Abstract
The thermo-sensitive genic male sterility (TGMS) system plays a key role in the production of two-line hybrids in rapeseed (Brassica napus). To uncover key cellular events and genetic regulation associated with TGMS, a combined study using cytological methods and RNA-sequencing analysis was conducted for the rapeseed TGMS line 373S. Cytological studies showed that microspore cytoplasm of 373S plants was condensed, the microspore nucleus was degraded at an early stage, the exine was irregular, and the tapetum developed abnormally, eventually leading to male sterility. RNA-sequencing analysis identified 430 differentially expressed genes (298 upregulated and 132 downregulated) between the fertile and sterile samples. Gene ontology analysis demonstrated that the most highly represented biological processes included sporopollenin biosynthetic process, pollen exine formation, and extracellular matrix assembly. Kyoto encyclopedia of genes and genomes analysis indicated that the enriched pathways included amino acid metabolism, carbohydrate metabolism, and lipid metabolism. Moreover, 26 transcript factors were identified, which may be associated with abnormal tapetum degeneration and exine formation. Subsequently, 19 key genes were selected, which are considered to regulate pollen development and even participate in pollen exine formation. Our results will provide important insight into the molecular mechanisms underlying TGMS in rapeseed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rapeseed (Brassica napus L.) is an important oil crop worldwide, which provide both edible oil for human consumption and industrial materials such as livestock meal, lubricants, and biodiesel (Rondanini et al. 2012). Utilization of heterosis has become a major strategy to increase crop productivity, and hybrid rapeseed accounts for about 70% of the total planted area in China (Fu 2019). So far, genic male sterility (GMS), cytoplasmic male sterility (CMS), ecological male sterility (EMS), self-incompatibility (SI), and chemical hybridization agent (CHA) are the main pollination control systems of heterosis utilization in rapeseed. Of them, EMS, or referred as photoperiod and/or temperature (thermo-) sensitive genic male sterility (P/TGMS), is regarded as an efficient system that can produce two-line hybrids. This system has the following advantages: the firstly, almost all conventional inbred lines can restore the fertility and can serve as male parents; secondly, no negative effects associated with sterility-inducing cytoplasm have been observed, and the thirdly, the genes of this system can be easily transferred to other genetic backgrounds (Yu et al. 2015). P/TGMS germplasms have been explored and characterized in several crops, including wheat (Tang et al. 2011, 2012; Ru et al. 2015; Yuan et al. 2018; Wang et al. 2019; Yang et al. 2020), maize (Tang et al. 2006), cotton (Zhang et al. 2020b), and soybean (Frasch et al. 2011). In rice, the genes underlying the P/TGMS trait have been successfully cloned and characterized (Ding et al. 2012; Zhou et al. 2014; Fan et al. 2016; Sun et al. 2022; Wu et al. 2022). More recently, using the Arabidopsis system, slowing of development is proposed as a general mechanism applicable to the sterility-fertility conversion of P/TGMS lines (Zhang et al. 2020a; Zhu et al. 2020). Rapeseed P/TGMS germplasms has been exploited and applied to hybrid breeding. Until now, several two-line hybrid varieties of rapeseed, including Xiangzayou No. 5, Xiangzayou No. 7, Liangyou 586, Ganliangyou No. 2, Ganliangyou No. 3 and Ganliangyou No. 5 (Liu et al. 2000, 2007, 2013; Gao et al. 2010), have been successfully developed and approved in China, indicating a promising future of two-line hybrid in rapeseed hybrid breeding.
Male sterility exists widely in plant kingdom because pollen development can be hindered at various developmental stages, which starts at the anther cell division and differentiation, and ends at the dehiscence of anthers when the mature pollen grains are released (Sanders et al. 1999; Quilichini et al. 2014). Since the first male sterile gene MS2 was mapped-based cloned in plants (Aarts et al. 1993), several dozens of genes have been identified to play vital roles in anther and pollen development in Arabidopsis (Ma 2005; Ariizumi and Toriyama 2011; Zhu et al. 2011; Jiang et al. 2013; Liu and Fan 2013; Shi et al. 2015; Ma et al. 2021). These genes could be classified into two types, regulatory genes and structural genes. The first type included various protein kinases and transcription factors (TFs), such as SPL/NZZ, EMS1, TPD1, DYT1, TDF1 , AMS / TDR , AtMYB103/MS188/MYB80, and MS1. The second type included genes involved in different pathways related to tapetum and pollen development, and the formation of primexine. The genes and their functions in anther and pollen development are summarized in Table 1. These TFs and their regulated downstream genes form genetic regulatory networks controlling anther and pollen development (Jiang et al. 2013; Liu and Fan 2013; Shi et al. 2015; Lu et al. 2020; Ma et al. 2021).
RNA-sequencing (RNA-seq) is a powerful and cost efficient high-throughput technology for transcriptomic profiling (Wang et al. 2009; McGettigan 2013; Wan and Li 2019). This technology has been used to identify differential expressed genes and important pathways related to male sterility in different kinds of male sterile lines in rapeseed, which include recessive GMS (Qu et al. 2015; Jiang et al. 2022), dominant GMS (Jiang et al. 2022), dominant TGMS (Yan et al. 2016), the recessive TGMS (Liu et al. 2017), pol TCMS (Xiao et al. 2021), and Nsa CMS (Xing et al. 2022) in B. napus. The findings of these studies have laid foundation for understanding of the complex molecular mechanism of male sterility in Brassica crops.
Previously, we have developed a TGMS line named 373S from a spontaneous male sterile mutant in B. napus (Yu et al. 2007). Recently, studies of different temperature and photoperiod treatments in natural conditions and controlled environments have shown that the fertility alteration of 373S are mainly attributed to temperature changes, and the critical temperature range leading to fertility alteration is from 10°C (15°C/5°C) to 12°C (17°C /7°C), and the temperature-responding stage coincides with anther development from pollen mother cell (PMC) formation to meiosis stages (Sun et al. 2020). Genetic analysis indicated that the TGMS trait in 373S was controlled by one pair of genes, with male sterility as the recessive (Sun et al. 2020). However, the genome-wide transcriptional analysis of TGMS-related genes and pathways of the TGMS line 373S need to be investigated. In the present study, we compared anther ultrastructure between the line 373S and the fertile control line Zhongshuang No. 9 (ZS9) and found various abnormalities in meiotic behavior and organelle degradation. To gain a comprehensive understanding of molecular mechanism of its male sterility, we further performed RNA-Seq to explore DEGs in fertile and sterile flower buds and anthers between fertile and sterile parents, and between fertile and sterile offsprings in BC1 population derived from the TGMS line 373S. Based on bioinformatics analysis, we finally identified candidate genes and TFs involved in pollen development, and further analyzed by quantitative real-time polymerase chain reaction (qRT-PCR). Our results will contribute to an understanding of TGMS molecular mechanisms and provide useful information for heterosis breeding in B. napus.
Materials and methods
Plant materials
The plant materials used in this study included 5A214 (the male sterile parent, 373S), 5C95 (the fertile parent), 5A219 (the BC1, 5A214// 5A214/5C95), and ZS9. We previously revealed that the TGMS trait in 373S was controlled by one recessive allele Bnmst1 (Sun et al. 2020). So, in the BC1 population of 5A219, the fertile and sterile individuals only differ in the locus BnMst1 /Bnmst1. All the materials were provided by Rapeseed Research Center, College of Agronomy, Northwest A&F University, China. These plants were cultivated in crop season from September 2015 to June 2016 in the experimental field of Northwest A&F University in Yangling (34°16′N, 108°4′E, altitude 530 m), Shaanxi, China. Cultural practices including soil preparation, fertilizer, and irrigations were applied equally to all the entries/experiments.
Cytological study
In April 2016, when the male fertility of the first opened flowers of 373S plants was visually detectable for male sterility, flower buds of 373S and ZS9 plants were collected and divided into groups according to their size on the same day (Sun et al. 2020). Acetocarmine staining was performed to examine the correlation of the microspore developmental stages with the bud lengths. Subsequently, DAPI (4′,6-diamidino-2-phenylindole) and aniline blue staining were performed. For transmission electron microscopy, anthers at different microspore developmental stages were fixed and embedded in LR-White resin. The ultrathin sections were obtained using a diamond knife on the Leica EM UC7 ultramicrotome (Leica, Nussloch, Germany), and picked up on the formvar-coated grids and floated on 3% aqueous solution of UA for 10–15 min, and then re-floated by 4% Pb solution for 8–10 min. All sections were examined under JEM-1230 transmission electron microscope (JEOL, Tokyo, Japan) at 80 kV, and all the images were recorded with side-inserted BioScan Camera Model 792 (Gatan, Pleasanton, Calif., USA).
RNA extraction and Illumina sequencing
For transcriptome sequencing, young flower buds (length ≤ 1mm) and anthers (isolated from > 1mm buds) of 5A214, 5C95, sterile, and fertile plants from 5A219 were pooled separately, and immediately frozen in liquid nitrogen, and stored at −80°C for RNA extraction. As a result, 16 samples (eight specimens with two biological replications, each replication prepared from five plants) were used for RNA-seq analysis (Table S1). For example, 5A219F-A1, means the first replication of anthers from fertile plants of 5A219.
Total RNA was extracted from the 16 samples with plant RNA extraction kit (E.Z.N.A. ®Plant RNA Kit, R6827-01, OMEGA), according to the manufacturer’s instructions. The quality and purity of RNA samples were monitored on 1% agarose gels, and the RNA integrity number (RIN) of samples was assessed using Agilent 2100 Bioanalyzer. Following purification, the mRNA samples were fragmented into small pieces using divalent cations under an elevated temperature in Reaction Buffer (5X) and the cleaved RNA fragments were used for first-strand cDNA synthesis using reverse transcriptase and random primers. Second strand cDNA synthesis was then performed using DNA Polymerase I and RNase H. Short fragments were purified and resolved with Ethidium bromide buffer for end reparation and single nucleotide A (adenine) addition. After that, the short fragments were connected with adapters and the suitable fragments were selected for the PCR amplification. Agilent 2100 Bioanaylzer and ABI StepOnePlus Real-Time PCR System were used in quantification and qualification of the cDNA libraries. Finally, the prepared libraries were sequenced using a paired-end read protocol with 100 bp of data collected per run on the Illumina Hiseq 4000.
Sequencing data analysis and identification of DEGs
Clean reads were obtained from raw data by filtering the adaptor sequences, the low-quality sequences and unknown nucleotides N, and stored in FASTQ format (Cock et al. 2010). Then, the clean reads were mapped to the reference genome sequence of B. napus (http://www.brassica.info/resource/genome.php) (Chalhoub et al. 2014) using Bowtie2 v2.2.6 (http://bowtie-bio.sourceforge.net/bowtie2/index.shtml), with only a 1-bp mismatch allowed. After alignment, the count of mapped reads from each sample was derived and normalized to FPKM (Fragments Per Kilobase of transcript per million mapped reads). Based on the expression level, statistical significance in gene expression between sterile and fertile samples was assessed using the NOIseq program (Tarazona et al. 2011). The DEGs were identified using log2 (Fold change ratio of sterile/fertile) ≥ 1 and probability ≥ 0.8.
Bioinformatics analysis of DEGs
Functional annotation of DEGs was performed using BLASTX searches against the NR and Swiss-Prot databases (E-value < 1e−5). Venn diagram, Hierarchical Clustering, Gene ontology (GO), and Kyoto encyclopedia of genes and genomes (KEGG) pathway analysis were performed using the OmicShare tools, a free online platform for data analysis (www.omicshare.com/tools), to identify the putative biological functions and biochemical pathways for DEGs and find statistically overrepresented GO terms or KEGG pathways. BiNGO plugin (Maere et al. 2005) of Cytoscape software (http://www.cytoscape.org/) was used to draw networks of downregulated and upregulated genes (using Arabidopsis data). The known and predicted interactions among the DEGs were proved by STRING (https://string-db.org/) based on Arabidopsis data. Analysis of Cis-regulatory element of the 430 DEGs was performed using the online software Plant CARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/). Differentially expressed TFs were identified by online software Plant TFDB 4.0 (http://planttfdb.cbi.pku.edu.cn/) from the 430 DEGs.
qRT-PCR verification
To validate the Illumina sequencing results and characterize genes that are differentially expressed between the sterile and fertile samples, small buds (length ≤ 1mm), and anthers (from buds length > 1mm), the 19 genes identified to be related to the pollen development were selected for analysis using qRT-PCR. RNAs were extracted as mentioned above, and cDNAs were synthesized according to the manufacturer’s protocol of GoScript TM Reverse Transcription System (A5001, Promega). A housekeeping gene Ubiquitin-conjugating enzyme 21 (UBC21) was used as the reference gene, and all primer sequences used in this study are shown in Table S2. The qRT-PCR assays were performed in triplicate using GoTaq®qPCR master mix (A6001, Promega) on a QuantStudio™ 7 Flex Real-Time PCR System (Applied Biosystems). Statistical analysis was performed using the 2-ΔΔCT method (Livak and Schmittgen 2001). All data are expressed as mean ± standard deviation.
Verification of DEGs in 373S under restrictive condition (high temperature) and permissive condition (low temperature)
To verify the 430 DEGs identified above, the following experiment was further conducted. The seeds of 373S line were sown in the experimental field of Northwest A&F University in Yangling, Shaanxi, China in middle of September 2021. The seedlings were transplanted into pots to greenhouse after vernalization in the middle of January 2022. These plants were cultivated in a greenhouse for 2 weeks at 28 °C/14 °C, 14-h/10-h day/night cycle, light intensity: 4000–6000 Lux. According to our previous experimental results (Sun et al. 2020), the plants were then transferred to growth cabinets for two different temperature regime treatments (13 °C/3 °C (mean = 8 °C), permissive condition, and 17 °C/7 °C (12 °C), restrictive condition) under 14-h day/10-h night, light intensity: 4000 Lux, for 10 days and then returned to the greenhouse. The two treatments had three biological replications with three pots (two seedlings per pot) each. When the male fertility of the first opened flowers of the treated 373S plants was visually detectable for male fertility under permissive condition, or male sterility under restrictive condition, young flower buds (length ≤ 3 mm) of 373S plants were collected to perform RNA-seq analysis to verify the DEGs identified above. Totally, six samples (two treatments × three biological replications) were used for RNA-seq analysis following the same procedure as described above. Each replication was prepared from five plants.
Results
Phenotypic performance and cytological analysis of the TGMS line 373S
During the flowering period, the flowers of control line ZS9 plants had long filaments, full yellow anthers with sufficient pollen grains, whereas the flowers of 373S plants opened wide and flat, the filaments were shortened, and the stamens withered without pollens production under higher temperature (Sun et al. 2020).
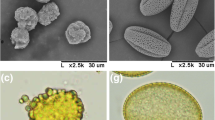
DAPI staining results indicated that, at PMC stage, the PMCs both in the control plants (Fig. 1A) and 373S plants (Fig. 1F) exhibited their chromosomes clearly. At tetrad stage, four bright DAPI signals were detected in control tetrads (Fig. 1B). By contrast, the tetrads of 373S plants showed very weak DAPI signals (Fig. 1G), indicating that their nuclei degenerated at this stage. At mature pollen stage, DAPI signals disappeared in 373S pollens (Fig. 1H), which indicated that their nuclei degraded completely compared to the control (Fig. 1C). In order to reveal the callose deposition pattern in 373S plants, we examined intact PMCs and tetrads isolated from anthers in PMC and tetrad stages. At PMC stage, a large percentage of PMCs were enclosed within thick callose wall in fertile anthers of control plants (Fig. 1D); however, PMCs of 373S plants were deformed or burst (Fig. 1I), and their callose walls were significantly thinner than their fertile counterparts (Table S3). In tetrad stage, tetrads from anthers of 373S plants were smaller than those in fertile counterparts, and callose walls of tetrads of 373S plants were also thinner than those in fertile counterparts (Figs. 1E, J; Table S3).
Abnormal chromosome and callose layer in 373S male sterile plants. A, F 4′,6-diamidino-2-phenylindole (DAPI) staining of pollen mother cells from male fertile and male sterile anthers; B, G DAPI staining of tetrads from male fertile and male sterile anthers; C, H DAPI staining of pollen grains from male fertile and male sterile anthers; D, I aniline blue staining of pollen mother cells from male fertile and male sterile anthers; E, J aniline blue staining of tetrads from male fertile and male sterile anthers
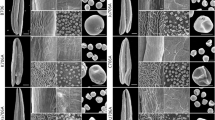
To gain insights into the cellular events during pollen development in 373S line, the anthers of 373S and fertile control plants at different developmental stages were examined by transmission electron microscopy. In the PMC stage, nuclear membranes, mitochondria, and vacuoles could be clearly observed in PMCs of fertile plants (Fig. 2A); however, these organelles could not be observed in PMCs of 373S plants (Fig. 2F). In the tetrad stage, PMCs of fertile control plants underwent meiosis to form tetrads surrounded by the characteristic callose wall, and numerous organelles were observed, including mitochondria and plastids in the tetrad cytoplasm (Fig. 2B). However, plasmolysis occurred in tetrads of 373S plants, and only a few tetrad cells had intact nuclei (Fig. 2G). In the early uninucleate stage, after the callose wall was dissolved, the microspores were released from the tetrads and covered with the exine wall in fertile control plants (Fig. 2C), whereas the microspores of 373S plants changed their shape, their pollen exine (tectum and baculum) did not form normally, and their cytoplasm was clearly concentrated (Fig. 2H). Thereafter, the microspores of 373S plants completely lost their cytoplasm and nuclei, leaving only irregular exine (Fig. 2I, J) compared with those of fertile control plants (Fig. 2D, E). The tryphine was not fully deposited in the pollen wall of sterile pollens of 373S plants during the middle uninucleate (Fig. 2I, V) and bicellular pollen stages (Fig. 2J, X) compared with fertile controls (Fig. 2D, U, E, W). In addition, the pollen intine of 373S plants could not be observed (Fig. 2V, X) compared with controls (Fig. 2U, W).
Transmission electron microscope micrographs of the anthers from control and 373S male sterile plants. A–E, U, W pollens from control plants; F–J, V, X pollens from sterile plants; K–O tapetal cells from control plants; P–T tapetal cells from sterile plants. A, F, K, P pollen mother cell stage; B, G, L, Q tetrad stage; C, H, M, R early microspore stage; D, I, N, S, U, V vacuolate microspore stage; E, J, O, T, W, and X bicellular pollen stage; U–X magnifications of the section indicated by the rectangle in D, I, E, and J, respectively. Ca, callose; Nu, nucleus; P, plastid; M, mitochondrion; G, Golgi body; Msp, microspore; Ex, exine; V, vacuole; L, lipid droplet; Epl, elaioplast; Ts, tapetosome; ER, endoplasmic reticulum; Ba, baculum; Te, tectum; In, intine; Ne, nexine; Try, tryphine
The observation of tapetum development indicated that there was no obvious difference between tapetal cells of 373S plants (Fig. 2P, Q) and the fertile control plants (Fig. 2K, L) before tetrad stage. In the tapetal cells, numerous organelles, including endoplasmic reticulum (ER), plastids, Golgi bodies, and vacuoles, could be observed. In the early uninucleate stage, abundant elaioplasts and tapetosomes were observed in fertile control plants (Fig. 2M). However, the tapetum of anthers from 373S plants showed abnormal developmental process, with only a large number of vacuoles formed (Fig. 2R). In the middle uninucleate stage, when the tapetal cells in the control plants just started to degrade (Fig. 2N), the tapetal cells in 373S plants were greatly degraded (Fig. 2S). In bicellular pollen stage, the tapetal cells of 373S plants were completely degraded (Fig. 2T) compared with those of the control plants (Fig. 2O). The results indicated that the tapetal cells in control underwent the normal programmed cell death (PCD) process, while the tapetal cells in 373S plants did not. Finally, the fertile control plants produced mature pollens, while 373S plants did not.
The transcriptome of the buds and anthers from fertile and sterile parents and their offspring
We sampled small buds (length ≤ 1mm) and anthers (dissected from > 1mm buds) from two parents (male sterile parent 5A214 and fertile parent 5C95) as well as male sterile and fertile plants from 5A219 (BC1 population, 5A214//5A214/5C95) for RNA-seq analysis (Table S1). Except for one sample (5C95-B2, Table S1) failed to be sequenced, all other samples were sequenced and contained 6.56-Gb raw reads. After filtering out reads containing unknown base N, low-quality data, and only adaptor reads, a total of 306,684,766 and 305,848,672 clean reads were obtained from fertile (the fertile parent and fertile plants of BC1 population) and sterile (the sterile parent and sterile plants of BC1 population) samples, respectively (Table S4). We mapped these clean reads to the B. napus reference sequences (http://www.brassica.info/resource/genome.php) and obtained average 70.06% and 69.70% reads matched (including perfect match and 1-bp or 2-bp mismatch) for fertile and sterile samples, respectively. Out of the reads mapped to the reference sequences, average 65.49% and 65.44% reads were uniquely matched. By sequence alignment, we found that a total of 427,988 and 427,713 genes were hit by the unique reads from fertile and sterile samples, respectively, which accounted for >94% of the known gene models. An overview of the Illumina sequencing results and the distribution of distinct clean reads is shown in Table S4.
The FPKM value was calculated to test the expression levels of the unigenes. The heatmap of sample correlations, which based on FPKM of all genes in the 15 samples tested (Additional file 1), showed that the two biological replicates of the same sample have higher correlation coefficience, which indicating good reproducibility between (Fig. S1A). Principal component analysis (PCA) showed that, the first four principal components (PC) cumulatively explained 88.99% of the total variance (Fig. S1C), with PC1 accounting for 34.411% and PC2 accounting for 30.077% of total variance, respectively. PCA analysis classified the tested samples into two groups (anther and bud samples) corresponding to their developmental stages, suggesting similar expression patterns in similar developmental stages (Fig. S1B).
Identification and validation of DEGs between fertile and sterile samples
We employed a two-step comparison strategy to identify the genes associated with male sterility in the TGMS line 373S. In the first step (Fig. S2), four pairwise comparisons were conducted between male fertile (5C95) and sterile (5A214) parents, and between male fertile (5A219F) and sterile (5A219S) plants of BC1 population at two stages (bud and anther). In the second step, we compared the DEGs from the comparisons of both parents with those from the comparison of male sterile and fertile plants of BC1, their intersect was DEGs of interest to us for further analysis (Fig. 3).
Strategy for identification of differentially expressed genes (DEGs) between fertile and sterile samples. Venn diagrams showing the resultant DEGs between fertile and sterile samples. The DEGs resulted from the comparisons between both parents as control, to remove the DEGs that showed differentially expressed between male sterile and fertile plants of BC1, but did not between both parents. Upregulated genes involved in four pairs of comparisons were shown in the left, and downregulated genes in the right
As a result, the pairwise comparisons in the first step revealed 4536 DEGs (2069 upregulated, 2467 downregulated) between 5A214-B and 5C95-B (5A214-B/5C95-B), 6005 DEGs (3140 upregulated, 2,865 downregulated) between 5A214-A and 5C95-A, 505 DEGs (228 upregulated, 277 downregulated) between 5A219S-B and 5A219F-B, and 648 DEGs (546 upregulated, 102 downregulated) between 5A219S-A and 5A219F-A (Fig. S2, Additional file 1). In total, 10,092 DEGs were identified. The second step comparison resulted in 430 DEGs (298 upregulated, 132 downregulated), which were common in pairwise comparisons between both parents and fertile and sterile plants of BC1 population (Fig. 3). These 430 DEGs included 242 in anthers, 90 in buds, and 98 common in both anthers and buds (Fig. S3, Additional file 2). Therefore, they were annotated to the TAIR database (http://www.arabidopsis.org/) (Additional file 3) and selected for further analysis.
Hierarchical clustering and functional enrichment analysis of the 430 DEGs
The selected 430 DEGs were subjected to a hierarchical clustering analysis. The results showed that 5A219F-A and 5C95-A, 5A219S-B and 5A214-B, 5A219F-B and 5C95-B, 5A219S-A and 5A214-A were first clustered together, and the group (5A219S-A and 5A214-A) was relatively far away from the other three groups, which indicated that samples were clustered according to their developmental stages and their fertility. Based on their expression patterns, the 430 DEGs were classified into six groups (Fig. 4). GO analysis of the overrepresented biological processes in each group indicated that each group encompassed several significant GO terms (Fig. 4, Additional file 4). The group 1 included 50 genes which were preferentially upregulated in 5A219F-A and 5C95-A, while downregulated in other samples, the most enriched GO term in this group was associated with regulation of multicellular organism growth. The group 2 included 72 genes which were preferentially upregulated in 5A219F-B and 5C95-B, the most enriched GO term in this group was related to anatomical structure formation involved in morphogenesis. The group 3 contained 92 genes which were upregulated in 5A214-A, the most enriched GO term was associated with response to organic substance. The group 4 included 128 genes which were preferentially upregulated in 5A219S-A, and the most enrichment GO term was related with regulation of lateral root development. The group 5 included 25 genes which were preferentially upregulated in 5A219S-B, the most enriched GO term was associated with protein targeting to chloroplast. The group 6 contained 63 genes which were preferentially upregulated in 5A219S-B, 5A214-B, 5A219F-B, and 5C95-B, and the most enriched GO term in this group was associated with pollen exine formation.
Cluster analysis of the 430 differentially expressed genes between the sterile and fertile samples. The color key represents log10 (FPKM+1). Color intensities (from green to red shading) increased with elevated expression level, as indicated at upper left. The red dotted line is used as reference line for grouping. The most significant GO (Gene ontology) term is indicated in each group
According to their GO annotation, these 430 DEGs were divided into three categories of biological process, cellular component, and molecular function, and further divided into 45 different sub-categories (Fig. S4, Additional file 5). For the biological process category, genes involved in cellular process were the predominant (208/430, 48.4%), followed by those involved in metabolic process (191/430, 44.4%), single-organism process (187/430, 43.5%), response to stimulus (138/430, 32.1%) and biological regulation (108/430, 25.1%). In the cellular component category, 65.6% (282/430) of DEGs targeted cell and cell part, 45.3% (195/430) were directed toward organelle, 29.3% (126/430) were directed toward membrane, 17.2% (74/430) were directed toward extracellular region, and 16.7% (72/430) to membrane part. In the molecular function category, 36.5% (157/430) of DEGs were assigned to binding, 34.4% (148/430) were assigned to catalytic activity, 6.5% (28/430) were assigned to nucleic acid binding transcription factor activity, 6.0% (26/430) were assigned to transporter activity, and 2.6% (11/430) to molecular function regulator.
In order to further analyze the important biological functions of the 430 DEGs, a detailed GO network of these DEGs was drawn by using the BiNGO plugin of Cytoscape software (Fig. S5). Based on the results of BiNGO analysis (Fig. S5) and GO enrichment analysis (Fig. S6), the most highly represented biological processes included sporopollenin biosynthetic process, pollen exine formation, extracellular matrix assembly, post-embryonic plant organ development, cellular component assembly and anatomical structure formation involved in morphogenesis, response to organic substance, asparagine biosynthetic process and glycine biosynthetic process. It is worth mentioned that, DEGs were mostly enriched in pollen exine formation in both GO enrichment analysis and BiNGO analysis.
We further investigated the known and predicted interactions among the DEGs by STRING. In the derived interaction network, these 430 DEGs were divided into 13 pathways (Additional file 6, Fig. S7). The genes in the pathway involved in response to stress were the predominant activity (47/430, 10.9%), followed by those involved in response to abiotic stimulus (32/430, 7.4%), multi-organism process (31/430, 7.2%), response to other organism (29/430, 6.7%), and response to oxygen-containing compound (27/430, 6.3%). The most interesting pathways included pollen wall assembly (including genes CYP703A2, FAR2, SHT, SWEET8, TKPR1, and TKPR2), carbohydrate transmembrane transport (NIP3;1, NIP7;1, STP1, STP14, STP2, SWEET1, SWEET8), pollen exine formation (CYP703A2, FAR2, SHT, TKPR1, TKPR2), and sporopollenin biosynthetic process (CYP703A2, TKPR1, TKPR2). The genes involved in these pathways are very important to microspore and pollen development.
Genes usually play a role in special biological functions by interacting with each other. Pathway-based analysis helps to further elucidate the biological functions of the DEGs. In the present study, KEGG analysis indicated that amino acid metabolism, carbohydrate metabolism, lipid metabolism, translation, signal transduction, transport, and catabolism were overrepresented metabolic activity, while pathways involved in global and overview, folding, sorting and degradation, metabolism of terpenoids and polyketides were also present (Fig. S8A). The KEGG enrichment analysis revealed that the top 20 of significantly overrepresented pathways related to fertility included photosynthesis-antenna proteins, amino acid metabolism, cutin, suberine and wax biosynthesis, flavoniod biosynthesis, terpenoid backbone biosynthesis, fatty acid elongation, pentose, and glucoronate interconversions (Fig. S8B). Further studies of these genes involved in certain pathways would help to elucidate the underlying mechanisms of male sterility in 373S.
TFs involved in anther and pollen development
In the anther and pollen development processes, TFs are generally thought to be important regulators. In this study, 26 TFs (23 upregulated, 3 downregulated) were identified from the 430 DEGs with online software Plant TFDB 4.0 (http://planttfdb.cbi.pku.edu.cn/) (Table 2, Additional file 3). Among these TFs, 20 genes were specifically upregulated in sterile anthers, and three genes upregulated in both sterile anthers and buds; two genes were specifically downregulated in sterile buds, and one gene was downregulated in both sterile anthers and buds. The TFs included HD-ZIP family (seven genes), NAC family (four genes), bZIP family (three genes), RAV family (two genes), C3H family (two genes), WRKY family (one gene), LBD family (one gene), bHLH family (one gene), B3 family (one gene), ERF family (one gene), TALE family (one gene), C2H2 family (one gene), and MYB family (one gene). Among these TFs, AMS is the key regulator in pollen development (Liu and Fan 2013; Shi et al. 2015; Ma et al. 2021). The other TFs identified may provide good candidate for investigation the pollen and anther functions.
In addition, the 430 DEGs were analyzed for the cis-regulatory elements in their promoter sequences. The identified cis-regulatory elements included temperature responsive elements (CAAT-box, MYC, TATA-box, MYB, G-box, DRE, and ABRE), light responsive elements (GT1-motif, G-box, GATA-box, and I-box), drought responsive elements (CAAT-box, GT1-motif, MYC, ABRE, MYB, G-box, DRE, and W-box), hormone responsive elements (ABRE, DRE, TATA-box, CAAT-box, W-box, G-box, and GT1-motif), and so on (Additional file 7). The presence of above-mentioned regulatory elements indicated that the expression of the 430 DEGs may be influenced by temperature, drought stress, hormone and light. Furthermore, in the promoter sequences of the 19 pollen development-related DEGs identified below (Table 3), the most cis-regulatory element was TATA-box (617/1272, 48.5%), followed by AT~ TATA-box (126/1272, 9.9%), MYB (104/1272, 8.2%), CAAT-box (74/1272, 5.8%), and MYC (67/1272, 5.3%), indicating that the expression of the 19 genes may be induced mostly by temperature, drought stress and hormone. Furthermore, the TFs of bHLHs, NACs, WRKYs, ERFs, and bZIPs may be bound to G-box, MYC-like, W-box, DRE, and ABRE, respectively, and they were responded to temperature, drought stress and light factors.
Genes associated with pollen development in 373S
In combination of TAIR database annotation, clustering analysis, GO, and KEGG analysis, we finally identified 19 genes which are involved in pollen development and pollen wall formation (Table 3, Additional file 3). The 19 DEGs were clustered into six groups according to their expression patterns in the four pair samples tested (Fig. S9). The group 1 comprised two genes, BnaA03g23380D and BnaC03g27700D, both were annotated to CEP1. The group 2 comprised four genes, BnaA10g04790D (STP2), BnaCnng08630D (CYP703A2), BnaC01g03140D (TKPR1), and BnaA10g19830D (TKPR2). The group 3 comprised two genes, BnaA03g41380D and BnaC07g32300D, both were annotated to Lipid Transfer Protein 12 (LTP12). The group 4 comprised five genes, BnaC05g00670D (CYP703A2), BnaA02g17470D (SKS18), BnaC02g23450D (SKS18), BnaA03g41660D (KCS15), and BnaC07g32730D (KCS15). The group 5 comprised two genes, BnaC07g41240D and BnaA03g49210D, both were annotated to ANTHER 7 (A7). The group 6 comprised four genes, BnaAnng22360D (SHT), BnaC09g09810D (SHT), BnaA02g17600D (ATA27), and BnaC03g46740D (AMS). Generally, genes annotated to the same orthologous gene in Arabidopsis were clustered together, except two genes annotated to CYP703A2, one (BnaCnng08630D) in group 2, another (BnaC05g00670D) in group 4.
qRT-PCR verification
The 19 genes related to pollen development were chosen to verify the accuracy of the RNA-Seq result by qRT-PCR. The results indicated that 78.95% (15/19) of the chosen genes showed significantly positive correlations (r=0.7392–0.9997) between the two results. This showed that the majority of these 19 genes had similar expression pattern by two approaches, indicating that the RNA-Seq results in this study are reliable (Fig. 5), which enhanced the reliability of our data in a relatively wide range.
qRT-PCR verification of the 19 selected differentially expressed genes involved in pollen development. Values were estimated from 2-△△Ct values in qRT-PCR and the standard deviation for each sample with three replicates was indicated by error bars. r, Pearson correlation coefficient between the results of qRT-PCR and RNA-Seq. * and ** mean significant at 0.05 and 0.01 level, respectively
Verification of DEGs in 373S under restrictive condition (high temperature) and permissive condition (low temperature)
To verify the 430 DEGs identified above, the field grown 373S seedlings were transplanted to greenhouse after vernalization and treated with two different temperature regimes (13 °C/3 °C (mean = 8 °C, permissive condition) and 17 °C/7 °C (12 °C, restrictive condition) for 10 days, according to our previous experimental results (Sun et al. 2020). When the male fertility of the first opened flowers of the treated 373S plants was visually detectable for male fertility under permissive condition, or male sterility under restrictive condition, young flower buds (length ≤ 3 mm) of 373S plants under two treatments were collected to perform RNA-seq analysis. As a results, 127 of the 430 genes (29.53%) were detected as differentially expressed when cutoff value as log2 (fold change) ≥ 1 and p =0.05 (Additional file 8, Table S5). Among the 127 differentially expressed genes in common, only 36 genes (28.35%) showed the same change trend (Table S5). These results indicated that DEGs identified between sterile and fertile samples, and those identified in 373S under permissive and restrictive conditions were largely different (Table S5, Additional file 3).
Discussion
Photoperiod and/or temperature sensitive genic male sterility (P/TGMS) is one of the most important pollination control systems for rapeseed hybrid production. Some valuable rapeseed P/TGMS germplasms have been identified and used for development of several two-line hybrid varieties of rapeseed (Liu et al. 2000, 2007, 2013; Gao et al. 2010). However, the molecular mechanism underlying the male sterility in rapeseed P/TGMS is unclear, which therefore limits the utilization of such system in hybrid breeding. In the present study, a combined study of cytological observation and RNA-seq based transcriptome profiling analysis was conducted to uncover the key cellular events and molecular regulation mechanism in the TGMS line 373S (B. napus). Many novel abnormalities were observed during 373S anther abortion, including microspore nucleus degradation, abnormal tapetum degradation, less elaioplast and tapetosome formation in tapetal cells, and irregular pollen wall formation (Fig. 1 and Fig. 2). Comparative transcriptome analysis identified 430 DEGs between the fertile and sterile samples, with 298 being upregulated and 132 downregulated (Fig. 3). GO analysis identified the most highly represented biological processes including sporopollenin biosynthetic process, pollen exine formation, extracellular matrix assembly, post-embryonic plant organ development, cellular component assembly, anatomical structure formation involved in morphogenesis, response to organic substance, asparagine biosynthetic process and glycine biosynthetic process (Fig. S5). KEGG analysis indicated that the most enriched pathways included amino acid metabolism, carbohydrate metabolism, lipid metabolism, translation, signal transduction, transport and catabolism (Fig. S8B). The 26 differentially expressed TFs were identified, which may be involved in abnormal formation of pollen wall, abnormal degeneration of tapetum, and ultimately lead to pollen abortion (Table 2, Additional file 3). These results suggested that the degradation of microspore nuclei due to abnormal development of chromosomes and callose wall, and abnormal pollen wall formation due to the abnormal development of tapetum are the main causes of pollen abortion, and the aberrant gene expression regulation revealed by RNA-seq analysis corresponded well with the observed cytological abnormalities.
Cytological characteristics of 373S anther abortion
In flowering plants, successful male reproductive development requires a sequence of developmental events such as cell differentiation, cell-to-cell communication, meiosis, and mitosis (McCormick 2004; Scott et al. 2004). Several studies have been conducted to examine ultrastructure changes in anthers of rapeseed EMS lines. The abortion of microspores in the EMS line H50S in B. napus started after the microspores were released from the tetrads. No obvious vacuoles were observed in the aborted microspores, and nuclei of the aborted microspores collapsed (Sun et al. 2009). For the EMS line Huiyou 50S, the microspore abortion occurred at the uninucleate stage. The microspore cytoplasm was disintegrated, and microspores became an empty cell at later time. Meanwhile, the tapetum degraded rapidly at late uninucleate stage (Xu et al. 2014b). Cytological observation of the TGMS line SP2S revealed many abnormal cytological phenomena, such as excessive vacuolation of tapetal cells at the microsporocyte stage, delayed tetrad wall degradation, vacuolization of uninucleate microspores, later deposition of exine, and absence of nexine (Yu et al. 2015; Liu et al. 2017). Ultrastructure analysis of the thermo-sensitive DGMS line TE5A in B. napus showed that its male gamete development was stopped at meiosis prophase I. Homologous chromosomes could not pair, synapse, condense, and form bivalents in TE5A (Yan et al. 2016). Compared with the results of anther development in these EMS lines, our cytological observations revealed some novel and noteworthy features during the anther development of the line 373S (Fig. 1 and Fig. 2). Our results suggested that abnormal anther development of 373S first appeared at the PMC stage. The PMCs of 373S plants had less cytoplasm, early degradation of nuclei, and deformation of the callose wall. However, some anthers in 373S could develop to tetrad and uninucleate microspore stages. Finally, the microspores in 373S deformed and were only surrounded by an irregular exine, and their nuclei, cytoplasm and intine were completely degraded. These findings enriched our previous results of semi-thin section and scanning electron microscope observation (Sun et al. 2020). Pollen wall consists of three layers including an inner pectocellulosic intine, an outer sporopollenin based exine and a lipid and protein-rich pollen coat in the crevices of exine, and its formation is an essential metabolic process throughout pollen development (Liu and Fan 2013; Shi et al. 2015; Ma et al. 2021). For tapetum development, previous studies have reported that elaioplasts are specialized plastids derived from proplastids which contain few internal membranes that were packed with globules of steryl esters enclosed by structural proteins, and they are abundant in tapetal cells during the active stage of pollen maturation in Brassicaceae species, including Arabidopsis (Platt et al. 1998; Ting et al. 1998; Wu et al. 1999; Kim et al. 2001). After degradation of tapetal cells, elaioplasts were deposited onto the maturing pollens to form the pollen coat, which is the outermost layer of the pollen wall (Ting et al. 1998). Tapetosomes, another specific organelle in cruciferous plants, act as transporters for the tryphine components in Arabidopsis and Brassicaceae plants, and they also accumulate ER-derived flavonoids, alkanes, oleosins for the formation of the pollen wall, as well as regulate tapetum programmed cell death (Xu et al. 2014a; Shi et al. 2015). Our study revealed that in the tapetal cells of 373S line, elaioplasts and tapetosomes disappeared, the rest of subcellular structures were invisible, and only a large number of vacuoles remained. All these phenomena we observed in 373S were not reported previously in other rapeseed EMS lines (Dong et al. 2004; Sun et al. 2009; Xu et al. 2014b; Yu et al. 2015; Yan et al. 2016; Liu et al. 2017). The novel results may be due to the different genetic backgrounds of the plant materials and the different environments in which the plant materials were grown.
Pathways and genes involved in anther abortion of 373S
Comparative transcriptomic analysis facilitates the systematic identification of DEGs between fertile and sterile samples, and enhances exploration of molecular mechanism involved in anther and pollen development (Wan and Li 2019). Although some valuable rapeseed P/TGMS germplasms have been discovered and utilized in rapeseed hybrid breeding (Sun et al. 2020), a few studies combining cytological and transcriptomic analysis were carried out to reveal the underlying molecular mechanism of P/TGMS in rapeseed (Yan et al. 2016; Liu et al. 2017). To reveal the molecular mechanism of the TGMS TE5A in B. napus, Yan et al. (2016) performed RNA-seq analysis to identify DEGs participating in the control of fertility. As a result, they obtained a total of 3841 DEGs between TE5A and its fertile NILs. Some of the DEGs were associated with homologous chromosome behavior and cycle control, which corresponded well with the abnormal meiosis prophase I observed in TE5A. Liu et al. (2017) conducted transcriptomic comparisons between young flower buds of the TGMS line SP2S and its NIL SP2F plants, and identified 465 differentially expressed transcripts. They suggested that the TGMS genes in SP2S may affect key proteins that are required for microsporocyte meiosis and tapetum development. The aberrant genetic regulation corresponded well with the observed cytological abnormalities. To reveal the underlying fertility conversion mechanism that controls fertility restoration of the pol TCMS in rapeseed, Xiao et al. (2021) identified 1637 DEGs in the fertile flowers of the TGMS 596 line under low temperature (16°C) when compared to the sterile flower of the temperature-stable pol CMS 1318 line and the TGMS 596 line under high temperature (25°C) by RNA-seq. These DEGs were involved in temperature response, ROS accumulation, anther development, and mitochondrial function. They also found that alternative oxidase, type II NAD(P)H dehydrogenases, and transcription factor Hsfs might play an important role in male fertility under the low-temperature condition. In the present study, we identified 430 DEGs (298 upregulated and 132 downregulated) between male sterile parent (373S line) and fertile parent, and between male sterile and fertile plants of the BC1 population derived from 373S line (Fig. 3, Additional files 2 and 3). These DEGs are mainly involved in sporopollenin biosynthetic process, pollen exine formation, extracellular matrix assembly, post-embryonic plant organ development, cellular component assembly and anatomical structure formation involved in morphogenesis, response to organic substance, asparagine biosynthetic process and glycine biosynthetic process. Among them, the process of pollen exine formation is the only pathway shared to another rapeseed TGMS line SP2S (Liu et al. 2017), indicating that this pathway is important for anther and pollen formation.
Anther and pollen development is a complex process governed by genetic regulatory networks consisting of many TFs and their downstream genes (Jiang et al. 2013; Liu and Fan 2013; Shi et al. 2015; Ma et al. 2021). Previous studies indicated that the genetic regulatory network DYT1-TDF1-AMS-MS188/MS103-MS1, and their downstream genes, including CYP703A2, ACOS5, TKPR1/2, controls the formation of the sexine of pollen wall (Jiang et al. 2013; Liu and Fan 2013; Shi et al. 2015; Lu et al. 2020; Ma et al. 2021). In addition, another pathway DYT1-TDF1-AMS-TEK-APGs is responsible for synthesizing the nexine of the pollen wall. So, AMS plays a central role in tapetum development and pollen wall formation in Arabidopsis (Sorensen et al. 2003; Xu et al. 2010; Xu et al. 2014a; Shi et al. 2015; Ma et al. 2021). Lipid synthesis-related genes CYP703A2 (Morant et al. 2007), CYP704B1 (Dobritsa et al. 2009), and MS2 (MALE STERILE2) (Chen et al. 2011) in Arabidopsis and the corresponding orthologs CYP704B2 (Li et al. 2010), CYP703A3 (Yang et al. 2014), and DPW (DEFECTIVE POLLEN WALL) (Xu et al. 2017) in rice are essential for the pollen wall formation. MS2, ACOS5, CYP703A, and CYP704B work in cascade to synthesize the main components of the sporopollenin precursor-fatty acid monomers, and their expressions are regulated by the TF AMS (Xu et al. 2010; Xu et al. 2014a). In the present study, we identified 26 TFs from the 430 DEGs (Table 2), including the members of HD-ZIP, NAC, bZIP, RAV, C3H, WRKY, LBD, bHLH, B3, ERF, TALE, C2H2, and MYB family. Through clustering, GO, and KEGG analysis of the 430 DEGs, we excavated 19 genes related to pollen development in the 373S line (Table 3). These genes included the TF AMS and some of its target genes reported previously, including CYP703A2,KCS15, TKPR1/DRL1, and LTP12 (Xu et al. 2010;Xu et al. 2014a). Furthermore, we have also identified some other TFs and DEGs not involved in the above AMS pathways in the present study, which are worthy of further study.
In the present study, in addition to genes that regulate the exine formation of the pollen wall synthesized in the tapetum, we have found some other genes, including ATA27 and STP2, which influence the exine formation of the pollen wall by regulating callose. Callose is the main component of the tetrad wall and forms at the early stage of meiosis. The synthesis and degradation of callose are important for the formation of pollen exine. ATA27 encodes a pollen coat protein similar to the BGL4 -glucosidase in B. napus. It only expressed in both tapetum and pollen during microspore and pollen development (Rubinelli et al. 1998; Lu et al. 2020). The STP2 gene of A. thaliana encodes a high affinity, low specificity monosaccharide carrier that can transport a number of hexoses and pentoses at similar rates. Its expression is confined to the early stages of gametophyte development from the beginning of callose degradation to microspore release from the tetrads, suggesting its role in the uptake of glucose units resulting from callose degradation during pollen maturation (Truernit et al. 1999). The aberrant expression of these genes may be related to deformation of the callose wall and intine observed in the present study (Fig. 1 and Fig. 2). Together, these abnormal gene expressions and metabolism caused cytoplasmic condensation, abnormal callose, irregular exine, and abnormal degradation of microspore nuclei and tapetum, ultimately leading to pollen abortion in the TGMS 373S. Of course, many genes, especially those mentioned above, are involved in this process. In the future, the cloning and functional characterization of the TGMS gene (s) underlying the TGMS trait in 373S line will help to reveal the genetic mechanism of thermo-sensitive male sterility in rapeseed.
Conclusions
In this study, cytological observation and comparative transcriptome analysis were conducted to reveal the mechanism underlying temperature inducing male sterility in the rapeseed line 373S. Cytological studies indicated that the condensed cytoplasm, nucleus degradation of microspore, irregular pollen wall formation, and abnormal degradation of tapetum were related to microspore abortion in 373S. The comparative transcriptome analysis identified 430 DEGs in buds and anthers between male sterile and fertile samples. Of them, 298 were upregulated and 132 downregulated. The most highly represented biological processes included sporopollenin biosynthetic process and pollen exine formation. Nineteen pollen development-related genes, 26 TFs, and lots of cis-regulatory elements were identified. Furthermore, the TFs of bHLHs, NACs, WRKYs, ERFs, and bZIPs probably bound to the cis-regulatory elements of G-box, MYC-like, W-box, DRE, and ABRE of the 430 DEGs. The findings will provide insights into the complex gene regulation network during pollen development and contribute to understanding of the molecular mechanism of male sterility in TGMS rapeseed.
Data availability
The RNA-seq raw read data have been submitted in the Sequence Read Archive of the NCBI (accession number: PRJNA612938)
Code availability
Not applicable.
Abbreviations
- CHA:
-
chemical hybridization agent
- CMS:
-
cytoplasmic male sterility
- DAPI:
-
4′,6-diamidino-2-phenylindole
- DEGs:
-
differentially expressed genes
- EMS:
-
ecological male sterility
- FPKM:
-
fragments per kilobase of transcript per million mapped reads
- GMS:
-
genic male sterility
- GO:
-
Gene Ontology
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- PMC:
-
pollen mother cell
- PTGMS:
-
photoperiod and temperature/thermo- sensitive genic male sterility
- P/TGMS:
-
photoperiod and/or temperature sensitive genic male sterility
- RGMS:
-
recessive genetic male sterility
- RIN:
-
RNA integrity number
- RNA-seq:
-
RNA-sequencing
- SI:
-
self-incompatibility
- TFs:
-
transcript factors
- TGMS:
-
thermo-sensitive genic male sterility
- ZS9:
-
Zhongshuang No. 9
References
Aarts MGM, Dirkse WG, Stiekema WJ, Pereira A (1993) Transposon tagging of a male sterility gene in Arabidopsis. Nature 363:715–717. https://doi.org/10.1038/363715a0
Aarts MGM, Hodge R, Kalantidis K, Florack D, Wilson ZA et al (1997) The Arabidopsis MALE STERILITY 2 protein shares similarity with reductases in elongation/condensation complexes. Plant J 12:615–623. https://doi.org/10.1046/j.1365-313X.1997.00615.x
Ariizumi T, Hatakeyama K, Hinata K, Sato S, Kato T, Tabata S, Toriyama K (2003) A novel male-sterile mutant of Arabidopsis thaliana, faceless pollen-1, produces pollen with a smooth surface and an acetolysis-sensitive exine. Plant Mol Biol 53:107–116. https://doi.org/10.1023/B:PLAN.0000009269.97773.70
Ariizumi T, Hatakeyama K, Hinata K, Inatsugi R, Nishida I, Sato S, Kato T, Tabata S, Toriyama K (2004) Disruption of the novel plant protein NEF1 affects lipid accumulation in the plastids of the tapetum and exine formation of pollen, resulting in male sterility in Arabidopsis thaliana. Plant J 39:170–181. https://doi.org/10.1111/j.1365-313X.2004.02118.x
Ariizumi T, Toriyama K (2011) Genetic regulation of sporopollenin synthesis and pollen exine development. Annu Rev Plant Biol 62:437–460. https://doi.org/10.1146/annurev-arplant-042809-112312
Chalhoub B, Denoeud F, Liu S et al (2014) Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 345:950–953. https://doi.org/10.1126/science.1253435
Chen W, Yu XH, Zhang K, Shi J, De Oliveira S, Schreiber L, Shanklin J, Zhang D (2011) Male Sterile 2 encodes a plastid-localized fatty acyl carrier protein reductase required for pollen exine development in Arabidopsis. Plant Physiol 157:842–853. https://doi.org/10.1104/pp.111.181693
Cock PJ, Fields CJ, Goto N, Heuer ML, Rice PM (2010) The Sanger FASTQ file format for sequences with quality scores, and the Solexa/Illumina FASTQ variants. Nucleic Acids Res 38:1767–1771. https://doi.org/10.1093/nar/gkp1137
De ASC, Kim SS, Koch S, Kienow L, Schneider K, McKim SM, Haughn GW, Kombrink E, Douglas CJ (2009) A novel fatty Acyl-coa synthetase is required for pollen development and sporopollenin biosynthesis in Arabidopsis. Plant Cell 21:507–525. https://doi.org/10.1105/tpc.108.062513
Ding J, Lu Q, Ouyang Y, Mao H, Zhang P, Yao L, Xu C, Li L, Xiao J, Zhang Q (2012) A long noncoding RNA regulates photoperiod-sensitive male sterility, an essential component of hybrid rice. Proc Natl Acda Sci USA 109:2654–2659. https://doi.org/10.1073/pnas.1121374109
Dobritsa AA, Shrestha J, Morant M, Pinot F, Matsuno M, Swanson R, Moller BL, Preuss D (2009) CYP704B1 is a long-chain fatty acid ω-hydroxylase essential for sporopollenin synthesis in pollen of Arabidopsis. Plant Physiol 151:574–589. https://doi.org/10.1104/pp.109.144469
Dobritsa AA, Lei Z, Nishikawa S, Urbanczyk-Wochniak E, Huhman DV, Preuss D, Sumner LW (2010) LAP5 and LAP6 encode anther-specific proteins with similarity to chalcone synthase essential for pollen exine development in Arabidopsis. Plant Physiol 153:937–955. https://doi.org/10.1104/pp.110.157446
Dong JG, Dong ZS, Liu XX, Liu CS, Li HB (2004) Cytological studies on anther development of ecological male sterile line 533S in Brassica napus L. J Northwest Sci-Tech Univ of Agri and For ( Nat Sci Ed ) 32:61–66. https://doi.org/10.1300/J064v24n01_09
Fan Y, Yang J, Mathioni SM, Yu J, Shen J, Yang X, Wang L, Zhang Q, Cai Z, Xu C, Li X, Xiao J, Meyers B, Zhang Q (2016) PMS1T, producing phased small-interfering RNAs, regulates photoperiod-sensitive male sterility in rice. Proc Natl Acda Sci USA 113:15144–15149. https://doi.org/10.1073/pnas.1619159114
Frasch RM, Weigand C, Perez PT, Palmer RG, Sandhu D (2011) Molecular mapping of 2 environmentally sensitive male-sterile mutants in soybean. J Hered 102:11–16. https://doi.org/10.1093/jhered/esq100
Fu T (2019) Genetics and breeding of rapeseed hybrid. Hubei Science and technology press, Wuhan, Hubei, China
Gao H, Liu Z, Yuan W, Wu P, Zhou J (2010) Breeding of two-line hybrid rape variety Ganliangyou No. 3. Hubei Agric Sci 49:2370–2371. https://doi.org/10.1201/b10279-23
Grienenberger E, Kim SS, Lallemand B, Geoffroy P, Heintz D, Souza C, Heitz T, Douglas CJ, Legrand M (2010) Analysis of TETRAKETIDE α-PYRONE REDUCTASE function in Arabidopsis thaliana reveals a previously unknown, but conserved, biochemical pathway in sporopollenin monomer biosynthesis. Plant Cell 22:4067–4083. https://doi.org/10.1105/tpc.110.080036
Higginson T, Li SF, Parish RW (2003) AtMYB103 regulates tapetum and trichome development in Arabidopsis thaliana. Plant J 35:177–192. https://doi.org/10.1046/j.1365-313X.2003.01791.x
Ito T, Shinozaki K (2002) The MALE STERILITY1 gene of Arabidopsis, encoding a nuclear protein with a PHD-finger motif, is expressed in tapetal cells and is required for pollen maturation. Plant Cell Physiol 43:1285–1292. https://doi.org/10.1093/pcp/pcf154
Ito T, Nagata N, Yoshiba Y, Ohme-Takagi M, Ma H, Shinozaki K (2007) Arabidopsis MALE STERILITY1 encodes a PHD-type transcription factor and regulates pollen and tapetum development. Plant Cell 19:3549–3562. https://doi.org/10.1105/tpc.107.054536
Jia GX, Liu XD, Owen HA, Zhao DZ (2008) Signaling of cell fate determination by the TPD1 small protein and EMS1 receptor kinase. Proc Natl Acda Sci USA 105:2220–2225. https://doi.org/10.1073/pnas.0708795105
Jiang J, Zhang Z, Cao J (2013) Pollen wall development: the associated enzymes and metabolic pathways. Plant Biol 15:249–263. https://doi.org/10.1111/j.1438-8677.2012.00706.x
Jiang M, Zhu J, Wang W, Yang L (2022) Global transcriptome analysis reveals potential genes associated with genic male sterility of rapeseed (Brassica napus L.). Front Plant Sci 13:1004781. https://doi.org/10.3389/fpls.2022.1004781
Kim HU, Wu SSH, Ratnayake C, Huang AHC (2001) Brassica rapa has three genes that encode proteins associated with different neutral lipids in plastids of specific tissues. Plant Physiol 126:330–341. https://doi.org/10.1104/pp.126.1.330
Kim SS, Grienenberger E, Lallemand B, Colpitts CC, Kim SY, de Azevedo SC, Geoffroy P, Heintz D, Krahn D, Kaiser M, Kombrink E, Heitz T, Suh DY, Legrand M, Douglas CJ (2010) LAP6/POLYK ETIDE SYN THASE A and LAP5/POLYK ETIDE SYNTHASE B encode hydroxyalkyl α-pyrone synthases required for pollen development and sporopollenin biosynthesis in Arabidopsis thaliana. Plant Cell 22:4045–4066. https://doi.org/10.1105/tpc.110.080028
Li N, Zhang DS, Liu HS, Yin CS, Li XX, Liang WQ, Yuan Z, Xu B, Chu HW, Wang J, Wen TQ, Huang H, Luo D, Ma H, Zhang DB (2006) The rice tapetum degeneration retardation gene is required for tapetum degradation and anther development. Plant Cell 18:2999–3014. https://doi.org/10.1105/tpc.106.044107
Li H, Pinot F, Sauveplane V, Werck-Reichhart D, Diehl P, Schreiber L, Franke R, Zhang P, Chen L, Gao Y, Liang W, Zhang D (2010) Cytochrome P450 family member CYP704B2 catalyzes the ω-hydroxylation of fatty acids and is required for anther cutin biosynthesis and pollen exine formation in rice. Plant Cell 22:173–190. https://doi.org/10.4161/psb.5.9.12562
Liu ZW, Yuan WH, Xing LW, Wu P, Zhou XP, Zhou JG, Fu JH (2000) Breeding of two-line system hybrid Liangyou 586 in Brassica napus. Chin J Oil Crop Sci 22:5–7
Liu ZW, Wu P, Zhang QX, Zhou JG, Yuan WH, Zhou XP (2007) Breeding of high quality two-line hybrid rape variety Ganliangyou 2. Acta Agric Univ Jiangxiensis 19:10–11
Liu X, Huang J, Parameswaran S, Ito T, Seubert B, Auer M, Rymaszewski A, Jia G, Owen HA, Zhao D (2009) The SPOROCYTELESS/NOZZLE gene is involved in controlling stamen identity in Arabidopsis. Plant Physiol 151:1401–1411. https://doi.org/10.1104/pp.109.145896
Liu L, Fan XD (2013) Tapetum: regulation and role in sporopollenin biosynthesis in Arabidopsis. Plant Mol Biol 83:165–175. https://doi.org/10.1007/s11103-013-0085-5
Liu N, Wu P, Yuan W, Zhou J, Liu Z, Wang F (2013) Breeding of high-quality two-line hybrid rape variety Ganliangyou No. 5. Acta Agric Jiangxi 25:25–26
Liu XQ, Liu ZQ, Yu CY, Dong JG, Hu SW, Xu AX (2017) TGMS in rapeseed (Brassica napus) resulted in aberrant transcriptional regulation, asynchronous microsporocyte meiosis, defective tapetum, and fused sexine. Front Plant Sci 8:1268. https://doi.org/10.3389/fpls.2017.01268
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Lu JY, Xiong SX, Yin W, Teng XD, Lou Y, Zhu J, Zhang C, Gu JN, Wilson ZA, Yang ZN (2020) MS1, a direct target of MS188, regulates the expression of key sporophytic pollen coat protein genes in Arabidopsis. J Exp Bot 71(16):4877–4889. https://doi.org/10.1093/jxb/eraa219
Ma H (2005) Molecular genetic analyses of microsporogenesis and microgametogenesis in flowering plants. Annu Rev Plant Biol 56:393–434. https://doi.org/10.1146/annurev.arplant.55.031903.141717
Ma XF, Wu Y, Zhang GF (2021) Formation pattern and regulatory mechanisms of pollen wall in Arabidopsis. J Plant Physiol 260:153388. https://doi.org/10.1016/j.jplph.2021.153388
Maere S, Heymans K, Kuiper M (2005) BiNGO: a Cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics 21:3448–3449. https://doi.org/10.1093/bioinformatics/bti551
McCormick S (2004) Control of male gametophyte development. Plant Cell 16(Suppl):S142–S153. https://doi.org/10.1105/tpc.016659
McGettigan PA (2013) Transcriptomics in the RNA-seq era. Curr Opin Chem Biol 17:4–11. https://doi.org/10.1016/j.cbpa.2012.12.008
Morant M, Jorgensen K, Schaller H, Pinot F, Moller BL, Werck-Reichhart D, Bak S (2007) CYP703 is an ancient cytochrome P450 in land plants catalyzing in-chain hydroxylation of lauric acid to provide building blocks for sporopollenin synthesis in pollen. Plant Cell 19:1473–1487. https://doi.org/10.1105/tpc.106.045948
Phan AH, Iacuone S, Li SF, Parish RW (2011) The MYB80 transcription factor is required for pollen development and the regulation of tapetal programmed cell death in Arabidopsis thaliana. Plant Cell 23:2209–2224. https://doi.org/10.1105/tpc.110.082651
Platt KA, Huang AHC, Thomson WW (1998) Ultrastructural study of lipid accumulation in tapetal cells of Brassica napus L. Cv. Westar during microsporogenesis. Int J Plant Sci 159:724–737. https://doi.org/10.2307/2475141
Qu C, Fu F, Liu M, Zhao H, Liu C, Li J, Tang Z, Xu X, Qiu X, Wang R, Lu K (2015) Comparative transcriptome analysis of recessive male sterility (RGMS) in sterile and fertile Brassica napus lines. PLoS One 10:e0144118. https://doi.org/10.1371/journal.pone.0144118
Quilichini TD, Douglas CJ, Samuels AL (2014) New views of tapetum ultrastructure and pollen exine development in Arabidopsis thaliana. Ann Bot 114:1189–1201. https://doi.org/10.1093/aob/mcu042
Rondanini DP, Gomez NV, Agosti MB, Miralles DJ (2012) Global trends of rapeseed grain yield stability and rapeseed-to-wheat yield ratio in the last four decades. Eur J Agron 37:56–65. https://doi.org/10.1016/j.eja.2011.10.005
Rowland O, Lee R, Franke R, Schreiber L, Kunst L (2007) The CER3 wax biosynthetic gene from Arabidopsis thaliana is allelic to WAX2/YRE/FLP1. FEBS Lett 581:3538–3544. https://doi.org/10.1016/j.febslet.2007.06.065
Ru ZG, Zhang LP, Hu TZ, Liu HY, Zhao CP (2015) Genetic analysis and chromosome mapping of a thermo-sensitive genic male sterile gene in wheat. Euphytica 201:321–327. https://doi.org/10.1007/s10681-014-1218-x
Rubinelli P, Hu Y, Ma H (1998) Identification, sequence analysis and expression studies of novel anther-specific genes of Arabidopsis thaliana. Plant Mol Biol 37:607–619. https://doi.org/10.1023/A:1005964431302
Sanders PM, Bui AQ, Weterings K, McIntire KN, Hsu YC, Lee PY, Yruong MT, Beals TP, Goldberg RB (1999) Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex Plant Reprod 11(29711):297. https://doi.org/10.1007/s004970050158
Schiefthaler U, Balasubramanian S, Sieber P, Chevalier D, Wisman E, Schneitz K (1999) Molecular analysis of NOZZLE, a gene involved in pattern formation and early sporogenesis during sex organ development in Arabidopsis thaliana. Proc Natl Acda Sci USA 96:11664–11669. https://doi.org/10.1073/pnas.96.20.11664
Scott RJ, Spielman M, Dickinson HG (2004) Stamen structure and function. Plant Cell 16(Suppl):S46–S60. https://doi.org/10.1016/j.laa.2009.09.016
Shi JX, Cui MH, Yang L, Kim YJ, Zhang DB (2015) Genetic and biochemical mechanisms of pollen wall development. Trends Plant Sci 20:741–753. https://doi.org/10.1016/j.tplants.2015.07.010
Sorensen AM, Krober S, Unte SU, Huijser P, Dekker K, Saedler H (2003) The Arabidopsis ABORTED MICROSPORES (AMS) gene encodes a MYC class transcription factor. Plant J 33:413–423. https://doi.org/10.1046/j.1365-313X.2003.01644.x
Sun X, Hu S, Yu C (2009) Cytological observation of anther development of an ecological male sterile line H50S in Brassica napus L. Acta Agric Bor-Occid Sin 18:153–158
Sun MX, Huang XY, Yang J, Guan YF, Yang ZN (2013) Arabidopsis RPG1 is important for primexine deposition and functions redundantly with RPG2 for plant fertility at the late reproductive stage. Plant Reprod 26:83–91. https://doi.org/10.1007/s00497-012-0208-1
Sun YY, Zhang DS, Wang ZZ, Guo Y, Sun XM, Li W, Zhi WL, Hu SW (2020) Cytological observation of anther structure and genetic investigation of a thermo-sensitive genic male sterile line 373S in Brassica napus L. BMC Plant Biol 20:8. https://doi.org/10.1186/s12870-019-2220-1
Sun YJ, Fu M, Wang L, Bai YX, Fang XL, Wang Q, He Y, Zeng HL (2022) OsSPLs regulate male fertility in response to different temperatures by flavonoid biosynthesis and tapetum PCD in PTGMS Rice. Int J Mol Sci 23:3744. https://doi.org/10.3390/ijms23073744
Tang JH, Fu ZY, Hu YM, Li JS, Sun LL, Ji HQ (2006) Genetic analyses and mapping of a new thermo-sensitive genic male sterile gene in maize. Theor Appl Genet 113:11. https://doi.org/10.1007/s00122-006-0262-x
Tang Z, Zhang L, Yang D, Zhao C, Zheng Y (2011) Cold stress contributes to aberrant cytokinesis during male meiosis I in a wheat thermosensitive genic male sterile line. Plant Cell Environ 34(3):389–405. https://doi.org/10.1111/j.1365-3040.2010.02250.x
Tang Z, Zhang L, Xu C, Yuan S, Zhang F, Zheng Y, Zhao C (2012) Uncovering small RNA-mediated responses to cold stress in a wheat thermosensitive genic male-sterile line by deep sequencing. Plant Physiol 159(2):721–738. https://doi.org/10.1104/pp.112.196048
Tarazona S, García F, Ferrer A, Dopazo J, Conesa A (2011) Differential expression in RNA-seq: A matter of depth. Genome Res 21:2213–2223. https://doi.org/10.1101/gr.124321.111
Ting JTL, Wu SSH, Ratnayake C, Huang AHC (1998) Constituents of the tapetosomes and elaioplasts in Brassica campestris tapetum and their degradation and retention during microsporogenesis. Plant J 16:541–551. https://doi.org/10.1046/j.1365-313x.1998.00325.x
Truernit E, Stadler R, Baier K, Sauer N (1999) A male gametophyte-specific monosaccharide transporter in Arabidopsis. Plant J 17:191–201. https://doi.org/10.1046/j.1365-313X.1999.00372.x
Vizcay-Barrena G, Wilson ZA (2006) Altered tapetal PCD and pollen wall development in the Arabidopsis ms1 mutant. J Exp Bot 57:2709–2717. https://doi.org/10.1093/jxb/erl032
Wan XY and Li ZW (2019) Plant comparative transcriptomics reveals functional mechanisms and gene regulatory networks involved in anther development and male sterility. In: Blumenberg M (ed) Transcriptome analysis. https://doi.org/10.5772/intechopen.88318.77860
Wang Z, Gerstein M, Snyder M (2009) RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 10:57–63. https://doi.org/10.1038/nrg2484
Wang Y, Duan W, Bai J, Wang P, Yuan S, Zhao C, Zhang L (2019) Constitutive expression of a wheat microRNA, TaemiR167a, confers male sterility in transgenic Arabidopsis. Plant Growth Regul 88(3):227–239. https://doi.org/10.1007/s10725-019-00503-4
Wilson ZA, Morroll SM, Dawson J, Swarup R, Tighe PJ (2001) The Arabidopsis MALE STERILITY1 (MS1) gene is a transcriptional regulator of male gametogenesis, with homology to the PHD-finger family of transcription factors. Plant J 28:27–39. https://doi.org/10.1046/j.1365-313x.2001.01125.x
Wu SSH, Moreau RA, Whitaker BD, Huang AHC (1999) Steryl esters in the elaioplasts of the tapetum in developing Brassica anthers and their recovery on the pollen surface. Lipids 34:517–523. https://doi.org/10.1007/s11745-999-0393-5
Wu LY, Jin XH, Zhang BL, Chen SJ, Xu R, Duan PG, Zou DN, Huang SJ, Zhou TB, An CC, Luo YH, Li YH (2022) A natural allele of OsMS1 responds to temperature changes and confers thermosensitive genic male sterility. Nat Commun 13(1):2055. https://doi.org/10.1038/s41467-022-29648-z
Xiao Q, Wang HD, Chen H, Wen J, Dai C, Ma CZ, Tu JX, Shen JX, Fu TD, Yi B (2021) Molecular analysis uncovers the mechanism of fertility restoration in temperature-sensitive Polima cytoplasmic male-sterile Brassica napus. Int J Mol Sci 22:12450. https://doi.org/10.3390/ijms222212450
Xing M, Guan C, Guan M (2022) Comparative cytological and transcriptome analyses of anther development in Nsa cytoplasmic male sterile (1258A) and maintainer lines in Brassica napus produced by distant hybridization. Int J Mol Sci 23:2004. https://doi.org/10.3390/ijms23042004
Xu J, Yang C, Yuan Z, Zhang D, Gondwe MY, Ding Z, Liang W, Zhang D, Wilson ZA (2010) The ABORTED MICROSPORES regulatory network is required for postmeiotic male reproductive development in Arabidopsis thaliana. Plant Cell 22:91–107. https://doi.org/10.1105/tpc.109.071803
Xu J, Ding Z, Vizcay-Barrena G, Shi J, Liang W, Yuan Z, Werck-Reichhart D, Schreiber L, Wilson ZA, Zhang D (2014a) ABORTED MICROSPORES acts as a master regulator of pollen wall formation in Arabidopsis. Plant Cell 26:1544–1556. https://doi.org/10.1105/tpc.114.122986
Xu XF, Hu YM, Yu CY, Ge J, Guo YF, Dong JG, Hu SW (2014b) Physiological characterization and genetic analysis of reverse thermo-sensitive genic male-sterile line Huiyou 50S in Brassica napus. Acta Agric Boreal Sin 29:147–152
Xu D, Shi J, Rautengarten C, Yang L, Qian X, Uzair M, Zhu L, Luo Q, An G, Wassmann F, Schreiber L, Heazlewood JL, Scheller HV, Hu J, Zhang D, Liang W (2017) Defective Pollen wall 2 (DPW2) encodes an acyl transferase required for rice pollen development. Plant Physiol 173:240–255. https://doi.org/10.1104/pp.16.00095
Yan XH, Zeng XH, Wang SS, Li KQ, Yuan R, Gao HF, Luo JL, Liu F, Wu YH, Li YJ, Zhu L, Wu G (2016) Aberrant meiotic prophase I leads to genic male sterility in the novel TE5A mutant of Brassica napus. Sci Rep 6:33955. https://doi.org/10.1038/srep33955
Yang WC, Ye D, Xu J, Sundaresan V (1999) The SPOROCYTELESS gene of Arabidopsis is required for initiation of sporogenesis and encodes a novel nuclear protein. Genes Dev 13:2108–2117. https://doi.org/10.1101/gad.13.16.2108
Yang SL, Xie LF, Mao HZ, Pauh CS, Yang WC, Jiang L, Sundaresan V, Ye D (2003) TAPETUM DETERMINANT 1 is required for cell specialization in the Arabidopsis anther. Plant Cell 15:2792–2804. https://doi.org/10.1105/tpc.016618
Yang SL, Jiang L, Puah CS, Xie LF, Zhang XQ, Chen LQ, Yang WC, Ye D (2005) Overexpression of TAPETUM DETERMINANT1 alters the cell fates in the Arabidopsis carpel and tapetum via genetic interaction with EXCESS MICROSPOROCYTES1/EXTRA SPOROGENOUS CELLS. Plant Physiol 139:186–191. https://doi.org/10.1104/pp.105.063529
Yang C, Vizcay-Barrena G, Conner K, Wilson ZA (2007) MALE STERILITY1 is required for tapetal development and pollen wall biosynthesis. Plant Cell 19:3530–3548. https://doi.org/10.1105/tpc.107.054981
Yang X, Wu D, Shi J, He Y, Pinot F, Grausem B, Yin C, Zhu L, Chen M, Luo Z, Liang W, Zhang D (2014) Rice CYP703A3, a cytochrome P450 hydroxylase, is essential for development of anther cuticle and pollen exine. J Integr Plant Biol 56:979–994. https://doi.org/10.1111/jipb.12212
Yang XT, Ye JL, Zhang LL, Song XY (2020) Blocked synthesis of sporopollenin and jasmonic acid leads to pollen wall defects and anther indehiscence in genic male sterile wheat line 4110S at high temperatures. Funct Integr Genomic 20:383–396. https://doi.org/10.1007/s10142-019-00722-y
Yu CY, Li W, Chang JJ, Hu SW (2007) Development of a thermo-sensitive male-sterile line 373S in Brassica napus L. Chin Agric Sci Bull 23:245–248. https://doi.org/10.3969/j.issn.1000-6850.2007.07.057
Yu CY, Guo YF, Ge J, Hu YM, Dong JG, Dong ZS (2015) Characterization of a new temperature-sensitive male sterile line SP2S in rapeseed (Brassica napus L.). Euphytica 206:473–485. https://doi.org/10.1007/s10681-015-1514-0
Yuan GL, Wang YK, Yuan SH, Wang P, Duan WJ, Bai JF, Sun H, Wang N, Zhang FT, Zhang LP, Zhao CP (2018) Functional Analysis of wheat TaPaO1 gene conferring pollen sterility under low temperature. J Plant Biol 61(1):25–32. https://doi.org/10.1007/s12374-017-0269-7
Zhao DZ, Wang GF, Speal B, Ma H (2002) The EXCESS MICROSPOROCYTES1 gene encodes a putative leucine-rich repeat receptor protein kinase that controls somatic and reproductive cell fates in the Arabidopsis anther. Genes Dev 16:2021–2031. https://doi.org/10.1101/gad.997902
Zhang W, Sun Y, Timofejeva L, Chen C, Grossniklaus U, Ma H (2006) Regulation of Arabidopsis tapetum development and function by DYSFUNCTIONAL TAPETUM1 (DYT1) encoding a putative bHLH transcription factor. Development 133:3085–3095. https://doi.org/10.1242/dev.02463
Zhang ZB, Zhu J, Gao JF, Wang C, Li H, Li H, Zhang HQ, Zhang S, Wang DM, Wang QX, Huang H, Xia HJ, Yang ZN (2007) Transcription factor AtMYB103 is required for anther development by regulating tapetum development, callose dissolution and exine formation in Arabidopsis. Plant J 52:528–538. https://doi.org/10.1111/j.1365-313x.2007.03254.x
Zhang C, Xu T, Ren MY, Zhu J, Shi QS, Zhang YF, Qi YW, Huang MJ, Song L, Xu P, Yang ZN (2020a) Slow development restores the fertility of photoperiod-sensitive male-sterile plant lines. Plant Physiol 184:923–932. https://doi.org/10.1104/pp.20.00951
Zhang M, Liu J, Ma Q, Qin Y, Wang HT, Chen PY, Ma L, Fu XK, Zhu LF, Wei HL, Yu SX (2020b) Deficiencies in the formation and regulation of anther cuticle and tryphine contribute to male sterility in cotton PGMS line. BMC Genomics 21:825. https://doi.org/10.21203/rs.3.rs-18152/v1
Zhou H, Zhou M, Yang Y et al (2014) RNase ZS1 processes UbL40 mRNAs and controls thermosensitive genic male sterility in rice. Nat Commun 5:4884. https://doi.org/10.1038/ncomms5884
Zhu J, Chen H, Li H, Gao JF, Jiang H, Wang C, Guan YF, Yang ZN (2008) Defective in Tapetal development and function 1 is essential for anther development and tapetal function for microspore maturation in Arabidopsis. Plant J 55:266–277. https://doi.org/10.1111/j.1365-313X.2008.03500.x
Zhu J, Lou Y, Xu X, Yang ZN (2011) A genetic pathway for tapetum development and function in Arabidopsis. J Integr Plant Biol 53:892–900. https://doi.org/10.1111/j.1744-7909.2011.01078.x
Zhu J, Lou Y, Shi QS, Zhang S, Zhou WT, Yang J, Zhang C, Yao XZ, Xu T, Liu JL (2020) Slowing development restores the fertility of thermos-sensitive male-sterile plant lines. Nature Plants 6:360–367. https://doi.org/10.1038/s41477-020-0622-6
Acknowledgements
The authors thank Prof. Huixian Zhao, College of Life Sciences, Northwest A&F University, for her suggestions in the course of preparing the manuscript. We are also grateful to our anonymous reviewers for their thoughtful and detailed reviews.
Funding
This research was funded by the National Natural Science Foundation of China (3217150949), the Key Research and Development Project in Shaanxi Province of China (2018ZDXM-NY-008; 2022NY-153), Modern Crop Seed Industry Project of Shaanxi Province (20171010000004), and the Key Research and Development Projects of Yangling Seed Industry Innovation Center (YLzy-yc2021-01).
Author information
Authors and Affiliations
Contributions
SH conceived and designed research. YS and DZ performed the experiments. YS and SH analyzed the data. YS and SH wrote the manuscript. HD, ZW, JW, HL and YG discussed and edited the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
The list of differentially expressed genes in pairwise comparisons between the fertile and sterile parent, as well as between fertile and sterile individuals in BC1 in two stages (bud and anther) (XLSX 1074 kb)
Additional file 2:
The 430 differentially expressed genes and their expression pattern in different stages (XLSX 123 kb)
Additional file 3:
Annotation of the 430 differentially expressed genes (XLSX 42 kb)
Additional file 4:
Gene ontology (GO) terms of each cluster in the heatmap (XLSX 261 kb)
Additional file 5:
Results of gene ontology term enrichment of the 430 differentially expressed genes (XLSX 18 kb)
Additional file 6:
The known and predicted interactions among the differentially expressed genes by String (XLSX 10 kb)
Additional file 7:
Cis-regulatory element analysis of the 430 differentially expressed genes (XLSX 1092 kb)
Additional file 8:
RNA-seq of DEGs in 373S under restrictive condition (high temperature) and permissive condition (low temperature) (XLSX 50 kb)
Additional file 9:
Fig. S1. Analysis of the relationship among all 15 samples according to RNA-seq based gene expression values. (A) The heatmap of sample correlation. The bar in right represents the scale of the relationship among samples, and the value in each pane represents the correlation coefficient between two samples. (B) Principal components analysis. All samples were clustered into two separate groups corresponding to developmental stages, buds and anthers shown by cyan and hermosa pink colors, respectively. The numbers in parentheses after PC (principal component) represent the proportion of variance explained by that PC. (C) The variance explained by the first four PCs. (PNG 298 kb)

Additional file 10:
Fig. S2. The pair-wise comparisons between fertile and sterile samples. The criteria for screening DEGs were probability≥0.8 and log2(fold change)≥1. 5A219F-A, anthers from fertile plants in BC1 (5A214// 5A214/5C95); 5A219F-B, buds from fertile plants in BC1; 5A219S-A, anthers from sterile plants in BC1; 5A219S-B, buds from sterile plants in BC1; 5C95-A, anthers from the fertile parent; 5C95-B, buds from the fertile parent; 5A214-A, anthers from the sterile parent; 5A214-B, buds from the sterile parent. (PNG 91 kb)

Additional file 11:
Fig. S3. Venn diagrams showing distribution of 430 differentially expressed genes between male fertile and sterile samples (PNG 50 kb)

Additional file 12:
Fig. S4. Classification of gene ontology annotations. The x-axis indicates categories and sub-categories, and the y-axis the number of genes. (PNG 395 kb)

Additional file 13:
Fig. S5. A network of differentially regulated gene ontology (GO) terms in biological processes. The scheme was drawn by using the BiNGO plugin of Cytoscape software. GO terms are presented as nodes and associations as edges, node size represents gene number, the bigger of the node size representing the more gene. The significant level of nodes is shown by different yellow colors as indicated in the scale bar. The lines with arrows mean the regulating relation. (PNG 491 kb)

Additional file 14:
Fig. S6. Gene ontology functional enrichment of differential expressed genes in biological process. Coloring indicates p-value (high: yellow, low: red). The lower p-value indicates the more significant enrichment. The lines with arrows mean the regulating relation. (PNG 276 kb)

Additional file 15:
Fig. S7. A network of protein-protein interactions based on analysis of the differentially expressed genes. The scheme was drawn using the STRING software. Genes are shown as nodes and interactions as edges (PNG 2745 kb)

Additional file 16:
Fig. S8. The most enriched pathways of the differentially expressed genes (DEGs) between sterile and fertile samples. (A) The most enriched metabolic activity between the sterile and fertile samples. (B) Pathway enrichment of the DEGs. The y-axis shows significantly enriched kyoto encyclopedia of genes and genomes pathways, and the x-axis shows the rich factor. Rich factor stands for the ratio of the number of DEGs belonging to a pathway to the number of all the annotated genes located in the pathway. The higher rich factor represents the higher level of enrichment. The size of the dot indicates the number of DEGs in the pathway, and the color of the dot reflects the different p-value range. (PNG 412 kb)

Additional file 17:
Fig. S9. Cluster analysis of 19 differentially expressed genes associated with pollen development. The color key represents log10 (FPKM+1). Color intensities (from green to red shading) increase with elevated expression level, as indicated at upper left. (PNG 207 kb)
Additional file 18:
Table S1. Description of the samples in the present study (PDF 39 kb)
Additional file 19:
Table S2. Target genes and their primer sequences for qRT-PCR (PDF 22 kb)
Additional file 20:
Table S3. Tetrad size and callose thickness in fertile control and 373S male sterile plants (PDF 104 kb)
Additional file 21:
Table S4. The statistics of RNA-sequencing data in individual samples (PDF 48 kb)
Additional file 22
Table S5 Verification of 430 DEGs identified between sterile samples and fertile samples by the RNA-seq experiment of 373S line under restrictive condition (high temperature) and permissive condition (low temperature) (PDF 106 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, Y., Zhang, D., Dong, H. et al. Comparative transcriptome analysis provides insight into the important pathways and key genes related to the pollen abortion in the thermo-sensitive genic male sterile line 373S in Brassica napus L.. Funct Integr Genomics 23, 26 (2023). https://doi.org/10.1007/s10142-022-00943-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10142-022-00943-8