Abstract
PHD-finger proteins, which belongs to the type of zinc finger family, and that play an important role in the regulation of both transcription and the chromatin state in eukaryotes. Currently, PHD-finger proteins have been well studied in animals, while few studies have been carried out on their function in plants. In the present study, 129 non-redundant PHD-finger genes were identified from 5 Rosaceae species (pear, apple, strawberry, mei, and peach); among them, 31 genes were identified in pear. Subsequently, we carried out a bioinformatics analysis of the PHD-finger genes. Thirty-one PbPHD genes were divided into 7 subfamilies based on the phylogenetic analysis, which are consistent with the intron-exon and conserved motif analyses. In addition, we identified five segmental duplication events, implying that the segmental duplications might be a crucial role in the expansion of the PHD-finger gene family in pear. The microsynteny analysis of five Rosaceae species showed that there were independent duplication events in addition to the genome-wide duplication of the pear genome. Subsequently, ten expressed PHD-finger genes of pear fruit were identified using qRT-PCR, and one of these genes, PbPHD10, was identified as an important candidate gene for the regulation of lignin synthesis. Our research provides useful information for the further analysis of the function of PHD-finger gene family in pear.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zinc finger proteins contain large family members and are widely distributed in eukaryotic organisms. Although rich in histidines or cysteines, the “finger” structural domains have been divided into different types, such as really interesting new genes (RING); Lin11, Isl-1, and Mec-3 (LIM); and plant homeodomain (PHD) according to their amino acids alignment (Takatsuji 1998). PHD-finger proteins are among the common zinc finger proteins with one or several PHD-finger domains, which contain approximately 60 amino acids and are characterized by the Cys4-His-cys3 zinc-binding motif (Kaadige and Ayer 2006). Among these PHD domains, the numbers of amino acids between the cysteine residues or between cysteine and histidine are usually conserved, and the second amino acid residue before the last pair of cysteines is usually aromatic amino acids, such as tryptophan (Bienz 2006). A cysteine-rich domain was first observed in the PHD-finger proteins of Arabidopsis thaliana HAT3.1 and maize Zmhox1 (Schindler et al. 1993). Subsequently, PHD-finger proteins were studied in humans, drosophila, nematodes, and yeasts (Gibbons et al. 1997; Kehle et al. 1998; Martin et al. 2006; Papoulas et al. 1998).
Many studies reported that the PHD-finger proteins regulated the chromatin by binding their PHD-finger domains to nucleosomes, such as ATP-dependent chromatin assembly factor 1 (ACF1), bromodomain PHD finger transcription factor (BPTF), and BHC80 (also known as PHF21A) in humans (Lan et al. 2007). The human BPTF showed binding activity with H3K4 me2/3 (Wysocka et al. 2006), while the BHC80 protein specifically bound to non-methylated H3K4 (H3K4 me0) (Lan et al. 2007). In yeast, Yng1, Yng2, and PHO23 of the YNG family exhibited a strong H3K4 me2/3 binding activity in the PHD domain (Shi et al. 2007). Some PHD-finger proteins could bind to protein subunits that lack histone in the PHD-finger domain. For example, the PHD-finger domain of the trans-activator protein Pygopus in flies bound to Legless/BCL9 (Kramps et al. 2002). In addition, the PHD-finger domain of the human transcriptional repressor KAP-1 bound to an isomer of the Mi-2 alpha subunit (Schultz et al. 2001). PHD-finger transcription factors are also involved in the regulation of plant growth and development. For example, MS1 (male sterility 1) is related to the development of pollen and the viscoelastic layer (Yang et al. 2007); VIN3 (Vernalization insensitive 3), VRN5 (Vernalization 5) (Greb et al. 2007), SHL (short life), and MMD1 (male meiocyte death 1) are involved in the regulation of genes during the developmental process and meiosis (Yang et al. 2003).
The PHD-finger gene family has been identified in some model plant species, such as Arabidopsis (Arabidopsis thaliana), maize (Zea mays), and poplar (Populus trichocarpa). However, the PHD-finger genes in Rosaceae have not been studied as compared to the extensive surveys that have been conducted in other plants. Recently, the whole genomes of apple (Malus × domestica) (Velasco et al. 2010), pear (Pyrus bretschneideri) (Wu et al. 2013), peach (Prunus persica) (Verde et al. 2013), mei (Prunus mume) (Zhang et al. 2012), and strawberry (Fragaria vesca) (Shulaev et al. 2011) have been fully sequenced. This resource provided an excellent opportunity for further understanding of gene structure and function of the PHD-finger gene family in five Rosaceae species. In the present study, the gene structures, chromosomal localizations, and collinearity analysis of the PHD-finger genes in pear and other Rosaceae species were analyzed. Furthermore, their expression profiles during the development of pear fruit were assessed. These results suggested that the expression trend of PbPHD10 was consistent with the changing trend of stone cell content in pear. Some previous studies have suggested that the content of the stone cell is an important factor that have influences on the quality of pear fruit (Jin et al. 2013). Current strategies focus to reduce lignin content for improving the quality of pear fruit (Cai et al. 2010; Jin et al. 2013). Our study will contribute by revealing the effect of the PHD-finger genes on lignin synthesis during the development of pear fruit and providing a reference for improving the quality of other fruits that contain lignin.
Materials and methods
Identification of PHD-finger containing genes
To identify the PHD-finger genes in five Rosaceae species, we obtained the Hidden Markov Model (HMM) profile of PHD-finger proteins from the Pfam database (http://pfam.sanger.ac.uk/). These sequences in PHD-finger HMM profiles were used as a query to search against the fully sequenced genome databases of the pear, peach, apple, strawberry, and mei using the BlastP program (p value = 0.001). Subsequently, the SMART database (http://smart.embl-heidelberg.de/) (Letunic et al. 2012) and Pfam database (http://xfam.org/) (Punta et al. 2011) were used to determine whether each candidate PHD-finger protein sequence was a member of the PHD-finger family. Subsequently, we removed all potentially redundant PHD-finger sequences according to the results of the sequence alignments. According to the starting positions of the PHD-finger genes on the pear chromosomes that were extracted from the genome annotation document ‘Pyrus_bretschneideri_Chr_gene.gff_V121010,’ which can be obtained from the website (http://gigadb.org/dataset/100083), the chromosome location images of these genes in the pear were generated using Circos (http://circos.ca/) (Krzywinski et al. 2009). At the same time, the exon-intron structures of the pear PHD-finger genes were produced using the online GSDS website (http://gsds.cbi.pku.edu.cn/) (Guo et al. 2007) according to the genome annotations. The conserved motifs that were encoded by each PHD-finger gene were also identified in pear using the online tool MEME (http://meme-suite.org/tools/meme) (Bailey et al. 2015) with the optimum motif width ranging from 15 to 200 and the maximum number of motifs set to 20.
Phylogenetic analysis
ClustalX was used with the default parameters to perform a coding sequence alignment. A neighbor-joining (N-J) tree was constructed using MEGA5 (Tamura et al. 2011), and the parameters were set as follows: the bootstrap was set to 1000, and the pairwise deletion option and the Poisson correction were set to the distance model. The tree included five types of Rosaceae species and was constructed by MEGA5 according to the data that were downloaded from the National Center for Biotechnology Information (NCBI) Taxonomy Common Tree (http://www.ncbi.nlm.nih.gov/Taxonomy/CommonTree/wwwcmt.cgi).
Collinearity analysis
In this research study, the pear genome sequences were downloaded to our local server to identify the collinearity. The microsynteny relationships between each pair of chromosomes were ascertained using MCscanX (Wang et al. 2012). Consequently, the microsynteny chains were evaluated with an E value of < e−10(Jain and Das 2016).
Ka/Ks analysis
The synonymous (Ks) and nonsynonymous (Ka) substitution rates were estimated using DnaSP 5.0 (Librado and Rozas 2009). Subsequently, the selection modes of the PbPHD paralogs were confirmed by analyzing the Ka/Ks ratios.
Functional annotation analysis
The functional annotations of the PbPHDs were performed using Blast2GO software (Conesa et al. 2005) with the default parameters. Based on the cellular localizations, molecular functions, and biological processes, 31 pear PHD-finger proteins were classified using the WEGO web site (Ye et al. 2006). The KEGG pathways of these PbPHDs were carried out using KEGG database (http://www.kegg.jp) (Kanehisa and Goto 1999).
Orthologous analysis
The position of each PbPHD was marked on the chromosomes using a Perl script. The orthologous PbPHDs among the pear (Pyrus bretschneideri), apple (Malus x domestica), strawberry (Fragaria vesca), mei (Prunus mume), and peach (Prunus persica) were identified using OrthoMCL (Li et al. 2003) with E values less than 1e−5. The orthologous pairs among the five species were drawn using Circos (Krzywinski et al. 2009).
Expression analysis based on the RNA-sequencing data
The RNA-seq raw data of five developmental periods of pear fruits (25 DAF, 55 DAF, 85 DAF, 115 DAF, and 145 DAF) were retrieved from the National Center for Biotechnology Information (NCBI: Accession number SRP008545) (Xie et al. 2013). An analysis of these data was performed according to a previous method (Muthamilarasan et al. 2014). The NGS toolkit was used to filter the RNA-seq data for delete low quality reads (Patel and Jain 2012). The RNA-seq data mapped onto the reference genome of pear using the Bowtie2 software. The expression levels of the PbPHD genes in the exon model were estimated as reads per kilobase per million mapped reads (RPKM). In the present study, we considered a PbPHD gene was expressed if the RPKM value was > or = 1 in an expression atlas based on the previously published papers (Lin et al. 2012). The hierarchical clustering was performed with R software (http://www.bioconductor.org/) to analyze the expression profile of the PHD genes in pear.
Quantitative real-time RT-PCR
To investigate the expression patterns of the PbPHD genes, pear root, stem, leaf, and fruit samples (15, 39, 47, 55, 63, 79, 102, and 145 days after flowering (DAF)) were collected in 2013. Three biological replicates of the samples were harvested from 40-year-old pear trees grown in a horticultural field in Dangshan, Anhui, China during each developmental stage. All fruit samples were taken to the laboratory, immediately frozen in liquid nitrogen, and stored at − 80 °C for RNA isolation. The total RNA of all the samples was extracted using the RNAprep pure Plant Kit (Tiangen, Beijing) according to the manufacturer’s instructions with DNase I (Tiangen, Beijing) to remove any genomic DNA contamination. Then, the RNA (1 μg) was used for the reverse transcription using the SuperScript III reverse transcription kit (Invitrogen). Gene-specific primers (Table S1) for the qRT-PCR were designed using the Beacon Designer 7 software, and the tubulin gene (Wu et al. 2012) was used as an internal control. These primers were synthesized by General Biosystems, Inc. (Chuzhou, China). The qRT-PCR was conducted on ABI7500 (Applied Biosystems) using the FastFire qPCR PreMix (Tiangen, Beijing) based on the manufacturer’s instructions. The 2-ΔΔCT method (Livak and Schmittgen 2001) was used to assess the relative expression levels of these PbPHD genes.
Results
Sequence collection and identification
To identify the PHD-finger family genes, the Hidden Markov Model (HMM) and BLASTP searches were performed against the reference genomes of pear (Wu et al. 2013), apple (Kalyanaraman et al. 2010), peach (Verde et al. 2013), mei (Zhang et al. 2012), and strawberry (Shulaev et al. 2011) using DNATools software. Totally, 156 candidate PHD-finger genes were identified from five Rosaceae species. Subsequently, all candidate PHD-finger proteins were surveyed to further determine whether they contained the PHD-finger domain using the Pfam database. Due to the absence of the PHD-finger binding domain, 27 candidate genes were discarded. Finally, 129 non-redundant PHD genes were identified, including 31 apple PHDs, 19 mei PHDs, 25 peach PHDs, 23 strawberry PHDs, and 31 pear PHDs (Fig. 1, Tables S2 and S3). Remarkably, 7 genes encode proteins that contain 3 PHD-finger domains, and 12 genes encode proteins that contain 2 PHD-finger domains in pear (Table S2). A phylogenic tree of these PHD-finger genes from the five Rosaceae species was constructed to deduce the genome-wide duplication between the progenitors of pear and apple (Fig. 1). Additionally, due to several genes of this gene family occurred alternative splicing, the longest splicing isoforms were selected for following analysis except specific description.
Comparison of the PHD-finger genes from five Rosaceae species (Pyrus bretschneideri, Malus x domestica, Fragaria vesca, Prunus mume, and Prunus persica) in phylogenetic relationships. In the present study, the genomes of five Rosaceae species were investigated. GIGADB: http://gigadb.org/site/index; GDR: http://www.rosaceae.org/; BFU: http://prunusmumegenome.bjfu.edu.cn/index.jsp; JGI: (http://www.jgi.doe.gov/)
Phylogenetic and structures analysis of the PbPHD genes
To investigate the evolutionary relationships of the PHD-finger family in plant species, the PHD-finger genes from the monocots Oryza sativa and Zea mays or the dicots Arabidopsis thaliana, Vitis vinifera, Populus trichocarpa, Malus × domestica, Pyrus bretschneideri, Prunus persica, Prunus mume, and Fragaria vesca were used to construct an N-J phylogenetic tree with the MEGA 5.0 software (Tamura et al. 2011). Bootstrapping tests were performed on this tree. Based on the bootstrap values and the topology of the tree, all the PHD-finger proteins were divided into the following five main clades: I, II, III, IV, and V (Figs. 2 and S1). Furthermore, clade I was categorized into the following four subfamilies: I (a)–I (d); clade II classified into five subfamilies: II (a)–II (e); and clade IV divided into the following five subfamilies: IV (a)–IV (e), according to the phylogenetic tree (Figs. 2 and S1). The I (c) subfamily was not present in the monocots Oryza sativa and Zea mays; however, the I (c) subfamily was identified in the dicots species, including pear species. In pear species, the II (a), II (c), II (d), II (e), IV (c), and IV (d) subfamilies were absent (Figs. 2 and S1).
Phylogenetic tree of the PHD-finger proteins in the monocots Oryza sativa and Zea mays and the dicots Arabidopsis thaliana, Populus trichocarpa, Vitis vinifera, Malus × domestica, Pyrus bretschneideri, Prunus persica, Prunus mume, and Fragaria vesca. For the ten-species PHD-finger gene tree, the PHD-finger proteins of Oryza sativa and Vitis vinifera were obtained from the PLAZA 3.0 database (Proost et al. 2015). The neighbor-joining tree was constructed using MEGA5 software
Previous studies have shown that the gene structure diversity provides the primary resource for the evolution of multi-gene families (Cao et al. 2016a, b). To obtain further insights into the structural diversity of the PHD-finger genes in pear, an exon-intron analysis was performed. In the present study, the exon-intron structures (Fig. 3) showed that the intron numbers in PbPHDs varied from 1 to 23, and PbPHD23 contained the most introns (23). Moreover, most members clustered in the same group shared highly similar exon-intron structures, including the numbers and lengths of the introns. For instance, PbPHD14 and PbPHD24, which are located in subfamily III, all contained 22 introns, while all genes in subfamily I (a) included seven introns. In contrast, the PbPHDs in subfamily I (c) showed a high diversity in their intron lengths and numbers (Fig. 3). To sum up, the exon-intron structures of the PHD genes in pear substantially conformed to their phylogenetic relationships.
Phylogenetic relationships and gene structures of pear PHD-finger genes. The numbers next to the branches represent the bootstrap values based on 1000 replications. Blue boxes indicate UTRs, green boxes represent exons, and the introns are indicated by gray lines. The scale on the bottom is used to estimate the sizes of the exons and introns
As a common mechanism, alternative splicing could produce different subtypes of proteins, which could likely affect the diversities of proteomic and transcriptomics, and finally affecting the regulation of protein function and gene expression (Black 2003; Pan et al. 2008). In the present study, we also revealed the occurrence of alternative splicing events (such as PbPHD22 and PbPHD23) in the PHD family during the evolutionary process. The mRNAs of PbPHD22 and PbPHD23, which are produced by variable splicing, are different in the 3′-end (Fig. S2). Their first 20 exons and introns are all identical; however, an intron is inserted into the twenty-first exon, resulting in PbPHD23 being longer than PbPHD22 by 12 nucleotides.
The domain sequence and conserved motifs analysis of the PbPHD proteins
In general, the protein domain is associated with protein function; therefore, the domain sequence characteristics of 31 PbPHDs were analyzed. The sequence alignment suggested that the length of the PHD-finger domains ranged from 40 to 60 amino acids and contained 7 cysteine and 1 histidine residues for the binding of Zn ions and maintaining the stability of the domain. Additionally, the conserved residues on both sides are thought to be related to the different histone methylation patterns. In contrast, there are great differences between the two loop areas, including their lengths and the constitutions of amino acids. Moreover, the PbPHD proteins that contained common sequences were C-X (1–2)—C-X (8–14)—C-X (2–3)—C-X 4—H-x 2—C x (12–18) C-X 2—C (Figs. S3 and 4), which formed the C4HC3 type zinc finger structure. This common sequence was basically consistent with the conserved residues reported in a previous study (Capili et al. 2001). Remarkably, it was also found that there was a great difference in the nucleotide composition among the PHD domain genes, except for a few conserved T or G nucleotides (Fig. S4).
The sequence logos of the PHD-finger domains. These logos were based on a multiple alignment analysis of 31 PbPHDs (Fig. S2). The bit score indicates the information content of each position in the sequence. The asterisks indicate that the typically conserved PHD finger domains in pear were C-X (1–2) -C-X (8–14) -C-X (2–3) -C-X 4 -H-× 2 -C x (12–18) C-X 2 –C
Regarding the fact that the PHD-finger proteins function via their conserved domains, a study on the PHD-finger protein domains in pear was carried out. In total, 31 pear PHD-finger proteins were analyzed by MEME. As a result, 20 motifs were identified. The detailed information regarding each motif is shown in Table S4. The results showed that members with highly conserved motifs were included in the same subgroup, and most members of the same subgroup had at least one homologous protein with completely coincident motifs (Fig. 5), such as PbPHD13/PbPHD31 and PbPHD22/PbPHD23. Because of their sequence similarity, these paralogous proteins possibly exhibited a functional redundancy, resulting in a difficulty in analyzing their functions using single gene mutants. In the case of most PHD-finger proteins within diverse subgroups, the proteins were different not only in their motifs but also in their locations (Fig. 5). The known domains, except for the PHD-finger domain, were identified and annotated using SMART and Pfam (Fig. 5 and Table S4). Among these domains, motif 5, which encodes the TPL domain (De Geyter et al. 2012; Pauwels et al. 2010), was identified in all members of groups I (a), I (b), and I (c), suggesting that these subgroup members might be involved in regulating the jasmine acid signaling pathway. Motif 9 encoded the DDT domain (Doerks et al. 2001), which was predicted to be a DNA binding domain in different transcription and chromosome remodeling factors and was only distributed in group I (c). Both PbPHD10 and PbPHD11 in group I (d) contained motifs 18 and 19, which encode an SNF domain (Eisen et al. 1995; Linder et al. 2004), suggesting that they might be related to the transcriptional regulation of genes (Eisen et al. 1995; Linder et al. 2004). Additionally, a number of other interesting structural domains were also identified, such as SANT and PWWP. The identification of these motifs and domains will be helpful for further studies on the biological functions of the PHD-finger family in pear.
Distribution of the motifs in the PbPHD family members. The domains in the A, B, C, D, and E subgroups were determined by both SMART and Pfam searches. Different motifs are represented by different color boxes. The length of each box did not relate to the actual size of each motif. The detailed information regarding each motif is annotated on the right
Functional annotations analysis of the PbPHD proteins
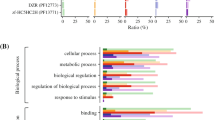
In our study, to better understand the function of the different PbPHDs, a GO annotation analysis was performed to predict the functions of the putative PbPHD proteins. In total, 24 of the 31 PbPHD proteins were classified into 19 functional categories within 3 main ontologies, including molecular function, biological process, and cellular component (Fig. 6, Table S5). The GO annotation of the PbPHD proteins showed that the binding, metabolic process, cell, catalytic, and cell part annotations were predominant among the functional categories. Several proteins were assigned to the macromolecular complex, organelle part, anatomical structure formation, and cellular component biogenesis. Furthermore, the molecular function annotations suggested that all PbPHDs functioned in the binding and catalytic categories, while the cellular component annotations suggested that these proteins were predominantly localized in the nucleus.
Gene ontology classification of the PHD-finger proteins of pear. The Y-axis (left) shows the percentages of genes, and the Y-axis (right) indicates the actual gene number. In total, 31 pear PHD-finger genes were annotated in three main categories (cellular component, molecular function, and biological process (X-axis)) using the Blast2GO software
To further evaluate the active pathways of PbPHDs, these genes were mapped to the canonical pathway in KEGG database. Subsequently, we found that the most represented pathways by these PbPHDs were cellular processes, cell cycle, environmental information processing, andspliceosome (Table S5). The pathway annotations of PbPHDs not only support the GO enrichment analysis but also provide a valuable resource for gaining insights into the functions, specific processes, and pathways in pear growth and development.
Chromosomal locations and duplications of the PbPHD genes
Pear is the third important fruit tree after grape and apple, which has very important economic value (Wu et al. 2013). At least one whole genome duplication (WGD) event was discovered in the pear genome during the evolutionary process (Cao et al. 2016c; Wu et al. 2013). In order to further understand the effect of gene differentiation and gene duplication on the PbPHD gene family, we investigated the localization of PbPHD in the pear genome and the types of its gene duplication events. According to the initial positions of the genes in the chromosomes, 21 of the 31 PbPHD genes were unevenly distributed on 11 chromosomes in pear, while PbPHD22, -23, -24, -25, -26, -27, -28, -29, -30, and -31 were mapped to currently unattributed scaffolds. The number of PbPHD genes that were mapped on each chromosome varied from 1 to 5 (Fig. 7). Chromosome 11 contained five PbPHD genes, while chromosomes 4, 5, 6, 8, 9, 12, and 15 included only one PbPHD gene (Fig. 7). These uneven distribution patterns were also observed in the genomes of maize, poplar, and Arabidopsis thaliana (Fan et al. 2009; Wang et al. 2015; Wu et al. 2016b).
In plants, segmental duplication or tandem duplication are the main ways to expand the number of family members. To understand the expanded mechanism of the PHD-finger gene family in pear during the evolution process, we investigated their duplication event potential. Based on the collinearity analysis of the PbPHD genes, eight gene pairs (PbPHD4/17, PbPHD5/8, PbPHD7/19, PbPHD9/19, PbPHD10/11, PbPHD12/26, PbPHD14/24, and PbPHD17/31) were identified as involved in the segmental duplication events (Fig. 7), among them PbPHD4/17 and PbPHD7/19 were involved in WGD events (Table 1). However, we did not find any tandem duplication events. PbPHD12/26, PbPHD14/24, and PbPHD17/31 were not shown in the figure because PbPHD26, PbPHD24, and PbPHD31 are mapped on the unattributed scaffolds.
To evaluate the driving force underlying the PHD-finger gene evolution, the synonymous (Ks) substitution rate ratio versus the nonsynonymous (Ka) were calculated for the duplicated PbPHD genes. Generally, Ka/Ks > 1 indicated a positive selection with an accelerated evolution; Ka/Ks < 1 indicated functional constraints with a negative selection; and Ka/Ks = 1 signified a neutral selection. The results showed that the Ka/Ks ratios of eight duplicated PbPHD gene pairs ranged from 0.090 to 0.696 with an average of 0.343 (Table 1). Additionally, the majority of the ratios were less than 0.4, suggesting that the duplicated PbPHD genes were under a strong negative selection during evolution.
Microsynteny analysis among pear, apple, peach, mei, and strawberry
To further insight into the evolutionary history of the PbPHD genes, we carried out the microsynteny analysis among five Rosaceae species. Finally, orthologous of all 90 pear genome PHDs were found in the genomes of the other Rosaceae species (i.e., apple, peach, mei, and strawberry) (Table S6). Among these orthologous, 25 orthologous gene pairs were identified in pear, apple, and peach, while 20 gene pairs were found in pear, mei, and strawberry (Fig. S4 and Table S6). In addition, to better observe the microsynteny of the PHDs in the different species, PbPHD8 was used as examples to display the microsynteny relationship in different Rosaceae species (Fig. 8).
Microsynteny relationships of the PHD-finger genes in pear. The relative positions of all flanking protein-coding genes were defined by the anchored PHD-finger genes and are highlighted in red. The broad line with the arrowhead represents the gene transcriptional orientation. The conserved gene pairs among the segments were connected by a gray line
Remarkably, we observed that certain collinear gene pairs in pear and apple were missing between pear and peach, mei, and strawberry, such as PbPHD6/MDP0000120221, PbPHD18/MDP0000232962, PbPHD22/MDP0000125254, PbPHD23/MDP0000125254, PbPHD25/MDP0000246432, PbPHD28/MDP0000272113, and PbPHD30/MDP0000311077. Similarly, certain uniquely duplicated genes also appeared between pear, peach, mei, and strawberry (Fig. S5 and Table S6). Moreover, the result indicated that two or more PHD-finger genes in apple, peach, mei, and strawberry matched one pear PHD-finger gene, i.e., mrna00537 and mrna19503, Pm009703 and Pm015135, and ppa011288m and ppa011295m are orthologous to PbPHD8, and MDP0000193753 and MDP0000120221 are orthologous to PbPHD6 (Table S6).
Expression analysis of the PbPHD genes
To determine the role of the PHD-finger gene family during the development of the pear fruit, the expression of the PbPHD genes were analyzed using Illumina RNA-Seq data. Figure S5 shows that the transcripts of the most PbPHD genes (67.74%) were not detected in each developmental stage of pear fruit, proposing that these genes might be express in other organs, such as the leaf, root, and flower or these genes are pseudogenes. Ten PbPHDs (32.26%) were expressed in one or more developmental stages. PbPHD10 and PbPHD11 were highly expressed in all stages (Fig. S6). Six of the ten PHD-finger genes that expressed during pear fruit development belonged to subgroup E (Fig. S5), indicating that this subfamily might play an important role in the developmental period of pear fruit.
In order to study the dynamic of PbPHD genes, the expression levels of ten PbPHDs in the root, stem, and leaf, and during the pear fruit development were investigated by qRT-PCR. The results showed that PbPHD12, 15, and 29 were expressed predominantly in the leaf, while the expression of PbPHD11 was not detected in the leaf (Fig. 9). The expression of PbPHD8 and PbPHD25 was higher at the 102 DAF (days after flowering) stage as compared to other like root, stem, and leaf in other developmental stages (15 DAF, 39 DAF, 47 DAF, 55 DAF, 63 DAF, 79 DAF, and 145 DAF) of pear fruit. Figure 9 demonstrated the expression of PbPHD5, 10, 11, and 25 was higher during the different developmental stages of pear fruit than that in the root, stem, or leaf. Remarkably, the expression of PbPHD10 was basically consistent with the lignin content of the pear fruit during the different developmental stages, which increased rapidly during the early stages of pear fruit development, highest peak in the middle stage of the development (55 DAF), and decrease gradually during the mature period (Cai et al. 2010; Jin et al. 2013).
Expression analysis of PbPHD genes in different plant tissues. The sample identities are as follows: 1, root; 2, stem; 3, leaf; 4, 15 days after flowering (DAF); 5, 39 DAF; 6, 47 DAF; 7, 55 DAF; 8, 63 DAF; 9, 79 DAF; 10, 102 DAF; and 11, 145 DAF. Error bars show the standard error between three replicates
Discussion
Due to the completion of pear genome sequencing, the analysis of a gene family in terms of its biological information has laid a steady foundation for exploring the origin, evolution, and gene function of pear. Currently, numerous transcription factor families have been identified in the pear genome, such as the WRKY, MYB, MADS families, etc. However, comprehensive analysis of the PHD-finger transcription factor gene family has not been reported in pear. The PHD-finger transcription factor is a zinc finger protein with a characteristic Cys4-His-Cys3 structure, which widely exists in transcriptional regulatory proteins in eukaryotes. The PHD-finger proteins, a class of transcription factors, have been thought to play an important role in plant development process.
In our study, 129 PHD-finger genes were screened from 5 Rosaceae species, among them various numbers of PHD-finger genes were identified in pear (31), apple (31), mei (20), peach (25), and strawberry (23). The number of PHD-finger genes in pear and apple were almost two-fold duplications as compare to mei, peach, and strawberry. This gene family has undergone an expansion at different extensions, which corresponds to the variation in chromosome number (Shulaev et al. 2011; Velasco et al. 2010; Verde et al. 2013; Wu et al. 2013; Zhang et al. 2012). For example, the chromosome number in apple and pear is 34, but there are only 16 chromosomes in peach and mei and 14 chromosomes in the strawberry. A recent genome-wide duplication event was shared by the apple and pear 30–45 MYA (million years ago) (Fig. 1) (Wu et al. 2013), the PHD-finger gene family expansion in the apple and pear might occur at that time. Remarkably, we also observed that there was no positive association between genome sizes and the number of PHD-finger gene family members in these Rosaceae species. For example, although there was no significant difference in the genome size of pear (271.9 Mb) and strawberry (240 Mb), while the number of PHD-finger genes obviously changed. In contrast, the number of PHD-finger genes in peach (224.6 Mb) and strawberry (240 Mb) have a positive correlation with their genome size.
To better understand the evolutionary relationships, a comparative phylogenetic analysis was performed. Then this tree was divided into 5 clades with 16 subfamilies. We found that most of the subfamilies were dominated by monocots and dicots in addition to subfamily I (c). These results suggested that the evolution of the PHD-finger gene families in plants (monocots and dicots) was relatively conservative. However, the PHD-finger genes from dicots, such as the poplar and pear, were classified in subfamily I (c), indicating that the PHD-finger genes in these subfamilies might have species-specific roles. These genes were lost in the monocots maize and rice or obtained in dicot lineages after the divergence from their last common ancestor. In addition, the phylogenetic tree was also showed that pear have strong association/relationship with apple as compared to other Rosaceae species. This result was supported by the interspecific microsynteny analysis. For example, 25 orthologous genes were found between pear and apple, but we found only 20 orthologous genes between pear and mei and 20 orthologous genes between pear and strawberry.
Gene duplication is the main way to produce new genes, which provide the raw material for the evolution of species (Faris et al. 2008; Moore and Purugganan 2003). Gene duplications, including tandem duplication, segmental duplication, and transposition events, were the primary driving force of the gene family expansion during the evolution process, such as the GRF, MYB, and WRKY family in pear (Cao et al. 2016c, d; Huang et al. 2015), the CHS family in maize (Han et al. 2016), the HD-Zip family in soybean (Belamkar et al. 2014), the 14-3-3 gene family in cotton (Sun et al. 2011), and γ-gliadin gene family in wheat (Sun et al. 2011). In the present study, the PHD-finger genes were assigned to segmental/WGD duplication blocks, and no PHD-finger genes were assigned to tandem duplication blocks, which indicated that segmental/WGD duplication was the main expansion mechanism of this PHD-finger gene family. Certain PHD-finger subfamilies have increased rapidly during the evolution process. In addition, the Ka/Ks ratios of eight PbPHD duplications were < 1, indicating that the PbPHD gene family has gone through purifying selection and highly conserved evolution. Remarkably, previous studies have indicated that the genome of the pear has undergone two genome-wide duplication events. The first genome-wide duplication event occurred at ~ 140 MYA (Ks ~ 1.5–1.8) (Fawcett et al. 2009), while the latest duplication event occurred in the period of 30–45 MYA (Ks ~ 0.15–0.3) (Wu et al. 2013). As shown in Table 1, these PHD-finger gene duplications may have been derived from the same recent genome-wide duplication events and the same ancient genome-wide duplication events, indicating that the PHD-finger family is an ancient gene family that expanded with the recent genome-wide duplication events.
Previous studies showed that the consensus sequences of the PHD-finger domain were C-X(1–2)-C-X(8–19)-C-X(2–4)-C-X(4–6)-H-X2-C-X(11–26)-C-X(2–3)-C in Arabidopsis (Fan et al. 2009), C-X (1–2)-C-X(8–28)-C-X(2–4)-C-X(4–6)-H-X 2-C-X(12–34)-C-X 2-C in poplar (Wu et al. 2016a), C–X(1–2)-C–X (8–19)-C–X(2–4)-C–X(4–6)-H-X2-C–X(11–26)-C–X(2–3)-C in maize (Wang et al. 2015), and C-X (1–2) -C-X (8–14) -C-X (2–8) -C-X (4–6) -H-X 2 -C-X (11–34) -C-X 2 –C in carrot (Wu et al. 2016b). In the present study, we found that the consensus sequence of the PHD-finger domain was C-X (1–2) -C-X (8–14) -C-X (2–3) -C-X 4- H-X 2 -C- X (12–18) -C-X 2 –C in pear. The difference among the five plants proposed that there have been significant changes in the PHD-finger proteins during the evolution process. The PbPHDs were divided into seven subfamilies, and the majority of these genes were similar in their exon-intron structures and included conserved motifs. The characteristics of each PHD sequence may be highly diverse due to the different numbers of PHD-finger motifs and other motifs, which is consist of many domains, such as the SNF domain (Eisen et al. 1995; Linder et al. 2004), DDT domain (Doerks et al. 2001), and PWWP domain (Qiu et al. 2002). In general, most PbPHDs in the same subgroup contain similar motifs, which was consistent with the phylogenetic analysis. Interestingly, several motifs only existed in specific-subgroups, which indicated that these motifs might be involved in the functional differentiation of PHD-fingers in pear.
The gene expression patterns could provide important clues for exploring gene function (Budak and Zhang 2017; Neilson et al. 2017; Thomas et al. 2018). Previous studies have confirmed that the expression of the PHD-finger genes was affected by salt, PEG, and ABA in maize or drought stress in poplar (Wang et al. 2015; Wu et al. 2016a). However, still nobody reported the PHD-finger genes roles during the fruit development. The stone cell is an important factor that affects the quality of pear, and lignin is the main component of stone cells (Cai et al. 2010; Jin et al. 2013). In the present study, ten genes were identified to be expressed in five developmental stages of pear fruit based on Illumina RNA-Seq data, suggesting their important roles in the development of pear fruit. Furthermore, the qRT-PCR showed that the expression level of PbPHD10 was consistent with the changing trend of lignin contents during different stages of pear fruit development (Cai et al. 2010; Jin et al. 2013). Interestingly, the expression of PbPHD10 was obviously increased at 55 DAF, and it showed an expression pattern that was similar to that of key genes that are involved in the regulation of the lignin synthesis pathway (Xie et al. 2013). These results strongly implied that PbPHD10 might regulate lignin synthesis in pear fruit. Remarkably, we found that a segmental duplication gene pair (PbPHD14 and PbPHD24) exhibited similar expression levels not only in the root, stem, and leaf but also during pear fruit development, indicating that these genes did not diverge much along with the evolution of each gene after the duplication.
Conclusions
In the present study, 129 non-redundant PHD-finger members were identified in 5 Rosaceae species. Among these members, 31 PHD-finger proteins were from the pear. Using integrated methods, including a phylogenetic, gene structure, sequence feature, duplication event, and orthologous analyses, it was found that although with a certain degree of divergence during the evolution process, certain well-conserved motifs were sustained in members of the PHD-finger family. The expression analysis suggested that the PbPHD genes exhibited different expression patterns during pear fruit development. In addition, PbPHD10 was found to be possibly involved in regulating lignin synthesis in pear fruit. PbPHD10 may be selected as a target for a functional investigation to improve the quality of pear fruit in the future.
References
Bailey TL, Johnson J, Grant CE, Noble WS (2015) The MEME suite. Nucleic Acids Res 43:W39–W46
Belamkar V, Weeks NT, Bharti AK, Farmer AD, Graham MA, Cannon SB (2014) Comprehensive characterization and RNA-Seq profiling of the HD-Zip transcription factor family in soybean (Glycine max) during dehydration and salt stress. BMC Genomics 15:1
Bienz M (2006) The PHD finger, a nuclear protein-interaction domain. Trends Biochem Sci 31:35–40
Black DL (2003) Mechanisms of alternative pre-messenger RNA splicing. Annu Rev Biochem 72:291–336
Budak H, Zhang B (2017) MicroRNAs in model and complex organisms. Funct Integr Genomics 17:1–4
Cai Y, Li G, Nie J, Lin Y, Nie F, Zhang J, Xu Y (2010) Study of the structure and biosynthetic pathway of lignin in stone cells of pear. Sci Hortic 125:374–379
Cao Y, Han Y, Li D, Lin Y, Cai Y (2016a) Systematic analysis of the 4-Coumarate:coenzyme a ligase (4CL) related genes and expression profiling during fruit development in the Chinese pear. Genes 7:89
Cao Y, Han Y, Meng D, Li D, Jin Q, Lin Y, Cai Y (2016b) Structural, evolutionary, and functional analysis of the class III peroxidase gene family in Chinese pear (Pyrus bretschneideri). Front Plant Sci 7:1874
Cao YP, Han Y, Jin Q, Lin Y, Cai Y (2016c) Comparative genomic analysis of the GRF genes in Chinese pear (Pyrus bretschneideri Rehd), poplar (populous), grape (Vitis vinifera), Arabidopsis and rice (Oryza sativa). Front Plant Sci 7:1750. https://doi.org/10.3389/fpls.2016.01750
Cao YP, Han Y, Li D, Lin Y, Cai Y (2016d) MYB transcription factors in Chinese pear (Pyrus bretschneideri Rehd.): genome-wide identification, classification and expression profiling during fruit development. Front Plant Sci 7:577. https://doi.org/10.3389/fpls.2016.00577
Capili AD, Schultz DC, Rauscher FJ, Borden KL (2001) Solution structure of the PHD domain from the KAP-1 corepressor: structural determinants for PHD, RING and LIM zinc-binding domains. EMBO J 20:165–177
Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M (2005) Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21:3674–3676
De Geyter N, Gholami A, Goormachtig S, Goossens A (2012) Transcriptional machineries in jasmonate-elicited plant secondary metabolism. Trends Plant Sci 17:349–359
Doerks T, Copley R, Bork P (2001) DDT -- a novel domain in different transcription and chromosome remodeling factors. Trends Biochem Sci 26:145–146
Eisen JA, Sweder KS, Hanawalt PC (1995) Evolution of the SNF2 family of proteins: subfamilies with distinct sequences and functions. Nucleic Acids Res 23:2715–2723
Fan Z, ZhongNan Y, Sen Z (2009) Genome-wide analysis of PHD-finger protein family in Arabidopsis thaliana. Acta Bot Yunnanica 31:227–238
Faris JD, Zhang ZC, Fellers JP, Gill BS (2008) Micro-colinearity between rice, Brachypodium, and Triticum monococcum at the wheat domestication locus Q. Funct Integr Genomics 8:149–164
Fawcett JA, Maere S, Van de Peer Y (2009) Plants with double genomes might have had a better chance to survive the cretaceous–tertiary extinction event. Proc Natl Acad Sci 106:5737–5742
Gibbons RJ, Bachoo S, Picketts DJ, Aftimos S, Asenbauer B, Bergoffen JA, Berry SA, Dahl N, Fryer A, Keppler K, Kurosawa K, Levin ML, Masuno M, Neri G, Pierpont ME, Slaney SF, Higgs DR (1997) Mutations in transcriptional regulator ATRX establish the functional significance of a PHD-like domain. Nat Genet 17:146–148
Greb T, Mylne JS, Crevillen P, Geraldo N, An H, Gendall AR, Dean C (2007) The PHD finger protein VRN5 functions in the epigenetic silencing of Arabidopsis FLC. Curr Biol 17:73–78
Guo A, Zhu Q, Chen X, Luo J (2007) GSDS: a gene structure display server. Yi Chuan 29:1023–1026
Han Y, Ding T, Su B, Jiang H (2016) Genome-wide identification, characterization and expression analysis of the Chalcone synthase family in maize. Int J Mol Sci 17:161
Huang X, Li K, Xu X, Yao Z, Jin C, Zhang S (2015) Genome-wide analysis of WRKY transcription factors in white pear (Pyrus bretschneideri) reveals evolution and patterns under drought stress. BMC Genomics 16:1
Jain A, Das S (2016) Synteny and comparative analysis of miRNA retention, conservation, and structure across Brassicaceae reveals lineage- and sub-genome-specific changes. Funct Integr Genomics 16:253–268
Jin Q, Yan C, Qiu J, Zhang N, Lin Y, Cai Y (2013) Structural characterization and deposition of stone cell lignin in Dangshan Su pear. Sci Hortic 155:123–130
Kaadige MR, Ayer DE (2006) The polybasic region that follows the plant homeodomain zinc finger 1 of Pf1 is necessary and sufficient for specific phosphoinositide binding. J Biol Chem 281:28831–28836
Kalyanaraman A et al (2010) The genome of the domesticated apple (Malus 9 domestica Borkh.). Nat Genet 42:833–839
Kanehisa M, Goto S (1999) KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28:27–30
Kehle J, Beuchle D, Treuheit S, Christen B, Kennison JA, Bienz M, Müller J (1998) dMi-2, a hunchback-interacting protein that functions in polycomb repression. Science 282:1897–1900
Kramps T, Peter O, Brunner E, Nellen D, Froesch B, Chatterjee S, Murone M, Züllig S, Basler K (2002) Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear β-catenin-TCF complex. Cell 109:47–60
Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA (2009) Circos: an information aesthetic for comparative genomics. Genome Res 19:1639–1645
Lan F, Collins RE, de Cegli R, Alpatov R, Horton JR, Shi X, Gozani O, Cheng X, Shi Y (2007) Recognition of unmethylated histone H3 lysine 4 links BHC80 to LSD1-mediated gene repression. Nature 448:718–722
Letunic I, Doerks T, Bork P (2012) SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Res 40:D302–D305
Li L, Stoeckert CJ, Roos DS (2003) OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res 13:2178–2189
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452
Lin F, Xing K, Zhang J, He X (2012) Expression reduction in mammalian X chromosome evolution refutes Ohno’s hypothesis of dosage compensation. Proc Natl Acad Sci U S A 109:11752–11757
Linder B, Cabot RA, Schwickert T, Rupp RAW (2004) The SNF2 domain protein family in higher vertebrates displays dynamic expression patterns in Xenopus laevis embryos. Gene 326:59–66
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method methods 25:402–408
Martin DG et al (2006) The Yng1p plant homeodomain finger is a methyl-histone binding module that recognizes lysine 4-methylated histone H3. Mol Cell Biol 26:7871–7879
Moore RC, Purugganan MD (2003) The early stages of duplicate gene evolution. Proc Natl Acad Sci 100:15682–15687
Muthamilarasan M, Khandelwal R, Yadav CB, Bonthala VS, Khan Y, Prasad M (2014) Identification and molecular characterization of MYB transcription factor superfamily in C 4 model plant foxtail millet (Setaria italica L.)
Neilson J et al (2017) Gene expression profiles predictive of cold-induced sweetening in potato. Funct Integr Genomics 17:1–18
Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ (2008) Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet 40:1413–1415
Papoulas O, Beek SJ, Moseley SL, McCallum CM, Sarte M, Shearn A, Tamkun JW (1998) The Drosophila trithorax group proteins BRM, ASH1 and ASH2 are subunits of distinct protein complexes. Development 125:3955–3966
Patel RK, Jain M (2012) NGS QC toolkit: a toolkit for quality control of next generation sequencing data. PLoS One 7:544–548
Pauwels L, Barbero GF, Geerinck J, Tilleman S, Grunewald W, Pérez AC, Chico JM, Bossche RV, Sewell J, Gil E, García-Casado G, Witters E, Inzé D, Long JA, de Jaeger G, Solano R, Goossens A (2010) NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature 464:788–791
Proost S, Van BM, Vaneechoutte D, Van dPY, Inzé D, Mueller-Roeber B, Vandepoele K (2015) PLAZA 30: an access point for plant comparative genomics. Nucleic Acids Res 43:D974
Punta M et al. (2011) The Pfam protein families database. Nucleic Acids Res gkr1065
Qiu C, Sawada K, Zhang X, Cheng X (2002) The PWWP domain of mammalian DNA methyltransferase Dnmt3b defines a new family of DNA-binding folds. Nat Struct Biol 9:217–224
Schindler U, Beckmann H, Cashmore AR (1993) HAT3. 1, a novel Arabidopsis homeodomain protein containing a conserved cysteine-rich region. Plant J 4:137–150
Schultz DC, Friedman JR, Rauscher FJ (2001) Targeting histone deacetylase complexes via KRAB-zinc finger proteins: the PHD and bromodomains of KAP-1 form a cooperative unit that recruits a novel isoform of the mi-2α subunit of NuRD. Genes Dev 15:428–443
Shi X, Kachirskaia I, Walter KL, Kuo JHA, Lake A, Davrazou F, Chan SM, Martin DGE, Fingerman IM, Briggs SD, Howe LA, Utz PJ, Kutateladze TG, Lugovskoy AA, Bedford MT, Gozani O (2007) Proteome-wide analysis in Saccharomyces cerevisiae identifies several PHD fingers as novel direct and selective binding modules of histone H3 methylated at either lysine 4 or lysine 36. J Biol Chem 282:2450–2455
Shulaev V, Sargent DJ, Crowhurst RN, Mockler TC, Folkerts O, Delcher AL, Jaiswal P, Mockaitis K, Liston A, Mane SP, Burns P, Davis TM, Slovin JP, Bassil N, Hellens RP, Evans C, Harkins T, Kodira C, Desany B, Crasta OR, Jensen RV, Allan AC, Michael TP, Setubal JC, Celton JM, Rees DJG, Williams KP, Holt SH, Rojas JJR, Chatterjee M, Liu B, Silva H, Meisel L, Adato A, Filichkin SA, Troggio M, Viola R, Ashman TL, Wang H, Dharmawardhana P, Elser J, Raja R, Priest HD, Bryant DW, Fox SE, Givan SA, Wilhelm LJ, Naithani S, Christoffels A, Salama DY, Carter J, Girona EL, Zdepski A, Wang W, Kerstetter RA, Schwab W, Korban SS, Davik J, Monfort A, Denoyes-Rothan B, Arus P, Mittler R, Flinn B, Aharoni A, Bennetzen JL, Salzberg SL, Dickerman AW, Velasco R, Borodovsky M, Veilleux RE, Folta KM (2011) The genome of woodland strawberry (Fragaria vesca). Nat Genet 43:109–116
Sun G, Xie F, Zhang B (2011) Transcriptome-wide identification and stress properties of the 14-3-3 gene family in cotton (Gossypium hirsutum L.). Funct Integr Genomics 11:627–636
Takatsuji H (1998) Zinc-finger transcription factors in plants. Cell Mol Life Sci CMLS 54:582–596
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Thomas J, Bowman MJ, Vega A, Kim HR, Mukherjee A (2018) Comparative transcriptome analysis provides key insights into gene expression pattern during the formation of nodule-like structures in Brachypodium. Funct Integr Genomics:1–12
Velasco R, Zharkikh A, Affourtit J, Dhingra A, Cestaro A, Kalyanaraman A, Fontana P, Bhatnagar SK, Troggio M, Pruss D, Salvi S, Pindo M, Baldi P, Castelletti S, Cavaiuolo M, Coppola G, Costa F, Cova V, Dal Ri A, Goremykin V, Komjanc M, Longhi S, Magnago P, Malacarne G, Malnoy M, Micheletti D, Moretto M, Perazzolli M, Si-Ammour A, Vezzulli S, Zini E, Eldredge G, Fitzgerald LM, Gutin N, Lanchbury J, Macalma T, Mitchell JT, Reid J, Wardell B, Kodira C, Chen Z, Desany B, Niazi F, Palmer M, Koepke T, Jiwan D, Schaeffer S, Krishnan V, Wu C, Chu VT, King ST, Vick J, Tao Q, Mraz A, Stormo A, Stormo K, Bogden R, Ederle D, Stella A, Vecchietti A, Kater MM, Masiero S, Lasserre P, Lespinasse Y, Allan AC, Bus V, Chagné D, Crowhurst RN, Gleave AP, Lavezzo E, Fawcett JA, Proost S, Rouzé P, Sterck L, Toppo S, Lazzari B, Hellens RP, Durel CE, Gutin A, Bumgarner RE, Gardiner SE, Skolnick M, Egholm M, van de Peer Y, Salamini F, Viola R (2010) The genome of the domesticated apple (Malus [times] domestica Borkh.). Nat Genet 42:833–839
Verde I et al (2013) The high-quality draft genome of peach (Prunus persica) identifies unique patterns of genetic diversity, domestication and genome evolution. Nat Genet 45:487–494
Wang Y, Tang H, DeBarry JD, Tan X, Li J, Wang X, Lee TH, Jin H, Marler B, Guo H, Kissinger JC, Paterson AH (2012) MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res 40:e49–e49
Wang Q, Liu J, Wang Y, Zhao Y, Jiang H, Cheng B (2015) Systematic analysis of the maize PHD-finger gene family reveals a subfamily involved in abiotic stress response. Int J Mol Sci 16:23517–23544
Wu T, Zhang R, Gu C, Wu J, Wan H, Zhang S, Zhang S (2012) Evaluation of candidate reference genes for real time quantitative PCR normalization in pear fruit. Afr J Agric Res 7:3701–3704
Wu J, Wang Z, Shi Z, Zhang S, Ming R, Zhu S, Khan MA, Tao S, Korban SS, Wang H, Chen NJ, Nishio T, Xu X, Cong L, Qi K, Huang X, Wang Y, Zhao X, Wu J, Deng C, Gou C, Zhou W, Yin H, Qin G, Sha Y, Tao Y, Chen H, Yang Y, Song Y, Zhan D, Wang J, Li L, Dai M, Gu C, Wang Y, Shi D, Wang X, Zhang H, Zeng L, Zheng D, Wang C, Chen M, Wang G, Xie L, Sovero V, Sha S, Huang W, Zhang S, Zhang M, Sun J, Xu L, Li Y, Liu X, Li Q, Shen J, Wang J, Paull RE, Bennetzen JL, Wang J, Zhang S (2013) The genome of the pear (Pyrus bretschneideri Rehd). Genome Res 23:396–408
Wu S, Wu M, Dong Q, Jiang H, Cai R, Xiang Y (2016a) Genome-wide identification, classification and expression analysis of the PHD-finger protein family in Populus trichocarpa. Gene 575:75–89
Wu XJ, Li MY, Que F, Wang F, Xu ZS, Xiong AS (2016b) Genome-wide analysis of PHD family transcription factors in carrot ( Daucus carota L.) reveals evolution and response to abiotic stress. Acta Physiol Plant 38:1–15
Wysocka J, Swigut T, Xiao H, Milne TA, Kwon SY, Landry J, Kauer M, Tackett AJ, Chait BT, Badenhorst P, Wu C, Allis CD (2006) A PHD finger of NURF couples histone H3 lysine 4 trimethylation with chromatin remodelling. Nature 442:86–90
Xie M, Huang Y, Zhang Y, Wang X, Yang H, Yu O, Dai W, Fang C (2013) Transcriptome profiling of fruit development and maturation in Chinese white pear (Pyrus bretschneideri Rehd). BMC Genomics 14:823
Yang X, Makaroff CA, Ma H (2003) The Arabidopsis MALE MEIOCYTE DEATH1 gene encodes a PHD-finger protein that is required for male meiosis. Plant Cell 15:1281–1295
Yang C, Vizcay-Barrena G, Conner K, Wilson ZA (2007) Male sterility1 is required for tapetal development and pollen wall biosynthesis. Plant Cell 19:3530–3548
Ye J, Fang L, Zheng H, Zhang Y, Chen J, Zhang Z, Wang J, Li S, Li R, Bolund L, Wang J (2006) WEGO: a web tool for plotting GO annotations. Nucleic Acids Res 34:W293–W297
Zhang Q et al (2012) The genome of Prunus mume. Nat Commun 3:187–190
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grant 31640068) and the 2017 Graduate Innovation Fund of the Anhui Agriculture University (grant 2017yis-31).
Author information
Authors and Affiliations
Contributions
Yunpeng Cao and Yahui Han conceived and designed the experiments; Yunpeng Cao and Yahui Han performed the experiments; Yunpeng Cao, Qing Jin, Yahui Han, and Dandan Meng analyzed the data; Yunpeng Cao, Yahui Han, Dahui Li, Yi Lin, and Yongping Cai contributed reagents/materials/analysis tools; Yunpeng Cao and Yahui Han wrote the paper.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Figure S1
Phylogenetic tree of the PHD-finger genes in ten plant species. The neighbor-joining (NJ) tree was constructed using the MEGA5 software. (GIF 419 kb)
Figure S2
Schematic depictions of the alternatively spliced genes PbPHD22 and PbPHD23. (GIF 115 kb)
Figure S3
Multiple sequence alignment of the PbPHD domain amino acids. (GIF 493 kb)
Figure S4
Multiple sequence alignment of the PbPHD domain nucleotides. (GIF 64 kb)
Figure S5
Microsynteny of the PHD-finger regions across pear, apple, peach, mei and strawberry. The chromosomes in pear, apple, peach, mei and strawberry, shown in different colors, are labeled Pb, Md, Pp, Pm and Fv. Numbers along each chromosome box indicate the lengths in megabases. Black lines represented the syntenic relationships between the PHD-finger regions. (GIF 94 kb)
Figure S6
Heat map of the expression levels of the PbPHD genes in pear fruit 25, 55, 85, 115, and 145 days after flowering (DAF). The values 1.5, 1, 0.5, 0, −0.5, −1 and − 2 represent high, intermediate and low expression, respectively. (GIF 215 kb)
Table S1
Primers used in this study. (XLSX 10 kb)
Table S2
Annotation of the PbPHD genes. (XLSX 11 kb)
Table S3
Full-length PHD-finger genes identified from five Rosaceae species (pear, apple, peach, mei, and strawberry). (XLSX 152 kb)
Table S4
All MEME motif sequences in the pear PHD-finger proteins. (XLSX 12 kb)
Table S5
Functional annotation details of 21 PbPHD protein sequences. (XLS 25 kb)
Table S6
Orthologous analyses of the PHD-finger genes between pear and the other four plant species of apple, peach, strawberry and mei. (XLSX 14 kb)
Rights and permissions
About this article
Cite this article
Cao, Y., Han, Y., Meng, D. et al. Systematic analysis and comparison of the PHD-Finger gene family in Chinese pear (Pyrus bretschneideri) and its role in fruit development. Funct Integr Genomics 18, 519–531 (2018). https://doi.org/10.1007/s10142-018-0609-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10142-018-0609-9












