Abstract
Purpose
Multiple intracranial aneurysms (MIA) occur in one-third of patients with intracranial aneurysms (IA), and have been previously associated with an overall worse prognosis. Risk factors for IA formation and rupture in patients with a single IA are well-known. However, risk factors associated with rupture in patients with MIA have been less studied.
Methods
We performed a retrospective search of patients with MIA identified by computed tomography angiography (CTA) within a 10-year period. Patients with > 1 saccular aneurysm with size ≥ 2.0 mm were included. The location, size, number, and rupture status of the aneurysms were recorded. Patient demographics and cerebrovascular risk factors were obtained from electronic medical records. The primary endpoint of this study was to determine the association of these factors with aneurysmal rupture. The case-fatality rate was evaluated as a secondary outcome.
Results
Of the 2957 patients with IA in our CTA database, 425 patients were diagnosed with MIA and were therefore included in our study. A total of 1082 aneurysms were identified. Predictors of increased risk of aneurysmal rupture were age (OR 0.98, 95% CI, 0.96–0.99), size ≥ 5 mm (OR 4.4, 95% CI 2.76–7.0); and location in the anterior communicating artery complex (AcomC) (OR 2.62, 95% CI, 1.46–4.72) or posterior communicating artery (PCOM) (OR 2.66, 95% CI, 1.45–4.87).
Conclusions
Younger age, aneurysm size ≥ 5 mm, and location in the AcomC and PCOM were independently associated with aneurysmal rupture in patients with MIA. Identifying these features could help recognize patients who might benefit from early intervention.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Despite the implementation of multiple screening and therapeutic interventions in the past decades, the mortality of ruptured intracranial aneurysms (AI) remains high, with rates oscillating between 15 and 30% [1]. Aneurysmal subarachnoid hemorrhage (aSAH) carries high morbidity, and a recent study reports that up to 35% of these patients experience a reduction of quality of life [1]. Multiple intracranial aneurysms (MIA), defined by the concomitant presence of ≥ 2 aneurysms [2], are detected in approximately one-third of patients with IA [3,4,5,6,7]. [Fig. 1] Although relatively uncommon, they have been previously linked with increased risk of IA rupture [6, 8] and overall worse prognosis [5, 9, 10]. Therefore, identifying clinical and imaging characteristics that help identify MIA patients with a higher risk of rupture can ultimately aid in the selection of patients that could benefit from early intervention.
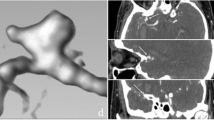
A 65-year-old woman with no significant past medical history presented to the emergency department with right-sided weakness and dizziness. A CTA was obtained, showing a 7-mm downward-pointing aneurysm arising from the right MCA (a), a 3-mm saccular aneurysm arising from the lateral aspect of the right terminal ICA (b), and a 11-mm posteriorly pointing aneurysm arising from the left posterior communicating artery (c)
IA is considered a complex pathology, implicating degenerative changes in the arterial wall [11], and it is thought to be caused by a combination of genetic and environmental risk factors [1]. Several studies have evaluated risk factors related to the rupture and formation of aneurysms, but these study groups are mainly composed of patients with single unruptured IA [12,13,14,15,16]. Most of the available information regarding MIA is derived from sub-population analyses within a larger cohort of SIA patients [13, 16, 17]. However, previous studies have identified significant differences between MIA and SIA patient populations [3, 9, 18, 19], suggesting that the MIA population should be examined independently. To our knowledge, very few studies are available about rupture risk factors focused specifically on patients with MIA. [5, 20,21,22,23,24].
The objective of this study was to analyze the clinical and aneurysmal imaging characteristics associated with rupture in patients with MIA. As a secondary endpoint, we aimed to determine the case-fatality rate for these patients.
Materials and methods
Patient selection
Our hospital’s Institutional Review Board approved this study and waived the requirement for patient consent. A retrospective search of our radiology database for patients with MIA, using the RENDER software [25], was performed for a time frame of 10 years. Patients who underwent a head CTA and presented with more than one IA larger than 2 mm in size, with or without SAH, were identified. For patients with multiple CTA studies, the first study was used for analysis; however, for patients with a ruptured IA, the first CTA performed after the rupture was analyzed. Cases with fusiform aneurysms, dissecting aneurysms, and infundibular dilatations were excluded. Aneurysms were considered incidental if the CTA was performed for an indication other than (1) subarachnoid hemorrhage on NCCT; (2) assessment for an IA; (3) evaluation of intracranial hemorrhage, or (4) follow-up of a known aneurysm.
Data collection
Electronic medical records were assessed to identify patient’s demographics and in-hospital case-fatality rate attributed to IA rupture, including discharge summaries, physician/nursing notes, and laboratory results. Smoking, hypertension (HTN), diabetes mellitus (DM), and hyperlipidemia (HLD) were recorded only if the diagnosis or related medications were mentioned within 3 months after the CTA. The presence of DM and HLD was recorded if established laboratory criteria were satisfied, according to the American Association of Diabetes [26]. Smoking history was divided into current, former, or never. A patient characteristic was described as missing if it was not available in medical history.
Imaging
NCCTs and CTAs were performed on all patients using multi-slice (16 and 64 slices) scanners by Siemens (Siemens, Erlangen, Germany) and GE (General Electric Medical Systems, Milwaukee, USA). The parameters for the NCCT scans of the brain were 120–140 kVp, 170–350 mA, 22–23 cm FOV, and 1.2–5.0-mm slice thickness. The CTA parameters were 120–140 kVp, 170–350 mA, 18–25 cm FOV, and 0.6–1.25-mm slice thickness. Iodinated contrast material (65–85 mL) was administered by a power injector at a rate of 4–5 mL per second into an antecubital vein with SmartPrep, a semiautomatic contrast bolus–triggering technique. CTA was performed by scanning from the aortic arch to the cranial vertex. Later, post-processing with 3D angiographic techniques was performed; this include maximum intensity projection, volume-rending, and multi-planar reconstructions.
Imaging evaluation
All NCCT and CTA images were re-evaluated separately by two neuroradiologists (AL and SS), each with two years of experience. In cases of discrepancy, the consensus was reached with a third neuroradiologist with 14 years of experience (JMR). An IA was defined as a saccular dilation arising from the luminal wall or bifurcation of the cerebral arteries that is ≥ 2.0 mm in diameter. The location, size, number, and rupture status of the aneurysms were recorded. Aneurysms located in the ICA were subdivided into six segments according to a classification system by Shapiro et al. as the following: petrous, cavernous, paraophthalmic, posterior communicating (PCOM) ICA, choroidal, and terminus [27]. We also assessed other anterior circulation aneurysms such as the M1 (pre-bifurcation and bifurcation segments) and M2 segments of the MCA, the anterior communicating artery complex (AcomC), the anterior cerebral artery, and the posterior circulation aneurysms. The likely site of aneurysm rupture was determined by associating the distribution of SAH, intraventricular hemorrhage, and intraparenchymal hematoma on NCCT with an aneurysm detected in the vicinity by CTA [28]. Neurosurgery or DSA reports were also used to assess the site of aneurysm rupture, which were available in 91% of the patients. In cases where discrepancies over the site of rupture were found based on the CTA, DSA, or surgery (5.7% of the cases), surgery and DSA results were used for statistical analysis.
Statistical analysis
Binary variables (sex, HTN, DM, HLD, and rupture status) and categorical variables (smoking and IA locations) were presented as percentages. Continuous variables (age, number of IAs per patient, and IA size) were summarized by their mean, standard deviation, median, range, and interquartile range. In order to evaluate the likelihood of IA rupture, odds ratios with 95% CI were calculated. Univariate analyses between a binary/continuous variable and binary outcomes were performed using the chi square test or logistic regression. Univariate analyses between a binary variable and a continuous outcome were performed using unpaired Student t test or Wilcoxon-Mann-Whitney test, according to the data distribution. Correlation between continuous variables was calculated using the Spearman rank test.
To determine the optimal IA size cutoff to use for the prediction of IA rupture, we compared the sensitivities and specificities using ≥ 5, ≥ 6, and ≥ 7 mm as cutoffs. Patient characteristics, chosen based on data from preexisting literature [19, 29], and five intradural aneurysm locations were included in the multivariable regression model. Missing values in datasets were replaced using multiple imputations by chained equations technique. Significant differences between complete and incomplete dataset analyses were calculated. If no significant differences were found, the imputed dataset analyses were reported. Stata statistical software (Release 14, College Station, TX: StataCorp LP) was used for analysis.
Results
Patient selection and baseline characteristics
Of the 2957 patients diagnosed with IAs, 425 (14%) patients had MIA and were included in the study. The total number of examined aneurysms was 1082. Common clinical indications that lead to a CTA in patients with MIA were evaluation or follow-up of an IA (57%), headache (28%), intracranial hemorrhage (21%), or altered mental status (9%). Nineteen percent (19%) of the patients with MIA had their aneurysms discovered incidentally on CTAs performed for other indications.
There were 331 females (mean age, 60.8 years; SD, 13.8) and 94 men (mean age, 60.1 years; SD, 12.8). Females had significantly more aneurysms than males (p = 0.010). No significant difference was found between the number of aneurysms and patient age (r = 0.013, p = 0.779). History of smoking (current and former smokers) was present in 73% of our patients. HTN, HLD, and DM were found in 63%, 53%, and 10% of these patients, respectively. In our cohort, 11.5% (49/425) of the patients diagnosed with MIA had missing data. Smoking history, HTN, HLD, and DM each represented about 8% of missing data. Patient demographics are shown in detail in Table 1.
Aneurysm features
The majority of patients presented with 2 IAs (62%), and the highest number of IAs found in a single patient was 7. The median size of all aneurysms was 4.0 mm (range, 2.0–57.0 mm) (Table 2). Most IAs were located in the paraophthalmic ICA (27%), followed by the M1 segments (pre-bifurcation and bifurcation) of the MCA (21%), AcomC (11%), cavernous ICA (10%), and PCOM (10%) (Table 3). IAs arising from the anterior circulation were significantly more frequent than IAs from the posterior circulation (91% versus 9%; p < 0.001).
Aneurysmal rupture
The total number of patients with ruptured IAs was 107/425 (25.2%), and the case-fatality rate was 20.1% (22/107). Patients with ruptured IA were significantly younger than patients with UIA, with a median age difference of 5.5 years (p = 0.019). Current smokers were more likely to have a ruptured IA compared with non-smokers (OR 2.08; 95% CI, 1.15–3.76). No association was found between the number of IAs per patient and IA rupture (p = 0.608). The rest of the baseline characteristics were not associated with rupture (Table 1).
Ruptured aneurysms had a larger diameter compared with their unruptured counterpart, with a median size difference of 3.0 mm (p < 0.001). Fifty-nine percent of IAs (n = 643) measured < 5 mm in diameter and only 4% of them ruptured. On the other hand, 10% of the IAs (n = 109) measured between 10 and 24.9 mm, and 28% of these ruptured. Increased occurrence of rupture was seen in IAs measuring between 5 and 6.9 mm, 7 and 9.9 mm, and 10 and 24.9 mm compared with IAs < 5 mm in size (Table 2). We determined that a cutoff diameter of ≥ 5 mm is the most accurate measurement to predict aneurysm rupture (OR 4.4; 95% CI, 2.8–7.0). The sensitivity and specificity for this cutoff size are 74% and 63%, respectively.
We found that posterior circulation aneurysms were more prone to rupture compared with their anterior circulation counterpart (OR 2.9; 95% CI, 1.6–5.1). Among the ruptured IAs, the majority were located in the AcomC (27%), followed by the PCOM (23%), M1 bifurcation (13%), and basilar artery tip (8%) respectively. IAs arising from the AcomC (OR 3.5; 95% CI, 2.1–5.7), PCOM (OR 3.3; 95% CI, 1.9–5.6), basilar artery tip (OR 2.8; 95% CI, 1.1–6.2), and PICA (OR 5.4; 95% CI, 1.1–21.5) were more likely to rupture compared with those in other locations. On the other hand, IAs arising from the M1 pre-bifurcation segment (OR 0.4; 95% CI, 0.1–1.0) and paraophthalmic ICA (OR 0.07; 95% CI, 0.0–0.2) rarely ruptured. Univariate analysis of MIA location and IA rupture is shown in detail in Table 3.
Multivariable analysis
The multivariable regression model analyzing the patient characteristics and aneurysm characteristics revealed that age (OR 0.98; 95% CI, 0.96–0.99), IA size ≥ 5 mm (OR 4.40; 95% CI, 2.76–7.0), and IA locations in the AcomC (OR 2.62; 95% CI, 1.46–4.72) and PCOM (OR 2.66; 95% CI, 1.45–4.87) were significantly associated with IA rupture in patients with MIA (Table 4). IAs located in the paraophthalmic ICA were negatively associated with IA rupture.
Discussion
We found an association between aneurysms emerging from AcomC and PCOM and rupture, and these findings are similar to previous literature [5, 10, 12, 20, 21, 24]. The size of the IA was also independently associated with the risk of rupture. In our population, ruptured IAs were significantly larger than the unruptured ones, and a cutoff diameter of ≥ 5 mm was associated with the incidence of rupture. Past MIA studies have demonstrated similar findings, two of which also determined cutoff sizes of 4 mm [5] and 7 mm [21] to be associated with rupture. We also noticed a higher rupture rate in the posterior circulation, which is consistent with previous literature regarding single IA, thus demonstrating that the risk is maintained for patients with MIA [12, 14, 15]. Recent research has attributed the increased posterior bleeding risk to the aneurysm’s intrinsic morphological features, such as an overall larger diameter, higher aspect ratio, and parent artery size [30, 31]. However, extrapolating information from single IA cohorts should be done with caution, since previous literature has identified significant differences between MIA and SIA patient populations [3, 9, 18, 19]. For this reason, studies that focus exclusively on MIA patients may provide important information. To our knowledge, our study has the largest MIA cohort to date.
Smoking was significantly associated with a higher risk of rupture in the univariate analysis, and our finding is similar to prior studies done both single AI and MIA cohorts [5, 32, 33]. In the multivariable analysis, the direction of the effect for the relationship of active smoking and rupture was also consistent towards an increased risk despite not reaching statistical significance. We believe this might be due to a dilution of effect, given the strength of association of aneurysm size and location in our multivariable model. No other association was found between demographic characteristics, and the risk of rupture was found in this cohort. Our population showed a higher prevalence of HTN and an overall older age compared with the rest of the studies dedicated to MIA patients. These findings may be explained by our study’s broader inclusion criteria.
We found that increasing age is inversely related to the risk of aneurysm rupture since patients with ruptured IAs had a significantly lower median age compared with those without rupture. Lu H-T. et al. reported a similar finding, showing that patients between the age of 45 and 65 had a higher incidence of rupture compared with those older than 65 in their MIA cohort [20]. A possible explanation might be the slower flow rate found in atherosclerotic or calcified walls of older patients [34].
We found no significant association between the number of IAs and the risk of rupture. Previous studies have yielded ambiguous evidence about this association; some have been able to successfully establish this association [16], while others have failed [12]. However, the fact that these studies were focused on patients with single unruptured IA further decreases the generalizability of this information to our study.
The case-fatality rate is comparable with the mortality rate reported in previous studies that focused exclusively on patients with MIA. While the UCAS study on unruptured IA patients reported a higher case-fatality rate of 35% [5, 10, 20, 21, 24], this discrepancy could be explained by the age of their population, since 53% of their cohort who suffered an IA rupture were ≥ 70 years old, and only 23% of our ruptured cases were ≥ 70 years old [13]
This study has potential limitations. Computed tomography angiography was used as our reference imaging modality instead of the gold-standard DSA. Nonetheless, CTA has demonstrated high sensitivity and specificity for depicting IAs compared with DSA, even for aneurysms as small as 3.0 mm [35]. The retrospective nature of our study and the fact that it was performed in a large neurovascular referral center may possibly lead to selection bias. Lastly, other known factors that may affect aneurysm ruptures, such as aneurysm irregularity, aspect ratio, or size ratio [22, 24], were not examined in our study.
Conclusions
Younger patients with IA in the AcomC and PCOM locations, and larger than 5 mm were significantly associated with aneurysm rupture in patients with MIA. Identifying these features could help identify those patients who might benefit from early intervention.
References
Muehlschlegel S (2018) Subarachnoid hemorrhage. Continuum: Lifelong Learning Neurol 24:1623–1657
Ramazan J (2018) Dinger Thiemo Florin, Darkwah Oppong Marvin, Pierscianek Daniela, Dammann Philipp, Wrede Karsten H., et al. Risk factors for and clinical consequences of multiple intracranial aneurysms. Stroke. 49:848–855
Rinne J, Hernesniemi J, Puranen M, Saari T (1994) Multiple intracranial aneurysms in a defined population: prospective angiographic and clinical study. Neurosurgery. 35:803–808
Nehls DG, Flom RA, Carter LP, Spetzler RF (1985) Multiple intracranial aneurysms: determining the site of rupture. J Neurosurg 63:342–348
Jiang H, Weng Y-X, Zhu Y, Shen J, Pan J-W, Zhan R-Y (2016) Patient and aneurysm characteristics associated with rupture risk of multiple intracranial aneurysms in the anterior circulation system. Acta Neurochir 158:1367–1375
Wilson FMA, Jaspan T, Holland IM (1989) Multiple cerebral aneurysms — a reappraisal. Neuroradiology. 31:232–236
Roethlisberger M, Achermann R, Bawarjan S, Stienen MN, Fung C, D’Alonzo D, et al. (2018) Impact of aneurysm multiplicity on treatment and outcome after aneurysmal subarachnoid hemorrhage. Neurosurgery
Vajda J (1992) Multiple intracranial aneurysms: a high risk condition. Acta Neurochir 118:59–75
Rinne J, Hernesniemi J, Niskanen M, Vapalahti M (1995) Management outcome for multiple intracranial aneurysms. Neurosurgery. 36:31–37 discussion 37-38
Makio K, Masahiro Y, Shobu S (2003) Incidence and outcome of multiple intracranial aneurysms in a defined population. Stroke. 34:16–21
Grobelny TJ (2011) Brain aneurysms: epidemiology, treatment options, and milestones of endovascular treatment evolution. Disease-a-Month. 57:647–655
Greving JP, Wermer MJH, Brown RD, Morita A, Juvela S, Yonekura M et al (2014) Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol 13:59–66
UCAS Japan Investigators, Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S et al (2012) The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med 366:2474–2482
International Study of Unruptured Intracranial Aneurysms Investigators (1998) Unruptured intracranial aneurysms--risk of rupture and risks of surgical intervention. N Engl J Med 339:1725–1733
Wermer MJH, van der Schaaf IC, Algra A, Rinkel GJE (2007) Risk of rupture of unruptured intracranial aneurysms in relation to patient and aneurysm characteristics: an updated meta-analysis. Stroke. 38:1404–1410
Sonobe M, Yamazaki T, Yonekura M, Kikuchi H (2010) Small unruptured intracranial aneurysm verification study: SUAVe study, Japan. Stroke. 41:1969–1977
Yasui N, Suzuki A, Nishimura H, Suzuki K, Abe T (1997) Long-term follow-up study of unruptured intracranial aneurysms. Neurosurgery. 40:1155–1159 discussion 1159-1160
McDowell MM, Zhao Y, Kellner CP, Barton SM, Sussman E, Claassen J et al (2018) Demographic and clinical predictors of multiple intracranial aneurysms in patients with subarachnoid hemorrhage. J Neurosurg 128:961–968
Ellamushi HE, Grieve JP, Jäger HR, Kitchen ND (2001) Risk factors for the formation of multiple intracranial aneurysms. J Neurosurg 94:728–732
Lu H-T, Tan H-Q, Gu B-X, Null W-W, Li M-H (2013) Risk factors for multiple intracranial aneurysms rupture: a retrospective study. Clin Neurol Neurosurg 115:690–694
Backes D, Vergouwen MDI, Velthuis BK, van der Schaaf IC, Bor ASE, Algra A et al (2014) Difference in aneurysm characteristics between ruptured and unruptured aneurysms in patients with multiple intracranial aneurysms. Stroke. 45:1299–1303
Wang G-X, Liu L-L, Wen L, Cao Y-X, Pei Y-C, Zhang D (2017) Morphological characteristics associated with rupture risk of multiple intracranial aneurysms. Asian Pac J Trop Med 10:1011–1014
Zhang Y, Yang X, Wang Y, Liu J, Li C, Jing L et al (2014) Influence of morphology and hemodynamic factors on rupture of multiple intracranial aneurysms: matched-pairs of ruptured-unruptured aneurysms located unilaterally on the anterior circulation. BMC Neurol 14:253
Bhogal P, AlMatter M, Hellstern V, Ganslandt O, Bäzner H, Henkes H, et al. Difference in aneurysm characteristics between ruptured and unruptured aneurysms in patients with multiple intracranial aneurysms. Surg Neurol Int [Internet]. 2018 [cited 2019 Jan 17];9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5778729/
Dang PA, Kalra MK, Schultz TJ, Graham SA, Dreyer KJ (2009) Informatics in radiology: Render: an online searchable radiology study repository. Radiographics. 29:1233–1246
Association AD (2011) Standards of medical care in diabetes—2011. Diabetes Care 34:S11–S61
Shapiro M, Becske T, Riina HA, Raz E, Zumofen D, Jafar JJ et al (2014) Toward an endovascular internal carotid artery classification system. AJNR Am J Neuroradiol 35:230–236
Long B, Koyfman A (2016) Controversies in the diagnosis of subarachnoid hemorrhage. J Emerg Med 50:839–847
Zacharia BE, Hickman ZL, Grobelny BT, DeRosa P, Kotchetkov I, Ducruet AF et al (2010) Epidemiology of aneurysmal subarachnoid hemorrhage. Neurosurg Clin N Am 21:221–233
Tykocki T, Kostkiewicz B (2014) Aneurysms of the anterior and posterior cerebral circulation: comparison of the morphometric features. Acta Neurochir 156:1647–1654
Wang G-X, Zhang D, Wang Z-P, Yang L-Q, Yang H, Li W (2018) Risk factors for ruptured intracranial aneurysms. Indian J Med Res 147:51–57
Can A, Castro VM, Ozdemir YH, Dagen S, Yu S, Dligach D et al (2017) Association of intracranial aneurysm rupture with smoking duration, intensity, and cessation. Neurology. 89:1408–1415
Juvela S, Korja M (2017) Intracranial aneurysm parameters for predicting a future subarachnoid hemorrhage: a long-term follow-up study. Neurosurgery. 81:432–440
Inagawa T (2010) Risk factors for the formation and rupture of intracranial saccular aneurysms in Shimane, Japan. World Neurosurg 73:155–164
Lu L, Zhang LJ, Poon CS, Wu SY, Zhou CS, Luo S et al (2012) Digital subtraction CT angiography for detection of intracranial aneurysms: comparison with three-dimensional digital subtraction angiography. Radiology. 262:605–612
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study is compliant with the Health Insurance Portability and Accountability Act (HIPAA) and was approved by the Institutional Review Board.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liberato, A.C.P., Xu, J., Montes, D. et al. Multivariable analysis on factors associated with aneurysm rupture in patients with multiple intracranial aneurysms. Emerg Radiol 27, 487–494 (2020). https://doi.org/10.1007/s10140-020-01790-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10140-020-01790-5