Abstract
This study investigates the performance of reverse transcription loop-mediated isothermal amplification (RT-LAMP) assay for the colorimetric detection of SARS-CoV-2 using fluorometric dye, namely, calcein. The detection limit (LoD) with the N-ID1 primer set resulted in superior performance, corresponding to ~ 2 copies/reaction or ~ 0.1 copies/μL of the RNA sample. The color development can be observed by the naked eye, using an ultraviolet (UV) transilluminator or a hand-UV light without the requirement of expensive devices. The average time-to-reaction (TTR) value was 26.2 min in high-copy number samples, while it was about 50 min in rRT-PCR. A mobile application was proposed to quantify the positive and negative results based on the three-color spaces (RGB, Lab, and HSB). Compared to rRT-PCR (n = 67), this assay allows fast and sensitive visual detection of SARS-CoV-2, with high sensitivity (90.9%), selectivity (100%), and accuracy (94.03%). Besides, the assay was sensitive regardless of variants. Since this assay uses a fluorescent dye for visual observation, it can be easily adapted in RT-LAMP assays with high sensitivity. Thus, it can be utilized in low-source centers and field testing such as conferences, sports meetings, refugee camps, companies, and schools.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The COVID-19 disease emerged in December, 2019, spread worldwide as a pandemic, and caused millions of infections and deaths (Khan et al. 2020). The infection affects several organs in the body and causes multi-organ failure (Rabaan et al. 2023a). Although intensive vaccination campaigns improved immunization and suppressed transmission, the pandemic waves continue and threaten the human population worldwide, especially in low-income countries with low/insufficient vaccination rates or those with inadequate immunity (Mousavi et al. 2022; Samanta et al. 2023; Rabaan et al. 2023b). Scientists expect that the virus will exist in communities in the long term and stay endemic over time with new variants due to the high mutation rate (Wang et al. 2021; Amicone et al. 2022; Telenti et al. 2021; Brom et al. 2022). Therefore, there is still a need for economical detection methods.
Among many techniques, the rRT-PCR remains the gold standard for diagnosing SARS-CoV-2. Despite high sensitivity, the necessity of expensive thermal cyclers with expensive reagents limits the use of rRT-PCR. Furthermore, on-demand testing and rapid results are complex due to their considerable technical complexity. Besides, rapid antigen tests (RATs), which identify viral proteins, dominate point-of-care testing (POCT). However, the key restriction is the reduced sensitivity of RATs and the higher likelihood of false negative results (Scohy et al. 2020; Nagura-Ikeda et al. 2020; Khalid et al. 2022; Furqoni et al. 2023). Alternatively, colorimetric reverse transcription loop–mediated isothermal amplification (RT-LAMP) assay allows a rapid, easy-to-use, accurate, and cheaper testing alternative without requiring expensive instruments. The technique is also listed as a nucleic acid amplification test (NAAT) by the US CDC for the SARS-CoV-2 diagnosis (https://www.cdc.gov/coronavirus/2019-ncov/lab/naats.html).
The RT-LAMP is a simple, rapid, and exact method for diagnosing viral infections. The reaction includes four to six primers and forms a dumbbell-shaped structure harboring self-priming sites for a strand-displacement DNA polymerase (Notomi 2000). It is a single tube reaction including the activity of reverse transcriptase and DNA polymerase enzymes simultaneously at constant temperature (60–65 °C). As a result, it eliminates the need for a thermal cycler, offering a less expensive testing option that keeps sensitivity and specificity. Moreover, the LAMP products can be observed using different techniques such as colorimetric, fluorometric, turbidity testing, or gel electrophoresis. For colorimetric detection, pH-dependent or -intercalating dyes show differences between positive and negative samples (Alhamid and Tombuloglu 2022). The naked eye observes the color development without needing expensive quantitative thermal cyclers, and the results can be obtained quickly (< 1 h). A traditional thermal block or water bath is enough to develop the color at a constant temperature. On the other hand, this method has some drawbacks, such as mis-amplification in negative specimens and a high rate of false positivity. Using six primers in a single run increases the probability of secondary structure formations such as homodimer, heterodimer, or hairpin structures, another concern for the assay application.
To date, colorimetric RT-LAMP assays have been used for the diagnosis of numerous kinds of pathogens, including Klebsiella pneumoniae (Nakano et al. 2015), Staphylococcus aureus (Hanaki et al. 2011), Ebola virus (Kurosaki et al. 2016), Zika virus (Wang et al. 2016) and Mpox virus (Iizuka et al. 2009) infections. The assay has also been used to diagnose SARS-CoV-2 (He et al. 2021; Jang et al. 2021; Amaral et al. 2021; Alhamid et al. 2022a, 2023; Dong et al. 2022). In those assays, various pH-sensitive dyes (phenol red, cresol red, hydroxy naphthol blue) or DNA-intercalating dyes (propidium iodide (PI), malachite green, SYBR I, ethidium bromide) have been used to obtain the best performance (Alhamid and Tombuloglu 2022). Among the dyes used in colorimetric RT-LAMP, calcein is a fluorescent dye that determines metal ions. The idea behind the calcein-based RT-LAMP assay is to use calcein in the reaction tube to find the reduction in Mg2+ concentration that results from creating magnesium pyrophosphate (Mg2P2O7), a byproduct. Manganese (Mn2+), added and kept for quenching, is the first substance that calcein binds to. As the LAMP reaction occurs and magnesium pyrophosphate is created, Mn ions quickly leave calcein to form an insoluble salt with magnesium pyrophosphate. As the reaction continues, the free calcein subsequently binds to Mg2+, and the color changes from orange to green in positive samples (Tomita et al. 2008) (Fig. 1). In addition to simple eye observation, color change can be observed under ultraviolet (UV) light. Until now, calcein-based colorimetric LAMP or RT-LAMP assays have been used to diagnose various pathogens hosting in humans (Wastling et al. 2010; Ali et al. 2017), plant (Niu et al. 2012; Fischbach et al. 2015), foods (Pang et al. 2019a), and marine animals (Suebsing et al. 2015, 2016). Pang et al. (2019b) used a mixture of calcein and hydroxynaphthol blue (HNB) dyes for the detection of foodborne pathogens. In addition, a calcein-based LAMP assay was developed to identify duck circovirus (DuCV) in farmed ducks (Xie et al. 2014). The widespread application allows the assay to develop new and rapid diagnostic methods. In addition, it emphasizes its potential applicability by providing advantages in cheapness, simplicity, portability, sensitivity, and specificity equivalent or superior to conventional methods.
The working principle of the calcein-based LAMP reaction. a The binding position of six LAMP primers, namely, LB, LF, FIP, BIP, F3, and B3, and the formation of stem-loop and dumbbell structures serve as starting points for the LAMP amplification cycles. b, c The input and output of the LAMP reaction upon activating the Bst DNA polymerase enzyme. The pyrophosphate (PPi−), the byproduct of the polymerization reaction, interacts with free Mg+2 and calcein-bounded Mn2+ (quenched form). The calcein-Mg+2 interaction results in fluorescence under UV light and the color turns from yellow to green. CAL, calcein; dNTPs, deoxyribonucleotide triphosphate; dNMPs, deoxyribonucleotide monophosphate; PPi−, pyrophosphate
Therefore, this study investigates the efficiency of calcein in detecting SARS-CoV-2 by using the RT-LAMP method. The assay was tested in pseudo-viral RNA, and 110 clinical SARS-CoV-2 RNA samples belong to the most recent variant under monitoring (VUM) and the variant of concern (VOC), including Delta (B.1.617.2 and AY lineages) and Omicron (B.1.1.529, BA.1, BA.1.1, BA.2, BA.3, BA.4, and BA.5 lineages) (https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html). Seven primer sets were tested to obtain the best performance. The results are compared to gold standard rRT-PCR and a smartphone color detector application.
Materials and methods
SARS-CoV-2 genome sequences and SARS-CoV-2 primers
SARS-CoV-2 genomes belong to the variant of interest (VOI) and the variant of concern (VOC), including B.1.1.7 (Alpha), B.1.351 (Beta), P.1 (Gamma), B.1.617.2 (Delta), and B.1.1.529 (Omicron), was downloaded from GISAID database (https://gisaid.org/). The sequences originated from different countries/continents were collected. MAFFT software (https://mafft.cbrc.jp/alignment/server) was used to align genomes with default settings. The gene sequences (N, RdRp, E, and S) were selected and screened using the JalView (v2.11.1.3) program to check possible mutation sites. Only the consensus sequences (100% alignments) were chosen to target the primers. Primer Explorer V5 by Eiken (https://primerexplorer.jp/e/) was used for primer design. Several LAMP primer sets were used to acquire the best sensitivity and specificity (Alhamid et al. 2023). Possible secondary structures like homodimer, heterodimer, and hairpins were inspected using the IDT DNA OligoAnalyzerTM tool (https://www.idtdna.com/pages/tools/oligoanalyzer).
Sample collection and RNA isolation
Total RNAs were extracted from nasopharyngeal swabs or combined nasopharyngeal/oral swabs collected from patients at King Fahad Specialist Hospital (KFSH) and Johns Hopkins Aramco Healthcare (JHAH), Dammam/KSA. The Institutional Review Board (IRB) at Imam Abdulrahman bin Faisal University (IAU) approved the study (IRB-2020-13-406). Also, it was approved by the institutional research committee, and the de-identified samples were left over after completion of diagnostic tests; hence, this study requires no consent as per institutional ethics committee regulations. The swabs were placed in a liquid viral transport medium (VTM) from (Capricorn Sci., Germany). The collected samples were brought to the diagnostic laboratory in sterile containers to proceed directly or kept at + 4 °C for further processing. RNA isolation was carried out using a QIAamp Viral RNA kit (Qiagen, Germany) following the manufacturer’s instructions, quantified by using NanoDrop 2000 (Thermo Sci.), and stored at − 80 °C for further experiments.
RT-LAMP conditions
All master-mix preparations for the RT-LAMP reaction were performed on ice inside a biosafety cabinet level-2 (BSL-2). Following the guidelines provided by New England BioLabs (NEB), RT-LAMP reactions were carried out using the following reagents: 2.5 μL 10 × isothermal amplification buffer II, 2.5 μL MgSO4 (100 mM), 3.5 μL dNTP (10 mM), 0.21 μL Bst 3.0 DNA polymerase (120,000 U), 2.5 μL 10 × primers mix (FIP and BIP (1.6 μM), F3 and B3 (0.2 μM), LF and/or LB (0.4 μM)), 1 μL guanidine hydrochloride (40 mM), 1 μL fluorescent calcein (2.5 mM), and 2 μL template RNA. Ultra-pure DNase/RNase-free distilled water was added in quantity enough to complete the final volume reaction of 25 μL. Isothermal amplification was performed on either TMTECHNE thermal cycler or a water bath at 63 °C constant temperature for up to 60 min. The products were monitored at 10-min intervals and stored at 4 °C.
Detection of SARS-CoV-2
Calcein, a metal indicator dye, allows visual detection of the reactant color change from orange to green based on substituting manganese chloride (MnCl2) to magnesium chloride (MgCl2). The reaction was considered positive when the naked eye observed an orange-to-green color change. In addition, the products were visualized both under a transilluminator and mini hand-UV with UV-protective glasses (Fig. 2c). Besides, RT-LAMP products were loaded on 2% agarose gel and run in a gel electrophoresis device for 40 min at 100 V constant voltage.
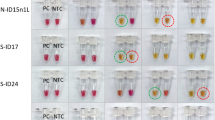
The observation of calcein-based LAMP results a by the naked eye, b under UV light provided by c a hand UV and a protective glass to safely see the color difference by the naked eye. d In addition, the color difference can be observed under a transilluminator. The SARS-CoV-2 positive control (PC) and negative control (NC) tubes were set for each reaction. e Detection of SARS-CoV-2 by using different LAMP primer sets (N-ID5, E-ID1, S-set11, RdRp-ID37, S-ID17, S-ID24, N-ID15, and N-ID15n1L) by naked eye. f The average detection time of calcein-based LAMP using different primer sets (n = 6)
Amplification efficiency and analytic sensitivity
The assay’s performance was assessed by calculating the limit of detection (LoD) using 10-fold diluted samples (from 100 to 106) of SARS-CoV-2 positive control (PC) RNA. Also, the effects of enzyme concentration in color development and the detection of SARS-CoV-2 were tested using different Bst3.0 enzyme concentrations: 1 ×, 2 ×, and 3 ×, corresponding to 8000, 16,000, and 24,000 U/mL, respectively. The analytic specificity of the primers used for RT-LAMP was determined using the RNA specimens isolated from other respiratory viruses, including parainfluenza virus 3, enterovirus, rhinovirus, human metapneumovirus A + B, parainfluenza virus 4, bocavirus, and coronavirus 229 E.
Diagnostic performance
Clinical RNA samples (n = 67) were tested using calcein-based RT-LAMP, multiplex RT-PCR targeting viral RdRp and N, and human RP genes (Tombuloglu et al. 2021, 2022). An RNA mixture containing synthetic copies of each target gene was used (Twist synthetic RNA control 51 (EPI_ISL_7718520), Twist Bioscience, USA) as the positive control (PC) sample. For negative control (NC), RNase/DNase-free dH2O was added instead of the RNA template. Reaction mixtures were segregated using 2% agarose gel electrophoresis stained with VisualaNA(A) DNA to confirm the LAMP results. Gels were imaged under UV with a transilluminator from Bio-Rad Laboratories. The sensitivity, specificity, accuracy, positive-percent agreement (PPA), and negative-percent agreement (NPA) were calculated according to the following equations:
where TP is true positive, FN is false negative, FP is false positive, and TN is true negative.
Results
Observation of the LAMP reaction
LAMP is a well-known diagnostic technique for screening infections in routine or field station laboratories. By integrating the LAMP technique with dye indicators, colorimetric LAMP can be produced for quick and accurate pathogen identification. In this study, a single-tube colorimetric LAMP assay was demonstrated and optimized for visual detection of the SARS-CoV-2 virus using calcein dye, resulting in a clear distinction between the reactions diagnosed as positive or negative. The metal indicator dye calcein allows visual observation by the naked eye or under UV light. A color change to green was observed in PC samples after colorimetric RT-LAMP reactions, while the non-template or negative control (NC) was orange (Fig. 2a). Under UV light, the color turns light green from dark green (Fig. 2b). However, a hand UV lamp with UV-protective glass must be utilized (Fig. 2c). In addition, the color change can be observed under a transilluminator, as depicted in Fig. 2d.
Detection of SARS-CoV-2 with calcein by using different primer sets
Different LAMP primer sets (n = 8) targeting four other genes (N, S, RdRp, and E), namely, N-ID5, E-ID1, S-set11, RdRp-ID37, S-ID17, S-ID34, N-ID15, and N-ID15n1L, were used to find out the efficient primer set for the best result (Supporting File 1; Fig. 2e). The reactions took place in a thermal block or a water bath. The average detection time of reaction mixtures, including different primers, ranged between 25 and 60 min, as shown in Fig. 2f. The mean detection time (n = 6) was about 26.2 min when using the N-ID5 set, followed by N-ID15n1L (34.4 min), N-ID15 (35.2 min), E-ID1 (44.8 min), and S-set11 (45.1 min). The mean detection time for the S-ID17, S-ID24, and RdRp-ID37 primer sets was relatively late (~ 55–57 min). Accordingly, N-ID5 was determined as the primer set giving the earliest result and used in later optimization steps. All primer sets showed an equally distinctive color difference between the positive and negative RT-LAMP reactions after calcein addition.
Effect of enzyme concentration on color development
To elucidate the effect of enzyme concentration on color development and intensity, three different Bst 3.0 enzyme concentrations (8000 U/mL, 16,000 U/mL, and 24,000 U/mL, corresponding to 1 ×, 2 ×, and 3 ×, respectively) were used with the N-ID5 primer set. When examined under a transilluminator, UV light, and by the naked eye, the triplicate concentration (24,000 U/mL) led to the highest color intensity among other engagements, as Fig. 3a shows the brightest PC reaction tubes with the highest enzyme concentration. The changes in the color intensity, corresponding to different enzyme concentrations, were also evident by reading the red, green, and blue (RGB) codes using a mobile application, as depicted in Fig. 3b–d. It is evident that increasing the enzyme concentration gradually raised the RGB value and color intensity in all tested observation assays (i.e., visual (Vis), transilluminator (Trans), and UV light (UV)). Compared to the visual (Vis) and transilluminator (Trans) tests, the UV light revealed the most notable RGB difference between the PC specimens at 1 × and 3 × enzyme concentrations.
Effect of enzyme concentration on color development and the detection of SARS-CoV-2 using N-ID5 primers. Bst3.0 enzyme in 1×, 2×, and 3× concentrations (8000 U/mL, 16,000 U/mL, and 24,000 U/mL) were used in reaction tubes. a The results were observed by the naked eye (visual or Vis) under a transilluminator (Trans) and UV light (UV). The positive control (PC) and negative control (NC) tubes were set for each reaction. b–d The RGB (red, green, and blue) color intensities were gradually increased by increasing enzyme concentration. Error bars show the standard deviations of the mean (n = 6)
Detection of SARS-CoV-2 on clinical samples
The color change after the calcein-LAMP reaction was observed by using clinically verified SARS-CoV-2 positive (n = 8) (Fig. 4a) and negative (n = 6) (Fig. 4b) samples. The N-ID5 primer set was used in the reactions. The color difference between the positive and negative tubes was evident in all observation assays, i.e., transilluminator, UV light, and naked eye (visual). Together with this, the color change was quantified by using a mobile color analyzer application (Color Wheel Analyzer, CWA), which numerically defines the RGB, Lab, and HSB values on a picture in either .jpeg or .png format (Fig. 4c). The expected colors in SARS-CoV-2 positive and negative samples upon the calcein-LAMP reaction are indicated in Fig. 4d. The results in visual observation showed that the red, green, blue, L, and B values were quite different between the SARS-CoV-2 negative and positive samples, but no results were obtained for the a, b, H, and S values (Fig. 4e–g). Under the transilluminator, the R, G, L, a, b, and B values were remarkably altered between the SARS-CoV-2 negative and positive reaction tubes (Fig. 4h–j). Under UV light, the CWA program resulted in the most remarkable color difference in green (RGB), a (Lab), and S (HSB) values (Fig. 4k–m). In addition to the naked eye option, the users can choose one or more of these color code models to identify positive and negative samples according to the existence of these facilities (i.e., UV, transilluminator, or visual). In addition, the CWA analysis on calcein-LAMP images from serially diluted RNA samples was conducted, and the RGB, Lab, and HSB color codes of each result from visual, transilluminator, and UV-light were determined (Supplementary Figs. S3, S4, and S5). The results showed that the CWA application successfully distinguished the results of the diluted samples and the negative control. The RGB color value in visual observation gradually decreased in parallel with the dilution series (Supplementary Fig. S3a). In addition, RGB values were only remarkably altered at the highest dilution series (105 and 106) at the transilluminator (Supplementary Fig. S3b) and UV-light observations (Supplementary Fig. S3c). These results demonstrate the sensitivity of the CWA application in distinguishing color formations at lower viral loads and intermediate results that are difficult for the human eye.
Clinically verified SARS-CoV-2 a positive (P1–P8) and b negative (N1–N6) samples were observed by using a transilluminator, UV light, and the naked eye (visual). The positive control (PC) and negative control (NC) tubes were set for each treatment. c The RGB, lab, and HSB values were determined using a mobile application (Color Wheel Analyzer, CWA). d Expected colors in PC and NC samples. The mean RGB, lab, and HSB codes in PC and NC samples were observed by e–g visually, h–j transilluminator, and k–m UV light. The error bar indicates the standard deviation of the technical replicates (n = 6).
Diagnostic performance of calcein-LAMP on clinical samples
Clinical samples (n = 67) were comparatively tested by RT-LAMP assay using an N-ID5 primer set and optimized LAMP protocol. Multiplex rRT-PCR assays targeting viral N and RdRp and human RP genes were used to verify the SARS-CoV-2 positive and negative samples. Among the tested samples, the calcein-LAMP assay was compatible with rRT-PCR results in 63 samples (94.03%), as 40 were detected as SARS-CoV-2 positives and 23 were negatives in both assays (Table 1). The sensitivity (90.91%), accuracy (94.03%), specificity (100%), positive-percent agreement (PPA) (100%), and negative-percent agreement (PPA) (85.19%) were calculated. Four samples were found inconclusive, which were positive in calcein RT-LAMP but negative in rRT-PCR (Supplementary Table 2; Supplementary Fig. 1). Also, no false-negative (FN) result was evident (Table 1).
True positive (TP), false positive (FP), true negative (TN), and false negative (FN) terms were utilized to evaluate the diagnostic accuracy of the RT-LAMP method
Analytic sensitivity on different variants: Delta and Omicron
An RNA sample of SARS-CoV-2 (Delta variant) with an unknown concentration was serially diluted (1-, 101-, 102-, 103-, 104-, and 105-fold) to determine the analytical sensitivity of LAMP reactions (Fig. 5a). The results showed that the color change from orange to green had been detected in 100-, 101-, 102-, 103-, and 104-fold dilutions, while the 105 and negative control (NC) samples were orange. The same result was evident under the transilluminator and UV light. Also, it was tested further via gel electrophoresis (Fig. S2), where the results are consistent with the colorimetric analyses. A ladder-type banding pattern detected dilutions, while no bands appeared for NCs. In Fig. 5b, a known concentration of pseudo viral SARS-CoV-2 RNA (Twist Bioscience, Omicron variant) was serially diluted (1, 101-, 102-, 103-, 104-, 105-, and 106-fold) and used as the template. After RT-LAMP reactions, the color change from orange to green was visually observed up to 106-fold dilutions. It was also consistent with the transilluminator and UV light observations. The LoD of the assay was calculated as ~ 2 copies/reaction or ~ 0.1 copies/μL of the RNA sample.
The detection limit of calcein-based colorimetric RT-LAMP assay. a An RNA sample of SARS-CoV-2 (Delta variant) with an unknown concentration was serially diluted (1-, 101-, 102-, 103-, 104-, and 105-fold). b A known concentration of pseudo viral SARS-CoV-2 RNA (Twist Bioscience, Omicron variant) was serially diluted (1-, 101-, 102-, 103-, 104-, 105-, and 106-fold). The color change was photographed under a transilluminator, UV light, and by the naked eye (visual). The PC and NC tubes were set for each treatment. c The target sequences of the F3 primer on the genome of Delta and Omicron variants. The binding region harbors nine nucleotides of mutation in the Omicron variant. d The average time-to-reaction (TTR) value of calcein-based RT-LAMP (minutes) (n = 53)
When the primer binding sites were checked in the Delta and Omicron variants, it was noted that the binding position of the F3 primer included deletions (nine nucleotides) in the Omicron variant. This mutation was expected to affect the binding of the F3 primer. However, it is seen that it does not disrupt the LAMP reaction. These results showed the successful amplification of the N gene by calcein RT-LAMP regardless of variants, including Delta and Omicron. The average time-to-reaction (TTR) value of calcein-based RT-LAMP was calculated as 40 minutes (n = 53) (Fig. 5d). It was previously detected that the mean TTR (n = 6) was about 26.2 min (Fig. 2f). The average TTR may vary depending on the copy number of viral RNA in the samples.
Discussion
In life-threatening outbreaks, identifying and detecting disease agents rapidly, specifically, and sensitively, is essential for preventing the spreading and protecting against disease effects. The approaches to see SARS-CoV-2 can be categorized based on types of test material and working principles. Nucleic acid-based techniques, mainly RT-PCR and gene sequencing, and protein-based techniques rely on the detection of antigen (RAT, specific peptide detecting test) and antibody (ELISA, IgM/IgG detecting serological test) are currently applicable and valid methods (Alhamid et al. 2022b; Heng et al. 2022). Also, as an adapted and developing technique, biosensors that recognize various biomarkers specific to the disease are available (Roberts et al. 2021). Each method has advantages and disadvantages; beyond all, there is a need to eliminate time-consuming equipment requirements, personnel qualification, expenditure, intensive data processing, and evaluation for accurate diagnostic tools (Loeffelholz and Tang 2021).
Current strategies for the visual detection of the LAMP products rely on pH-sensitive dyes such as phenol red, neutral red, and cresol red, or fluorometric dyes such as SYBR green I, propidium iodine, etc. However, the pH range of the dyes to change the color is so low, which makes the dyes very sensitive and necessitates specific pH values for the RNA sample. This drops detection efficacy, particularly in samples containing interfering substances and body fluids such as saliva.
In our experiments, we combined nucleic acid intercalating fluorescent dye calcein with RT-LAMP to develop and enhance the detection capacity of the technique. In addition to direct eye observations, color changes in reaction tubes have been visualized in a transilluminator and under UV. Eight primer sets have been tested in PC RNA samples belonging to the globally common variants Delta and Omicron. Although all primers allowed eye observation of positive signals for diagnosis, a fluorescent dye made data more precise and more accessible to detect without any suspicious circumstances. In addition, the results showed that the N-ID5 primer gave outstanding visual results among different primer sets and lasted less time than others, an average of 26.2 min in PC samples (n = 6) (Fig. 2). Even with Omicron’s deletion mutation at the F3 primer binding site, the N-ID5 set detected this variant efficiently. This indicates a further advantage of the LAMP technique in its ability to detect mutated regions with the help of the six primers, unlike the gold standard rRT-PCR, which uses two primers that must be continuously updated when new variants are emerging (Chen et al. 2022, Sharma et al. 2022). Thus, N-ID5 has been used as an optimum primer set for further experiments. When testing with clinical samples (n = 53), the TTR value was about 40 min, which depended on the viral load of the samples.
It was revealed that conditions such as Bst DNA polymerase enzyme concentration, incubation time, sample amount, and amplicon length effectively detect SARS-CoV-2 (Huang et al. 2022). Thus, Bst 3.0 enzyme concentration was tested for the calcein-based RT-LAMP technique to check the assay’s efficiency. Experiments were carried out by using previously identified SARS-CoV-2 PC RNA. As seen in Fig. 3, although all Bst 3.0 DNA polymerase concentrations gave detectable signals, the intensity of color changes was proportional to DNA polymerase enzyme concentration. Increasing the enzyme concentration improved the color intensity. Although this increases the test’s cost, improving the results’ robustness and preventing false negative results are priorities. Enzymes developed in-house could help reduce the cost of further studies.
Clinical samples’ sensitivity, accuracy, specificity, NPA, and PPA were compared with the rRT-PCR technique. At 94.03%, RT-LAMP results were compatible with reference rRT-PCR analysis (Table 1). In some circumstances, negative samples showed late positive signals (20–25 min subsequent detection of positive models), which were analyzed in a real-time thermal cycler, pointing out false-positive results (Mautner et al. 2020). In addition, the formation of secondary structures (i.e., homodimer, heterodimer, and hairpin) and mis-amplification may lead to false-positive reactions, which is the main disadvantage of the LAMP technique (Meagher et al. 2018, Odiwuor et al. 2022). While being more sensitive than Bst 2.0, Bst 3.0 is more susceptible to errors in amplification and false positives. In our experiments, negative samples showed negative signals even at late time points (60 min), indicating the avoidance of false-positive results (Table 1). On the other hand, four samples were found inconclusive, which are positive in calcein RT-LAMP but negative in rRT-PCR (Supplementary Table 2; Supplementary Fig. 1). The negative result in multiplex rRT-PCR could be related to the sensitivity of the rRT-PCR reaction, which has previously been detected as 35.13 and 20.31 copies/reaction for RdRP and N genes, respectively (Tombuloglu et al. 2022). It seems that the sensitivity of the calcein RT-LAMP assay is higher than the applied rRT-PCR approach.
The LoD was determined using different concentrations of PC RNA. In all diluted samples, even in lower ones (106-fold diluted sample), the positive reactions were observed clearly, corresponding to ~ 2 copies/reaction or ~ 0.1 copies/μL of the RNA sample. This points out the high sensitivity of calcein-based assays compared to LAMP assays using pH-depended dyes such as phenol red, cresol red, neutral red, m-Cresol purple, etc. (Tanner et al. 2015). Recently, Rivas-Macho et al. (2023) proposed an extraction-free RT-LAMP assay for the SAR-CoV-2 detection using calcein as the colorimetric dye. The assay targets the N gene with a LoD of 62 copies, while it was 1114 copies when using an extraction-free protocol. The pH-based LAMP assays release by-products, including a pyrophosphate moiety (PPi−) and a hydrogen ion (H+), reducing the pH and eventually changing the color. The color change in the reaction necessitates the accumulation of H+ ions to drop the pH. However, this is not always achievable in samples with a low copy number of viral RNA. Instead, the calcein-based assay relies on the interaction of Mg+2 ions with calcein, making the assay more sensitive than pH-dependent LAMP assays. Therefore, the assay can be exceptionally suitable for samples containing a low-copy number of viral RNA.
Colorimetric RT-LAMP SARS-CoV-2 diagnostic sensitivity relies on color interpretation and viral load (Aoki et al. 2021). In some circumstances, the interpretation of the color may vary from person to person. While the color change is observed, not only absolute black and white or absolute yellow and red color occurs. In addition to this, third intermediate color forms can also occur. It is known that intermediate color forms occur, especially in samples with low viral copy numbers (Aoki et al. 2021). For this reason, it may not always be easy to distinguish the color change and define positives and negatives by eye. Therefore, we proposed a quantitative color sorter based on RGB, Lab, and HSB codes in addition to the naked eye, hand-UV, and transilluminator observations (Fig. 3). The program can be accessed and easily used on mobile phones. The color codes to differentiate the color difference are varied based on the observation type. For instance, once the naked eye observes the reaction tubes, R, G, B, L, and B values should be considered. On the other hand, under the transilluminator, the R, G, L, a, b, and B values should be considered. Under UV light, G, a, and S values reveal the most remarkable difference. Calcein is known to be a partial inhibitor of LAMP (Scott et al. 2020). There will never be a “perfect” color-based indicator for everyone because of the genetic variability that makes human color discerning very variable (Neitz and Neitz 2011; Scott et al. 2020). Because of this visual subjectivity, a quantitative measure to define the color may be needed. We used a simple colorimetric analysis method via the smartphone app to solve this problem. Once used in serially diluted samples, the CWA successfully identified the color intensities between the samples even at the highest dilution series and the negative control (Supplementary Figs. S3, S4, and S5). These results indicate the sensitivity of the CWA application, which can distinguish the changes in color intensity that are difficult for the human eye. This setup can be easily adapted to LAMP devices for POC testing. This analysis allows easy color identification via simple applications that measure any RGB (red, green, blue), HSB (hue, saturation, brightness), and Lab (lightness, green–red opponents, and blue–yellow opponents) color space components.
Overall, RT-LAMP detected fluorescent signals quickly and in samples with lower copy numbers. For resource-poor countries/regions with more global cases and testing capacity, the RT-LAMP process is available for rapid, low-cost, visually detectable, accurate, and point-of-care tests. Improvement of sample preparation steps and making reagents more accessible in flexible conditions to facilitate transport and long-term storage are some topics for further studies. The assay can be utilized in field testing at conferences, sports meetings, stadiums, refugee camps, private companies, schools, etc.
Conclusions
The results demonstrated the superior performance of the calcein-based LAMP approach over the gold standard PCR technique. The assay can be implemented in the samples having low RNA copies. Also, using calcein as the dye to differentiate positive and negative reactions alternates the monitoring setups such as visual, hand UV, and transilluminator. In addition, smartphone applications that distinguish different color spaces, such as RGB, HNB, and Lab, can be used easily. This allows people with difficulty distinguishing colors due to genetic variability to differentiate samples easily. Also, the assay successfully identified different SARS-CoV-2 variants, including Delta and Omicron. The main benefits that many researchers are interested in are the lack of thermal cycler machine requirements and simple skill requirements. The ease of use, speed, high specificity, and sensitivity offer signs of acceptability, particularly in environments with limited resources. Moreover, calcein-LAMP approach can be applicable for the detection of other pathogens (viruses and bacteria), paving the way for vast diagnostic applications.
Data availability
Not applicable.
References
Alhamid G, Tombuloglu H (2022) Perspective chapter: recent progressions on the colorimetric diagnosis of SARS-CoV-2 by loop-mediated isothermal amplification (LAMP) assay. In: SARS-CoV-2 Variants-Two Years After. IntechOpen
Alhamid G, Tombuloglu H, Al-Suhaimi E (2023) Development of loop-mediated isothermal amplification (LAMP) assays using five primers reduces the false-positive rate in COVID-19 diagnosis. Sci Rep 13(1):1–13
Alhamid G, Tombuloglu H, Motabagani D, Motabagani D, Rabaan AA, Unver K et al (2022a) Colorimetric and fluorometric reverse transcription loop-mediated isothermal amplification (RT-LAMP) assay for diagnosis of SARS-CoV-2. Funct Integr Genomics 22(6):1391–1401
Alhamid G, Tombuloglu H, Rabaan AA, Al-Suhaimi E (2022b) SARS-CoV-2 detection methods: a comprehensive review. Saudi J Biol Sci:103465
Ali SA, Kaur G, Boby N, Sabarinath T, Solanki K, Pal D, Chaudhuri P (2017) Rapid and visual detection of Leptospira in urine by LigB-LAMP assay with pre-addition of dye. Mol Cell Probes 36:29–35
Amaral C, Antunes W, Moe E, Duarte AG, Lima LM, Santos C et al (2021) A molecular test based on RT-LAMP for rapid, sensitive and inexpensive colorimetric detection of SARS-CoV-2 in clinical samples. Sci Rep 11(1):16430
Amicone M, Borges V, Alves MJ, Isidro J, Zé-Zé L, Duarte S et al (2022) Mutation rate of SARS-CoV-2 and emergence of mutators during experimental evolution. Evol Med Public health 10(1):142–155
Aoki MN, de Oliveira Coelho B, Góes LGB, Minoprio P, Durigon EL et al (2021) Colorimetric RT-LAMP SARSCoV-2 diagnostic sensitivity relies on color interpretation and viral load. Sci Rep 11(1):9026
Brom F, de Hoog J, Knottnerus JA, Mampuys R, van der Lippe T (2022) Coronavirus disease 2019 scenarios for a long-term strategy under fundamental uncertainty. J Clin Epidemiol 148:196–199
Chen Y, Han Y, Yang J, Ma Y, Li J, Zhang R (2022) Impact of SARS-CoV-2 variants on the analytical sensitivity of rRT-PCR assays. J Clin Microbiol 60(4):e02374–e02321. https://doi.org/10.1128/jcm.02374-21
Dong Y, Zhao Y, Li S, Wan Z, Lu R, Yang X et al (2022) Multiplex, real-time, point-of-care RT-LAMP for SARS-CoV-2 detection using the HFman probe. ACS sensors 7(3):730–739
Fischbach J, Xander NC, Frohme M, Glökler JF (2015) Shining a light on LAMP assays’ a comparison of LAMP visualization methods including the novel use of berberine. Biotechniques 58(4):189–194
Furqoni AH, Amin M, Restifan YD, Putri SMD, Ferandra VA, Lusida MI (2023) The evaluation results of proposed antigen rapid diagnostic tests for COVID-19: some possible factors might influence. Infection:1–7
Hanaki KI, Sekiguchi JI, Shimada K, Sato A, Watari H, Kojima T et al (2011) Loop-mediated isothermal amplification assays for identification of antiseptic-and methicillin-resistant Staphylococcus aureus. J Microbiol Methods 84(2):251–254
He Y, Xie T, Tong Y (2021) Rapid and highly sensitive one-tube colorimetric RT-LAMP assay for visual detection of SARS-CoV-2 RNA. Biosens Bioelectron 187:113330
Heng L, Zhang H, Ding K, Wang X-H, Sun G-Y, Liu Z-X, Luo Y (2022) The evolving epidemiology of monkeypox virus. Cytokine Growth Factor Rev 68
Huang X, Tang G, Ismail N, Wang X (2022) Developing RT-LAMP assays for rapid diagnosis of SARS-CoV-2 in saliva. EBioMedicine 75:103736
Iizuka I, Saijo M, Shiota T, Ami Y, Suzaki Y, Nagata N et al (2009) Loop-mediated isothermal amplification-based diagnostic assay for monkeypox virus infections. J Med Virol 81(6):1102–1108
Jang WS, Lim DH, Yoon J, Kim A, Lim M, Nam J et al (2021) Development of a multiplex loop-mediated isothermal amplification (LAMP) assay for on-site diagnosis of SARS CoV-2. PloS One 16(3):e0248042
Khalid MF, Selvam K, Jeffry AJN, Salmi MF, Najib MA, Norhayati MN, Aziah I (2022) Performance of rapid antigen tests for COVID-19 diagnosis: a systematic review and meta-analysis. Diagnostics 12(1):110
Khan S, Tombuloglu H, Hassanein SE, Rehman S, Bozkurt A, Cevik E et al (2020) Coronavirus diseases 2019: current biological situation and potential therapeutic perspective. Eur J Pharmacol 886:173447
Kurosaki Y, Magassouba NF, Oloniniyi OK, Cherif MS, Sakabe S, Takada A et al (2016) Development and evaluation of reverse transcription-loop-mediated isothermal amplification (RT-LAMP) assay coupled with a portable device for rapid diagnosis of Ebola virus disease in Guinea. PLoS Negl Trop Dis 10(2):e0004472
Loeffelholz MJ, Tang YW (2021) Detection of SARS-CoV-2 at the point of care. Bioanalysis 13(15):1213–1223
Mautner L, Baillie CK, Herold HM et al (2020) Rapid point-of-care detection of SARS-CoV-2 using reverse transcription loop-mediated isothermal amplification (RT-LAMP). Virol J 17:160. https://doi.org/10.1186/s12985-020-01435-6
Meagher RJ, Priye A, Light YK, Huang C, Wang E (2018) Impact of primer dimers and self-amplifying hairpins on reverse transcription loop-mediated isothermal amplification detection of viral RNA. Analyst 143(8):1924–1933
Mousavi SZ, Rahmanian M, Sami A (2022) Organ-specific or personalized treatment for COVID-19: rationale, evidence, and potential candidates. Funct Integr Genomics 22(3):429–433
Nagura-Ikeda M, Imai K, Tabata S, Miyoshi K, Murahara N, Mizuno T et al (2020) Clinical evaluation of self-collected saliva by quantitative reverse transcription-PCR (RT-qPCR), direct RT-qPCR, reverse transcription–loop-mediated isothermal amplification, and a rapid antigen test to diagnose COVID-19. J Clin Microbiol 58(9):e01438–e01420
Nakano R, Nakano A, Ishii Y, Ubagai T, Kikuchi-Ueda T, Kikuchi H et al (2015) Rapid detection of the Klebsiella pneumoniae carbapenemase (KPC) gene by loop-mediated isothermal amplification (LAMP). J Infect Chemother 21(3):202–206
Neitz J, Neitz M (2011) The genetics of normal and defective color vision. Vision Res 51(7):633–651
Niu JH, Jian H, Guo QX, Chen CL, Wang XY, Liu Q, Guo YD (2012) Evaluation of loop-mediated isothermal amplification (LAMP) assays based on 5S rDNA-IGS2 regions for detecting Meloidogyne enterolobii. Plant Pathology 61(4):809–819
Notomi T (2000) Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 28(12):63e–663e. https://doi.org/10.1093/nar/28.12.e63
Odiwuor N, Xiong J, Ogolla F, Hong W, Li X, Khan FM, Wang N, Yu J, Wei H (2022) A point-of-care SARS-CoV-2 test based on reverse transcription loop-mediated isothermal amplification without RNA extraction with diagnostic performance same as RT-PCR. Anal Chim Acta 1200:339590
Pang B, Yao S, Xu K, Wang J, Song X, Mu Y et al (2019a) A novel visual-mixed-dye for LAMP and its application in the detection of foodborne pathogens. Anal Biochem 574:1–6
Pang B, Yao S, Xu K, Wang J, Song X, Mu Y et al (2019b) A novel visual-mixed-dye for LAMP and its application in the detection of foodborne pathogens. Anal Biochem 574:1–6
Rabaan AA, Alshahrani FS, Halwani MA, Nassar MS, Bakhrebah MA, Abdulaal WH et al (2023b) A narrative review on SARS-CoV-2 infection in pediatrics: concerns, challenges, management, and mitigation strategies. J Popul Ther Clin Pharmacol 30(17):187–205
Rabaan AA, Smajlović S, Tombuloglu H, Ćordić S, Hajdarević A, Kudić N et al (2023a) SARS-CoV-2 infection and multi-organ system damage: a review. Biomol Biomed 23(1):37–52
Rivas-Macho A, Sorarrain A, Marimón JM, Goñi-de-Cerio F, Olabarria G (2023) Extraction-free colorimetric RT-LAMP detection of SARS-CoV-2 in saliva. Diagnostics 13(14):2344
Roberts A, Chouhan RS, Shahdeo D, Shrikrishna NS, Kesarwani V, Horvat M, Gandhi S (2021) A recent update on advanced molecular diagnostic techniques for covid-19 pandemic: an overview. Front Immunol 12:732756. https://doi.org/10.3389/fimmu.2021.732756
Samanta A, Alam SSM, Ali S, Hoque M (2023) Evaluating the transmission feasibility of SARS-CoV-2 Omicron (B. 1.1. 529) variant to 143 mammalian hosts: insights from S protein RBD and host ACE2 interaction studies. Funct Integr Genomics 23(1):36
Scohy A, Anantharajah A, Bodéus M, Kabamba-Mukadi B, Verroken A, Rodriguez-Villalobos H (2020) Low performance of rapid antigen detection test as frontline testing for COVID-19 diagnosis. J Clin Virol 129:104455
Scott AT, Layne TR, O’Connell KC, Tanner NA, Landers JP (2020) Comparative evaluation and quantitative analysis of loop-mediated isothermal amplification indicators. Anal Chem 92(19):13343–13353
Sharma D, Ye C, Lippi G, Torrelles JB, Martinez-Sobrido L, Gromiha MM, Henry BM (2022) In silico evaluation of the impact of the Omicron variant on the sensitivity of RT-qPCR assays for SARS-CoV-2 detection using whole genome sequencing. https://doi.org/10.21203/rs.3.rs-1220446/v1
Suebsing R, Kampeera J, Sirithammajak S, Withyachumnarnkul B, Turner W, Kiatpathomchai W (2015) Colorimetric method of loop-mediated isothermal amplification with the pre-addition of calcein for detecting Flavobacterium columnare and its assessment in tilapia farms. J Aquat Anim Health 27(1):38–44
Suebsing R, Pradeep PJ, Jitrakorn S, Sirithammajak S, Kampeera J, Turner WA et al (2016) Detection of natural infection of infectious spleen and kidney necrosis virus in farmed tilapia by hydroxynapthol blue-loop-mediated isothermal amplification assay. J Appl Microbiol 121(1):55–67
Tanner NA, Zhang Y, Evans Jr TC (2015) Visual detection of isothermal nucleic acid amplification using pHsensitive dyes. Biotechniques 58(2):59–68
Telenti A, Arvin A, Corey L, Corti D, Diamond MS, García-Sastre A et al (2021) After the pandemic: perspectives on the future trajectory of COVID-19. Nature 596(7873):495–504
Tombuloglu H, Sabit H, Al-Khallaf H, Kabanja JH, Alsaeed M, Al-Saleh N, Al-Suhaimi E (2022) Multiplex real-time RT-PCR method for the diagnosis of SARS-CoV-2 by targeting viral N, RdRP and human RP genes. Sci Rep 12(1):2853
Tombuloglu H, Sabit H, Al-Suhaimi E, Al Jindan R, Alkharsah KR (2021) Development of multiplex real-time RT-PCR assay for the detection of SARS-CoV-2. PloS One 16(4):e0250942
Tomita N, Mori Y, Kanda H, Notomi T (2008) Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat Protoc 3(5):877–882
Wang S et al (2021) Molecular evolutionary characteristics of SARS-CoV-2 emerging in the United States. J Med Virol 94:310–317
Wang X, Yin F, Bi Y, Cheng G, Li J, Hou L et al (2016) Rapid and sensitive detection of Zika virus by reverse transcription loop-mediated isothermal amplification. J Virol Methods 238:86–93
Wastling SL, Picozzi K, Kakembo AS, Welburn SC (2010) LAMP for human African trypanosomiasis: a comparative study of detection formats. PLoS Negl Trop Dis 4(11):e865
Xie L, Xie Z, Zhao G, Liu J, Pang Y, Deng X et al (2014) A loop-mediated isothermal amplification assay for the visual detection of duck circovirus. Virol J 11:1–6
Funding
Imam Abdulrahman bin Faisal University funds this study under the projects of 2020-IRMC-S-3 and 2020-IRMC-S-4, and the Deanship of Scientific Research (DSR) of Imam Abdulrahman Bin Faisal University (IAU) Fast track fund of COVID-19 (COVID19-2020-026-IRMC). Huseyin Tombuloglu received the awards.
Author information
Authors and Affiliations
Contributions
HT conceptualized and designed the experiments. MA, GA, and HT carried out the experiments. JHK and AAR provided the clinical samples. MA, AK, GT, and HT wrote the manuscript. EA and TU revised the manuscript. All authors read and commented on the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The Institutional Review Board (IRB) at Imam Abdulrahman bin Faisal University (IAU) approved the study with an IRB number of IRB-2020-13-406. Also, it is approved by the institutional research committee, and the de-identified samples were left over after completion of diagnostic tests; hence, this study requires no consenting as per institutional ethics committee regulations.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 979 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alsaeed, M., Alhamid, G., Tombuloglu, H. et al. Ultrasensitive and fast detection of SARS-CoV-2 using RT-LAMP without pH-dependent dye. Funct Integr Genomics 24, 16 (2024). https://doi.org/10.1007/s10142-024-01297-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10142-024-01297-z