):1563–1570
Reiss RF, Katz AJ (1976) Optimizing recovery of platelets in platelet rich plasma by the simplex strategy. Transfusion 16(4):370–374
Sartoris DJ, Holmes RE, Bucholz RW, Mooney V, Resnick D (1987) Coralline hydroxyapatite bone-graft substitutes in a canine diaphyseal defect model. Radiographic-histometric correlation. Invest Radiol 22(7):590–596
Shors EC (1999) Coralline bone graft substitutes. Orthop Clin North Am 30(4):599–613
Walsh WR, Harrison J, Loefler A, Martin T, Van Sickle D, Brown MK, Sonnabend DH (2000) Mechanical and histologic evaluation of Collagraft in an ovine lumbar fusion model. Clin Orthop 375:258–266
Whitman DH, Berry RL, Green DM (1997) Platelet gel: an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxillofac Surg 55(11):1294–1299
Zdeblick TA, Cooke ME, Kunz DN, Wilson D, McCabe RP (1994) Anterior cervical discectomy and fusion using a porous hydroxyapatite bone graft substitute. Spine 19(20):2348–2357
Zdeblick TA, Ghanayem AJ, Rapoff AJ, Swain C, Bassett T, Cooke ME, Markel M (1998) Cervical interbody fusion cages. An animal model with and without bone morphogenetic protein. Spine 23(7):758–765; discussion 766
Acknowledgements
The authors would like to thank Interpore-Cross for material and support for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Walsh, W.R., Loefler, A., Nicklin, S. et al. Spinal fusion using an autologous growth factor gel and a porous resorbable ceramic. Eur Spine J 13, 359–366 (2004). https://doi.org/10.1007/s00586-003-0597-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-003-0597-9